Effects of Continuous Prenatal Low Dose Rate Irradiation on Neurobehavior, Hippocampal Cellularity, Messenger RNA and MicroRNA Expression on B6C3F1 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Irradiation
2.2. Behavioral Studies
2.2.1. The SmithKline, Harwell, Imperial College, Royal Hospital, Phenotype Assessment (SHIRPA) Test
2.2.2. Open Field (Locomotor) Test
2.2.3. Novel Object Recognition Test
2.2.4. Forced Swim Test
2.2.5. Tail Suspension Test
2.3. Pathological Examination
2.4. Immunohistochemical Staining of the Hippocampus
2.5. RNA Extraction from the Hippocampus and Whole Blood
2.6. Systematic miR Sequencing (miRSeq) and mRNA Sequencing Analysis
2.7. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) Analysis of miR
2.8. Real-Time RT-PCR Analysis for mRNA
2.9. Statistical Analyses
3. Results
3.1. Behavioral Studies
3.1.1. SHIRPA Test
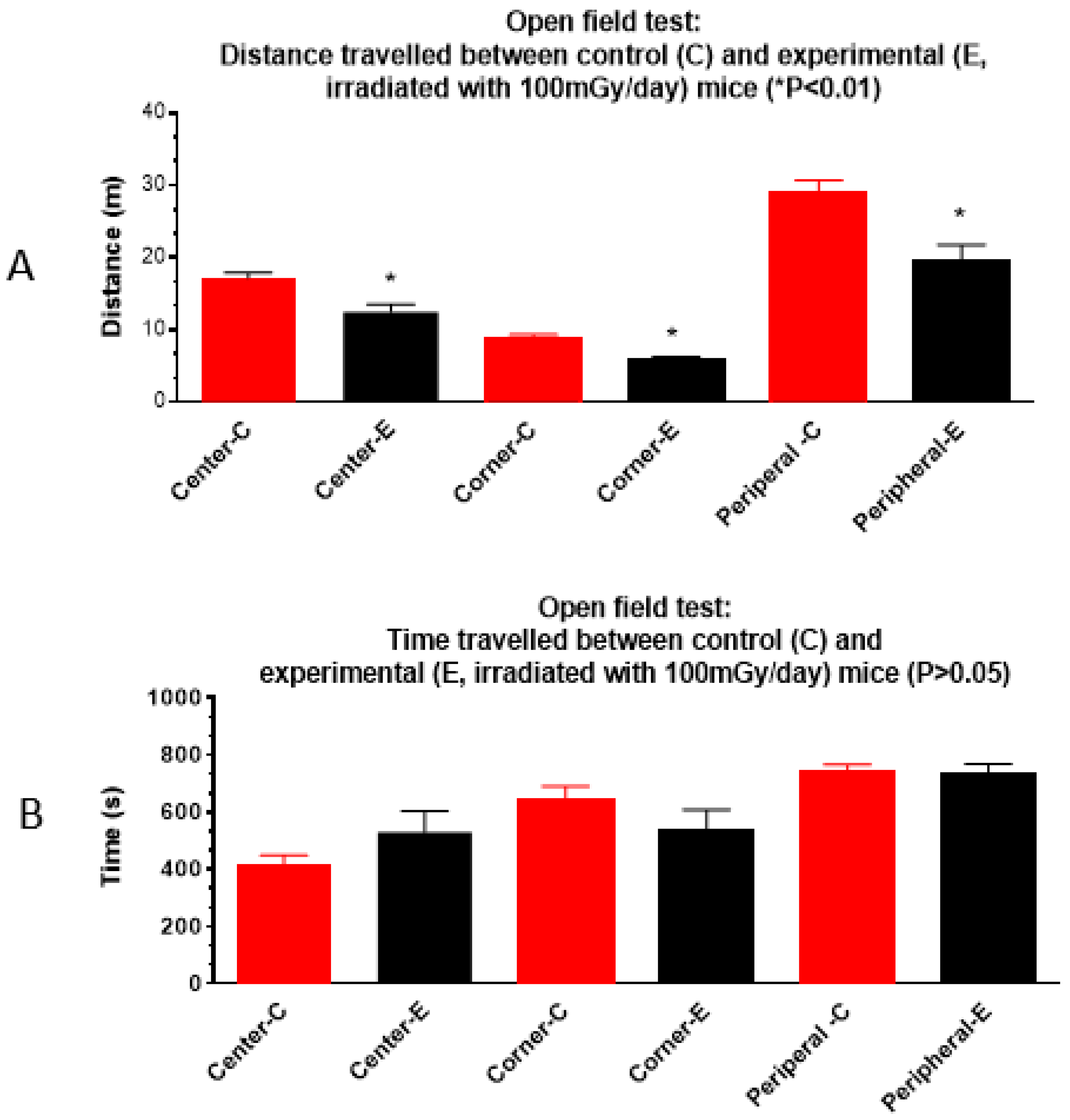
3.1.2. Open Field Test
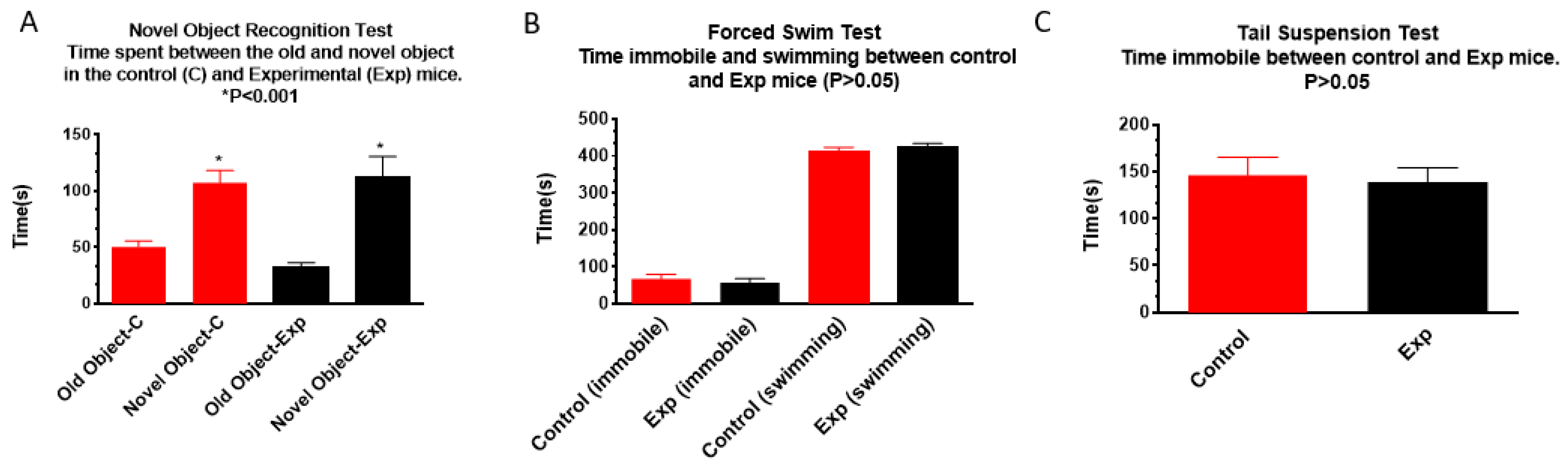
3.1.3. Novel Object Recognition, Forced Swim, and Tail Suspension Tests
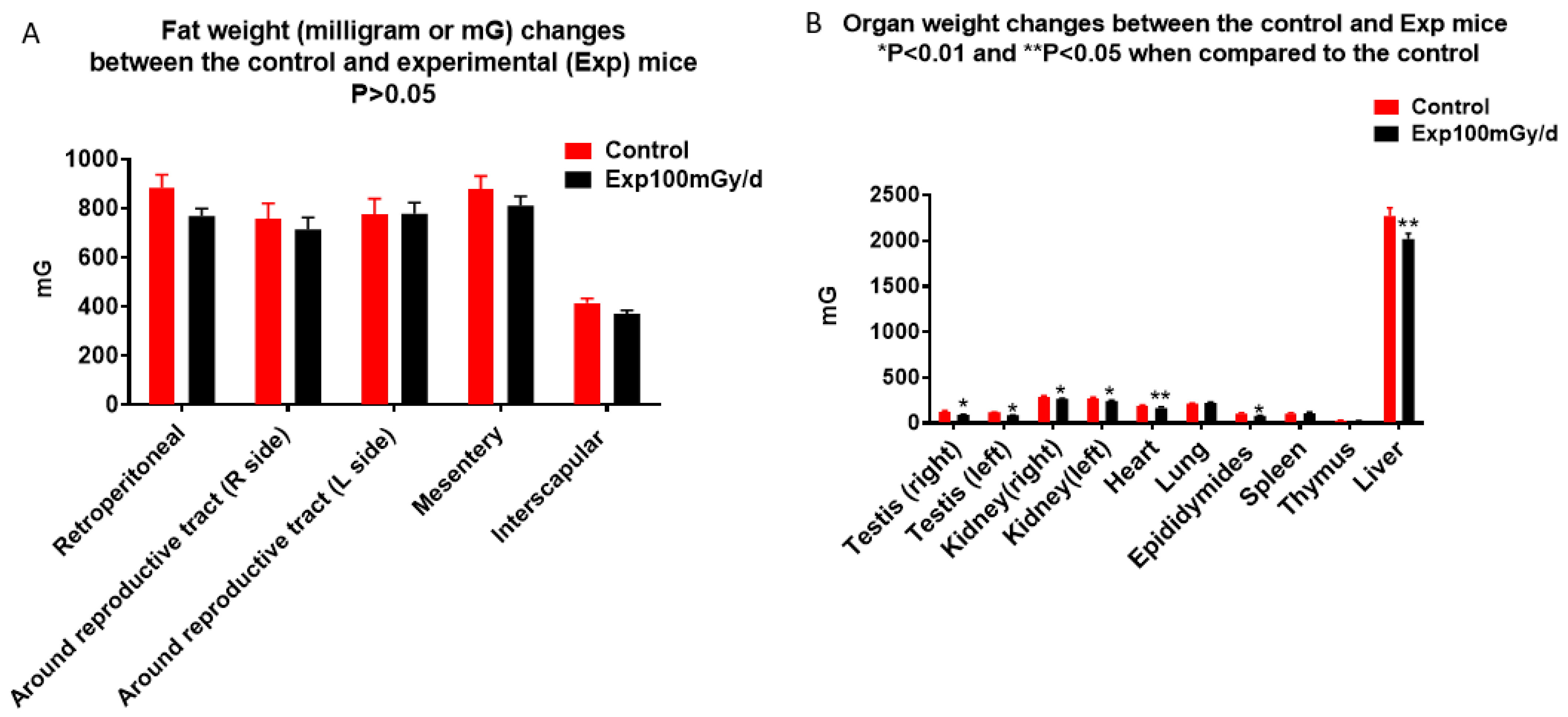
3.2. Pathology
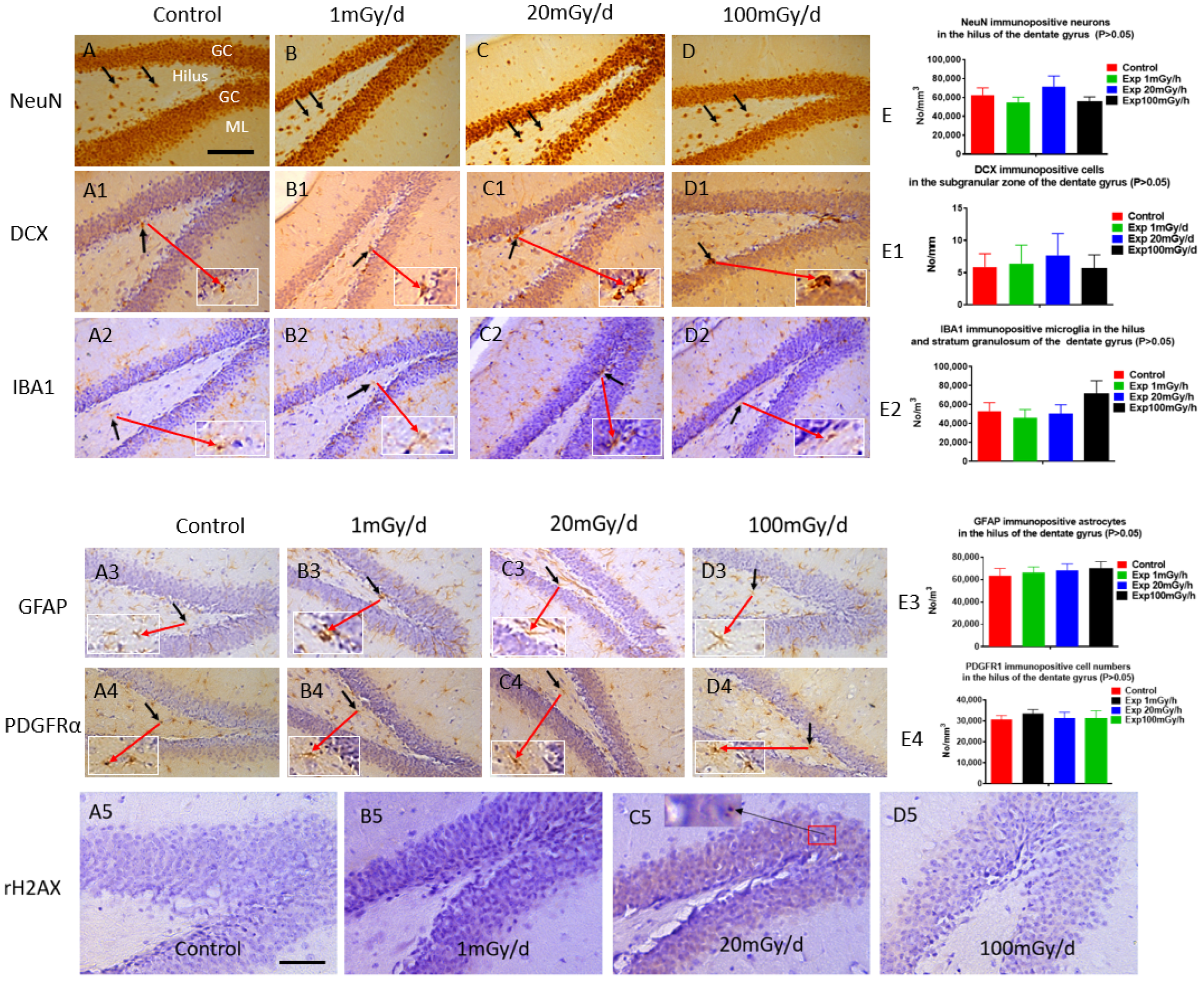
3.3. Immunohistochemistry
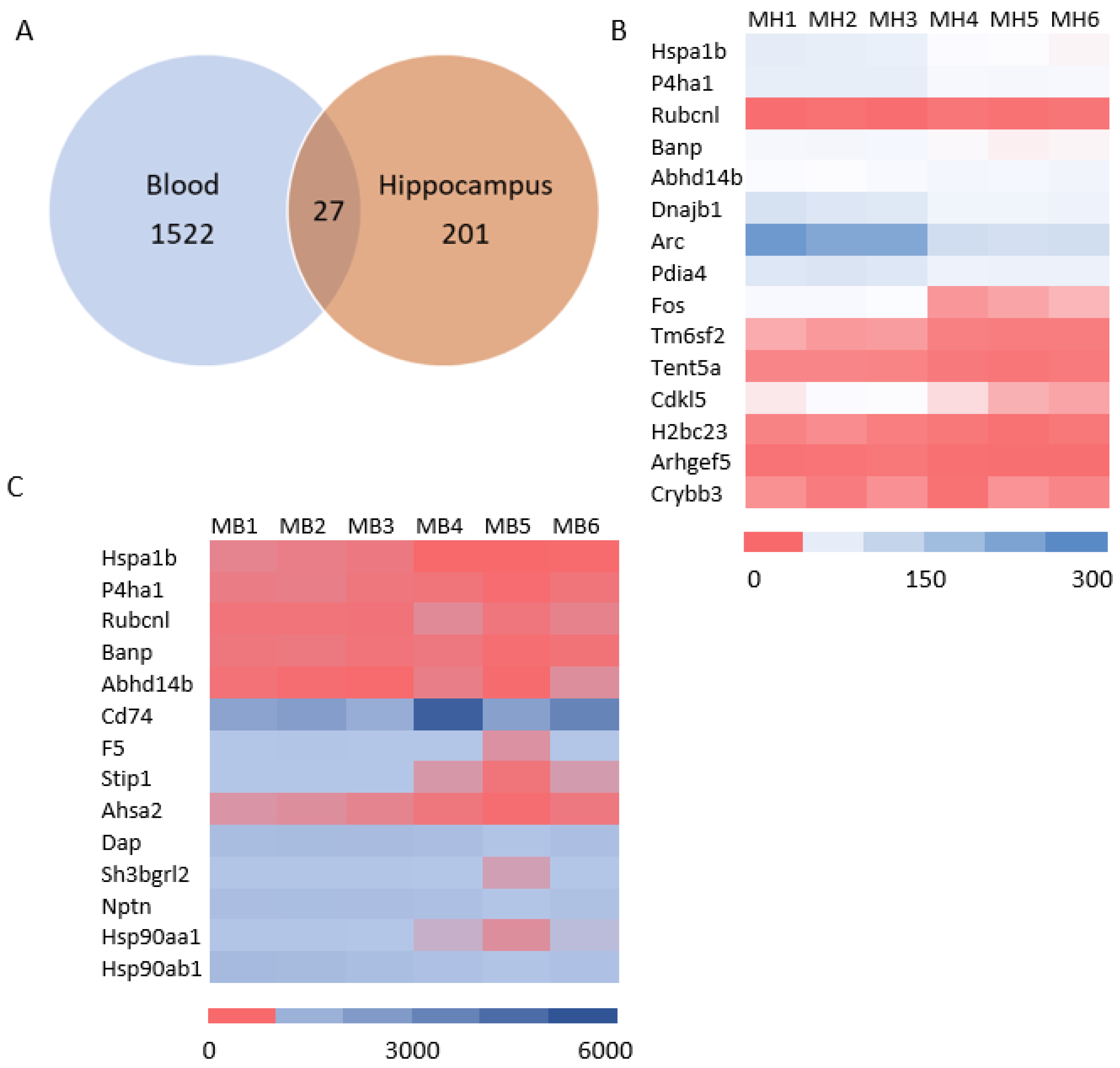
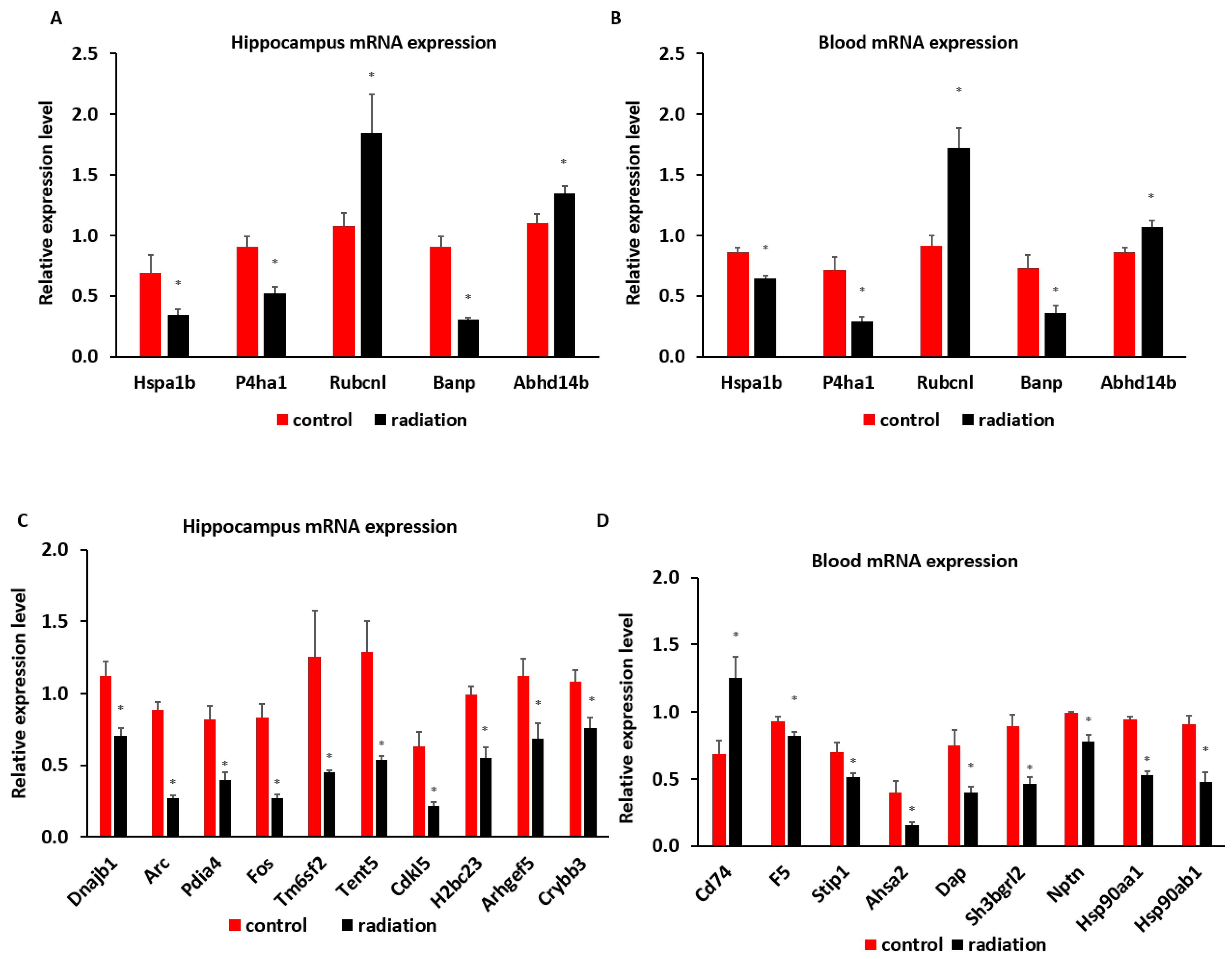
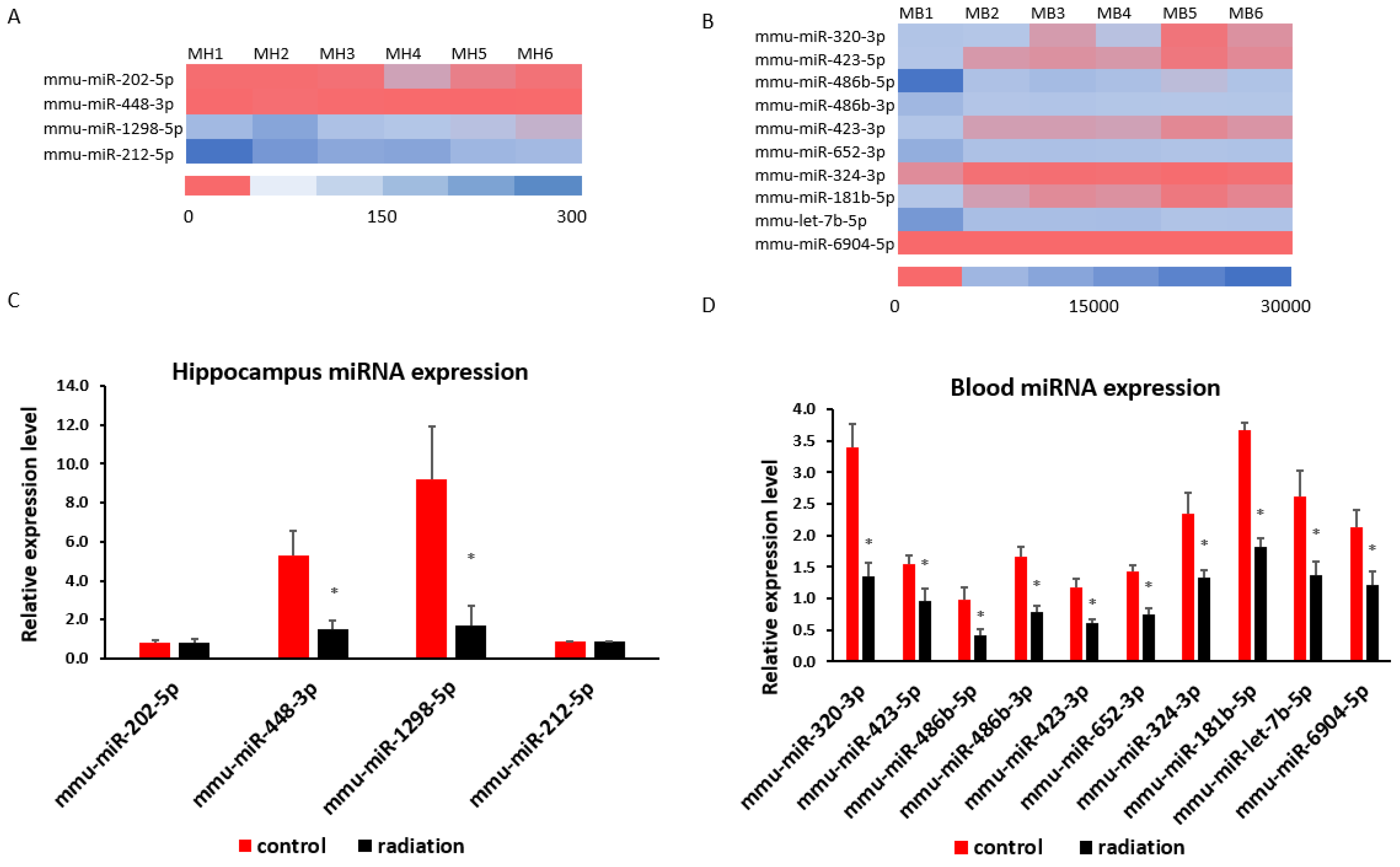
3.4. Systematic miR Sequencing (miRSeq) and mRNA Sequencing Analysis
4. Discussion
4.1. Main Findings
4.2. Pre- and Post-Natal (First 31 Days) Irradiation with a Low Dose Rate of 1 mGy/d and 20 mGy/d Did Not Induce Obvious Pathological Changes in the Brain and Other Organs
4.3. Prenatal Irradiation with a Dose Rate of 100 mGy/d (4.55 mGy/h, Total Accumulated Dose = 1.8 Gy) Did Not Induce Obvious Pathophysiological Changes in Middle-Aged Mice
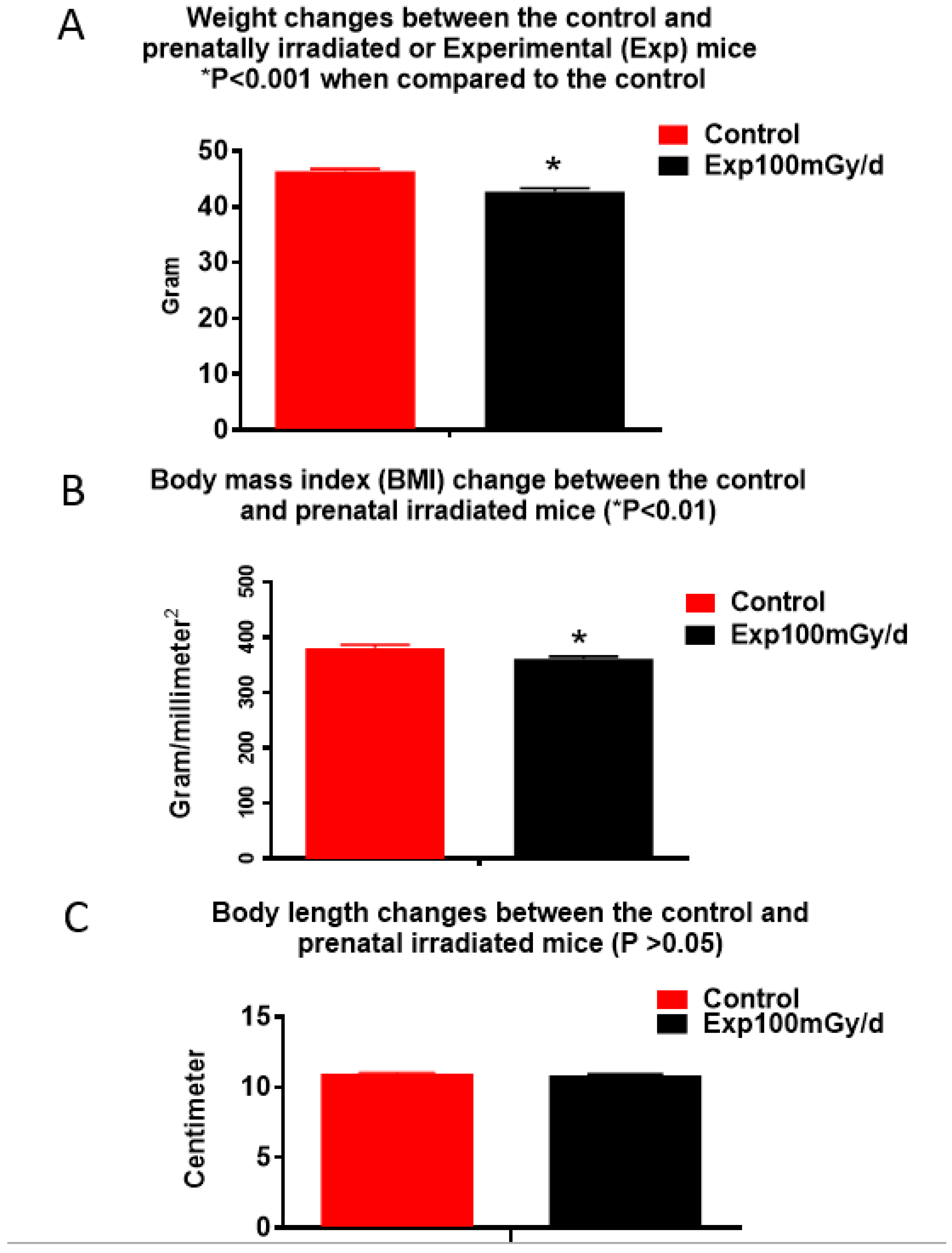
4.4. Low Dose/Dose Rate Radiation-Induced Animal Weight Changes
4.5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekaban, A.S. Abnormalities in children exposed to x-radiation during various stages of gestation: Tentative timetable of radiation injury to the human fetus. I. J. Nucl. Med. 1968, 9, 471–477. [Google Scholar]
- Goldstein, L.; Murphy, D.P. Etiology of ill health in children born after maternal pelvic irradiation. II. Defective children born after post conceptional maternal irradiation. Am. J. Roentgenol. 1929, 22, 322–331. [Google Scholar]
- Michalet, M.; Dejean, C.; Schick, U.; Durdux, C.; Fourquet, A.; Kirova, Y. Radiotherapy and pregnancy. Cancer Radiother. 2021, 26, 417–423. [Google Scholar] [CrossRef]
- Yang, B.; Ren, B.X.; Tang, F.R. Prenatal irradiation-induced brain neuropathology and cognitive impairment. Brain Dev. 2017, 39, 10–22. [Google Scholar] [CrossRef]
- Otake, M.; Schull, W.J. Radiation-related brain damage and growth retardation among the prenatally exposed atomic bomb survivors. Int. J. Radiat. Biol. 1998, 74, 159–171. [Google Scholar] [CrossRef]
- Otake, M.; Schull, W.J.; Yoshimaru, H. A review of forty-five years study of Hiroshima and Nagasaki atomic bomb survivors. Brain damage among the prenatally exposed. J. Radiat. Res. 1991, 32, 249–264. [Google Scholar] [CrossRef]
- Yamazaki, J.N.; Wright, S.W.; Wright, P.M. A study of the outcome of pregnancy in women exposed to the atomic bomb blast in Nagasaki. J. Cell. Comp. Physiol. 1954, 43 (Suppl. S1), 319–328. [Google Scholar] [CrossRef]
- Wit, F.; Vroonland, C.C.; Bijwaard, H. Prenatal X-ray Exposure and the Risk of Developing Pediatric Cancer-A Systematic Review of Risk Markers and a Comparison of International Guidelines. Health Phys. 2021, 121, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Rath, R.; Hammer, G.P.; Blettner, M. Are pre- or postnatal diagnostic X-rays a risk factor for childhood cancer? A systematic review. Radiat. Environ. Biophys. 2008, 47, 301–312. [Google Scholar] [CrossRef]
- Wakeford, R. Childhood leukaemia following medical diagnostic exposure to ionizing radiation in utero or after birth. Radiat. Prot. Dosim. 2008, 132, 166–174. [Google Scholar] [CrossRef]
- Zatsepin, I.; Verger, P.; Robert-Gnansia, E.; Gagnière, B.; Tirmarche, M.; Khmel, R.; Babicheva, I.; Lazjuk, G. Down syndrome time-clustering in January 1987 in Belarus: Link with the Chernobyl accident? Reprod. Toxicol. 2007, 24, 289–295. [Google Scholar] [CrossRef]
- Sperling, K.; Neitzel, H.; Scherb, H. Evidence for an increase in trisomy 21 (Down syndrome) in Europe after the Chernobyl reactor accident. Genet. Epidemiol. 2011, 36, 48–55. [Google Scholar] [CrossRef]
- Sperling, K.; Pelz, J.; Wegner, R.D.; Dorries, A.; Gruters, A.; Mikkelsen, M. Significant increase in trisomy 21 in Berlin nine months after the Chernobyl reactor accident: Temporal correlation or causal relation? BMJ 1994, 309, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Sperling, K.; Pelz, J.; Wegner, R.; Schulzke, I.; Struck, E. Frequency of trisomy 21 in Germany before and after the Chernobyl accident. Biomed. Pharmacother. 1991, 45, 255–262. [Google Scholar] [CrossRef]
- Wertelecki, W. Chornobyl radiation-congenital anomalies: A persisting dilemma. Congenit. Anom. 2020, 61, 9–13. [Google Scholar] [CrossRef]
- Wertelecki, W.; Chambers, C.D.; Yevtushok, L.; Zymak-Zakutnya, N.; Sosyniuk, Z.; Lapchenko, S.; Ievtushok, B.; Akhmedzhanova, D.; Komov, O. Chornobyl 30 years later: Radiation, pregnancies, and developmental anomalies in Rivne, Ukraine. Eur. J. Med. Genet. 2017, 60, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wertelecki, W.; Yevtushok, L.; Kuznietsov, I.; Komov, O.; Lapchenko, S.; Akhmedzanova, D.; Ostapchuk, L. Chornobyl, radiation, neural tube defects, and microcephaly. Eur. J. Med. Genet. 2018, 61, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Bonisoli-Alquati, A.; Rudolfsen, G.; Mousseau, T.A. Chernobyl birds have smaller brains. PLoS ONE 2011, 6, e16862. [Google Scholar] [CrossRef] [PubMed]
- Hayama, S.-I.; Tsuchiya, M.; Ochiai, K.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Kato, T.; Tanaka, A.; Konno, F.; Kawamoto, Y.; et al. Small head size and delayed body weight growth in wild Japanese monkey fetuses after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2017, 7, 3528. [Google Scholar] [CrossRef]
- Lorenz, E.; Gorgdon, C.; Deringer, M.; Hollcraft, J. Long-term effects of acute and chronic irradiation in mice: Survival and tumor incidence following chronic irradiation of 0.11 r per day. J. Natl. Cancer Inst. 1955, 15, 1049–1058. [Google Scholar]
- Spalding, J.; Thomas, R.; Tietjen, G. Life-Span of c57 Mice Ad Influenced by Radiation Dose: Dose Rate at Exposure; Rep No Vc 48-LA 9528; Los Alamos National Laboratory: Los Alamos, NM, USA, 1982.
- Carnes, B.A.; Fritz, T.E. Rsponses of the beagle to protracted irradiation. I. Effect of total dose and dose rate. Radiat. Res. 1991, 128, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Carnes, B.A.; Fritz, T.E. Continuous irradiation of beagles with gamma rays. Radiat. Res. 1993, 136, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Tanaka, I.B.; Sasagawa, S.; Ichinohe, K.; Takabatake, T.; Matsushita, S.; Matsumoto, T.; Otsu, H.; Sato, F. No lengthening of life span in mice continuously exposed to gamma rays at very low dose rates. Radiat. Res. 2003, 160, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, I.B., 3rd; Tanaka, S.; Ichinohe, K.; Matsushita, S.; Matsumoto, T.; Otsu, H.; Oghiso, Y.; Sato, F. Cause of death and neoplasia in mice continuously exposed to very low dose rates of gamma rays. Radiat. Res. 2007, 167, 417–437. [Google Scholar] [CrossRef]
- Tanaka, I.I.B.; Nakahira, R.; Komura, J.-I.; Tanaka, S. Life Span, Cause of Death and Neoplasia in B6C3F1 Mice Exposed in Utero to Low- and Medium-Dose-Rate Gamma Rays. Radiat. Res. 2022, 198, 553–572. [Google Scholar] [CrossRef]
- Gulay, K.C.M.; Tanaka, I.B., III; Komura, J.; Tanaka, S. Effects of continuous gamma-ray exposure in utero in B6c3f1 mice on gestation day 18 and at 10 weeks of age. Radiat. Res. 2018, 189, 425–440. [Google Scholar] [CrossRef]
- Benotmane, M.A.; Trott, K.R. Epidemiological and experimental evidence for radiation-induced health effects in the progeny after exposure in utero. Int. J. Radiat. Biol. 2023, 1–12. [Google Scholar] [CrossRef]
- Shiragai, A.; Saitou, M.; Kudo, I.; Kanaiwa-Kudo, S.; Matsumoto, T.; Furuse, T.; Yanai, T.; Ichinohe, K.; Sato, F.; Ohmomo, Y. Estimation of the Absorbed Dose to Mice in Prolonged Irradiation by Low-Dose Rate γ-Rays from 137Cs Sources. Radioisotopes 1997, 46, 904–911. [Google Scholar] [CrossRef][Green Version]
- Lalonde, R.; Filali, M.; Strazielle, C. SHIRPA as a Neurological Screening Battery in Mice. Curr. Protoc. 2021, 1, e135. [Google Scholar] [CrossRef]
- Masuya, H.; Inoue, M.; Wada, Y.; Shimizu, A.; Nagano, J.; Kawai, A.; Inoue, A.; Kagami, T.; Hirayama, T.; Yamaga, A.; et al. Implementation of the modified-SHIRPA protocol for screening of dominant phenotypes in a large-scale ENU mutagenesis program. Mamm. Genome 2005, 16, 829–837. [Google Scholar] [CrossRef]
- Tang, F.R.; Loke, W.K.; Wong, P.; Khoo, B.C. Radioprotective effect of ursolic acid in radiation-induced impairment of neurogenesis, learning and memory in adolescent BALB/c mouse. Physiol. Behav. 2017, 175, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mohr, U. International Classification of Rodent Tumors: The Mouse; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Maronpot, R.R. Pathology of the Mouse: Reference and Atlas; Cache River Press: Vienna, IL, USA, 1999. [Google Scholar]
- Wang, H.; Ma, Z.; Shen, H.; Wu, Z.; Liu, L.; Ren, B.; Wong, P.; Sethi, G.; Tang, F.R. Early Life Irradiation-Induced Hypoplasia and Impairment of Neurogenesis in the Dentate Gyrus and Adult Depression Are Mediated by MicroRNA- 34a-5p/T-Cell Intracytoplasmic Antigen-1 Pathway. Cells 2021, 10, 2476. [Google Scholar] [CrossRef]
- Konermann, G. Mouse germ development following continuous Co60 gamma irradiation during blastogenesis, organogenesis and fetal period. Strahlentherapie 1969, 137, 451–466. [Google Scholar] [PubMed]
- Russell, L.B.; Badgett, S.K.; Saylors, C.L. Comparison of the effects of acute, continuous and fractionated irradiation during embryonic development. In Immediate and Low-level Effects of Ionizing Radiation Conference Held in Venice; Buzzati-Traverso, A.A., Ed.; Taylor & Francis: London, UK, 1960; pp. 343–359. [Google Scholar]
- Vorisek, P. Effect of continuous intrauterine irradiation on perinatal mortality of the fetus. Strahlentherapie 1965, 127, 112–120. [Google Scholar] [PubMed]
- Cheng, X.; Sun, Q. RUBCNL/Pacer and RUBCN/Rubicon in regulation of autolysosome formation and lipid metabolism. Autophagy 2019, 15, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Q.; Yu, H.; Du, H.; Li, L.; He, Y.; Zhu, S.; Li, C.; Zhang, S.; Luo, B.; et al. Genetic association study of a novel indel polymorphism in HSPA1B with the risk of sudden cardiac death in the Chinese populations. Forensic Sci. Int. 2020, 318, 110637. [Google Scholar] [CrossRef] [PubMed]
- Solarz, A.; Majcher-Maślanka, I.; Kryst, J.; Chocyk, A. A Search for Biomarkers of Early-life Stress-related Psychopathology: Focus on 70-kDa Heat Shock Proteins. Neuroscience 2021, 463, 238–253. [Google Scholar] [CrossRef]
- He, Y.; Luo, J.; Zhang, G.; Jin, Y.; Wang, N.; Lu, J.; Li, C.; Guo, X.; Qin, N.; Dai, J.; et al. Single-cell profiling of human CD127(+) innate lymphoid cells reveals diverse immune phenotypes in hepatocellular carcinoma. Hepatology 2022, 76, 1013–1029. [Google Scholar] [CrossRef]
- Ali, H.S.; Boshra, M.S.; Agwa, S.H.A.; Hakeem, M.S.A.; El Meteini, M.S.; Matboli, M. Identification of a Multi-Messenger RNA Signature as Type 2 Diabetes Mellitus Candidate Genes Involved in Crosstalk between Inflammation and Insulin Resistance. Biomolecules 2022, 12, 1230. [Google Scholar] [CrossRef]
- Li, Y.; Shan, C.; Yang, B.; Wang, H. Up-regulation of HSPA1A and HSPA1B in the blood of tophi patients and its clinical significance. Acta Biochim. Pol. 2022, 69, 781–785. [Google Scholar] [CrossRef]
- Dou, J.; Cánovas, A.; Brito, L.F.; Yu, Y.; Schenkel, F.S.; Wang, Y. Comprehensive RNA-Seq Profiling Reveals Temporal and Tissue-Specific Changes in Gene Expression in Sprague-Dawley Rats as Response to Heat Stress Challenges. Front. Genet. 2021, 12, 651979. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Le Joncour, V.; Jahkola, T.; Juteau, S.; Laakkonen, P.; Saksela, O.; Hölttä, E. Prolyl 4-hydroxylase subunit alpha 1 (P4HA1) is a biomarker of poor prognosis in primary melanomas, and its depletion inhibits melanoma cell invasion and disrupts tumor blood vessel walls. Mol. Oncol. 2020, 14, 742–762. [Google Scholar] [CrossRef]
- Zhou, H.; He, Y.; Li, L.; Wu, C.; Hu, G. Overexpression of P4HA1 Is Correlated with Poor Survival and Immune Infiltrates in Lung Adenocarcinoma. BioMed Res. Int. 2020, 2020, 8024138. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dai, Z.; Xie, G.; Liu, G.; Guo, L.; Zhang, J. A novel metabolic-related gene signature for predicting clinical prognosis and immune microenvironment in head and neck squamous cell carcinoma. Exp. Cell Res. 2023, 428, 113628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Qiu, L.; Zhou, L.; Geng, R.; Yang, S.; Wu, J. P4HA1 activates HMGCS1 to promote nasopharyngeal carcinoma ferroptosis resistance and progression. Cell. Signal. 2023, 105, 110609. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, D.; Huang, K.; Liang, M. Hypoxia-induced P4HA1 overexpression promotes post-ischemic angiogenesis by enhancing endothelial glycolysis through downregulating FBP1. J. Transl. Med. 2024, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Villar, S.; Ariceta, B.; Agirre, X.; Urribarri, A.D.; Ayala, R.; Martínez-Cuadrón, D.; Bergua, J.M.; Vives, S.; Algarra, L.; Tormo, M.; et al. The transcriptomic landscape of elderly acute myeloid leukemia identifies B7H3 and BANP as a favorable signature in high-risk patients. Front. Oncol. 2022, 12, 1054458. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Anokhin, P.K.; Belov, O.V.; Gulyaev, M.V. Cortical glutamate/GABA imbalance after combined radiation exposure: Relevance to human deep-space missions. Neuroscience 2019, 416, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.E.; Younger, S.; Bertheau, E.; Fallgren, C.M.; Weil, M.M.; Raber, J. Effects of chronic exposure to a mixed field of neutrons and photons on behavioral and cognitive performance in mice. Behav. Brain Res. 2019, 379, 112377. [Google Scholar] [CrossRef]
- Nakamura, S.; Tanaka, I.B., III; Tanaka, S.; Nakaya, K.; Sakata, N.; Oghiso, Y. Adiposity in female B6C3F1 mice continuously irradiated with low-dose-rate gamma rays. Radiat. Res. 2010, 173, 333–341. [Google Scholar] [CrossRef]
- Shin, S.C.; Lee, K.-M.; Kang, Y.M.; Kim, K.; Yang, K.H.; Jin, Y.-W.; Kim, C.S.; Kim, H.S. Alteration of cytokine profiles in mice exposed to chronic low-dose ionizing radiation. Biochem. Biophys. Res. Commun. 2010, 397, 644–649. [Google Scholar] [CrossRef] [PubMed]
| miRNA Primers | Sequence |
|---|---|
| mmu-miR-68 | GCTGTACTGACTTGATGAAAGTAC |
| mmu-miR-202-5p | GCTTCCTATGCATATACTTCTTT |
| mmu-miR-448-3p | TTGCATATGTAGGATGTCCCAT |
| mmu-miR-1298-5p | TTCATTCGGCTGTCCAGATGTA |
| mmu-miR-212-5p | ACCTTGGCTCTAGACTGCTTACT |
| mmu-miR-320-3p | AAAAGCTGGGTTGAGAGGGCGA |
| mmu-miR-423-5p | TGAGGGGCAGAGAGCGAGACTTT |
| mmu-miR-486b-5p | TCCTGTACTGAGCTGCCCCGAG |
| mmu-miR-486b-3p | CGGGGCAGCTCAGTACAGGA |
| mmu-miR-423-3p | AGCTCGGTCTGAGGCCCCTCAGT |
| mmu-miR-652-3p | AATGGCGCCACTAGGGTTGTG |
| mmu-miR-324-3p | ATT CCA CTG CCC CAG GTG CTG CT |
| mmu-miR-122-5p | TGGAGTGTGACAATGGTGTTTG |
| mmu-miR-744-5p | TGCGGGGCTAGGGCTAACAGCA |
| mmu-miR-181b-5p | AACATTCATTGCTGTCGGTGGGTT |
| mmu-let-7b-5p | TGAGGTAGTAGGTTGTGTGGTT |
| mmu-miR-6904-5p | TCCTGGGGTTAGAGTTGAGTGG |
| mRNA Primers | Sequence | Direction |
|---|---|---|
| Dnajb1 F | CCAATGGGTATGGGTGGCTT | forward |
| Dnajb1 R | GCCTTCTCCAGGGACTTTCC | reverse |
| Arc F | GGAGGGAGGTCTTCTACCGT | forward |
| Arc R | TCCTCCTCAGCGTCCACATA | reverse |
| Pdia4 F | GGCCTCTTGGATGTGAATGC | forward |
| Pdia4 R | CAGGGCTGAAAGTGTGGTGA | reverse |
| Fos F | AGTCAAGGCCTGGTCTGTGT | forward |
| Fos R | TGGAACACGCTATTGCCAGG | reverse |
| Tm6sf2 F | TTCTCACACATGGGTGCCTC | forward |
| Tm6sf2 R | CTTGGTCCTGTGGCGAAGAT | reverse |
| Tent5a F | CTCCAGGACTGACCAAGGC | forward |
| Tent5a R | CGGACACCTATGCCCTTCTC | reverse |
| Cdkl5 F | AACGGCGAGAATCCAAGCAT | forward |
| Cdkl5 R | AAGGCGTTTGTTGGTCACTGT | reverse |
| H2bc23 F | TACAACAAGCGCTCGACCAT | forward |
| H2bc23 R | TGTCACTGAACACGTGCCTT | reverse |
| Hspb1 F | ATAGAGACCTGAAGCACCGC | forward |
| Hspb1 R | CGGTCATGTTCTTGGCTGGT | reverse |
| Crybb3 F | AAGCAGGTCTCTGCCTCCT | forward |
| Crybb3 R | TACGATCTCCATCTTGCGCC | reverse |
| Vmn1r58 F | GGTCAAAACACGGCCAAACC | forward |
| Vmn1r58 R | AGGAGAAACAGCCTTCTCTCAA | reverse |
| Scd1 F | GAGTAGCTGAGCTTTGGGCT | forward |
| Scd1 R | ACTTCATCAGCGGGGACTTG | reverse |
| Cd59b F | CTGTTGCCTTGGATCAGCCT | forward |
| Cd59b R | TGATACACTTGCCTTCCGGC | reverse |
| Stip1 F | GTGTTCAACCAGTGAGCAGG | forward |
| Stip1 R | CAGGTCTGACGGCTTGTTCT | reverse |
| Ahsa2 F | GACCAACGTGAACAACTGGC | forward |
| Ahsa2 R | CGTCTTGAGTGCCTTCAGGT | reverse |
| Dnaja1 F | GGCTCGGCTACAAAAGAGGT | forward |
| Dnaja1 R | ATGCGTTCTCCATGACCCTG | reverse |
| Dap F | TCCCTAAAGGGTCGTTGAACC | forward |
| Dap R | AGGAGCCCATCCCTCCTTAG | reverse |
| F5 F | GCTTGCCTTCTCAAGCGTTC | forward |
| F5 R | CCCAAGTGACTTTGCGTGTG | reverse |
| Hspa1b F | GGCACCGATTACTGTCAAGG | forward |
| Hspa1b R | ACAGTGCCAAGACGTTTGTT | reverse |
| P4ha1 F | AAGACTGTTCTGCCGCTACC | forward |
| P4ha1 R | TTCGTAGCCAGACAGCCAAG | reverse |
| Rubcnl F | AGGTGATCCGAACCTGTCG | forward |
| Rubcnl R | TCCGAGCATCACCTACGCC | reverse |
| Banp F | AACACCACGAGAATTCCGCA | forward |
| Banp R | GCACTTTGTTGCAGGTCTGG | reverse |
| Abhd14b F | TAGCACACGCCATTCTCCTG | forward |
| Abhd14b R | AAGGTATCCACCACAGCAGC | reverse |
| GAPDH F | ACC ACA GTC CAT GCC ATC AC | forward |
| GAPDH R | TCC ACC ACC CTG TTG CTG TA | reverse |
| Lesion | Organ | Lesion | Control (0 mGy) n = 16 (%) | 1 mGy/d (48 mGy) n = 16 (%) | 20 mGy/d (960 mGy) n = 16 (%) | 100 mGy/d (1800 mGy) n = 16 (%) |
|---|---|---|---|---|---|---|
| Neoplastic lesion | Liver | Adenoma, Hepatocellular | 1 (6.3) | 3(18.8) | 1 (6.3) | |
| Carcinoma, Hepatocellular | 1 (6.3) | 2 (12.5) | ||||
| Lung | Adenoma, Bronchiolo-Alveolar | 4 (25.0) | 3 (18.8) | 1 (6.3) | ||
| Non-neoplastic lesion | Adrenal gland | Accessory cortical tissue | 1 (6.3) | 1 (6.3) | 1 (6.3) | 1 (6.3) |
| Hyperplasia, subcapsular cell | 6 (37.5) | 5 (31.3) | 6 (37.5) | 10 (62.5) | ||
| Heart | Valvular endocardiosis | 6 (37.5) | 5 (31.3) | 3 (18.8) | 4 (25.0) | |
| Kidney | Cysts | 1 (6.3) | ||||
| Hyperplasia, renal tubular | 1 (6.3) | |||||
| Liver | Cellular alteration, foci | 1 (6.3) | 1 (6.3) | |||
| Cytoplasmic vacuolization | 14 (87.5) | 11 (68.8) | 12 (75.0) | 13 (81.3) | ||
| Granular degeneration | 9 (56.3) | 9 (56.3) | 12 (75.0) | 9 (56.3) | ||
| Inflammation | 1 (6.3) | 1 (6.3) | ||||
| Lung | Congestion | 1 (6.3) | 1 (6.3) | 1 (6.3) | 2 (12.5) | |
| Metaplasia, osseous | 1 (6.3) | |||||
| MNC inf, perivascular | 2 (12.5) | |||||
| Pneumonia, interstitial | 1 (6.3) | 2 (12.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, F.R.; Tanaka, I.B., III; Wang, H.; Lau, S.; Tanaka, S.; Tan, A.; Takai, D.; Abe, A. Effects of Continuous Prenatal Low Dose Rate Irradiation on Neurobehavior, Hippocampal Cellularity, Messenger RNA and MicroRNA Expression on B6C3F1 Mice. Cells 2024, 13, 1423. https://doi.org/10.3390/cells13171423
Tang FR, Tanaka IB III, Wang H, Lau S, Tanaka S, Tan A, Takai D, Abe A. Effects of Continuous Prenatal Low Dose Rate Irradiation on Neurobehavior, Hippocampal Cellularity, Messenger RNA and MicroRNA Expression on B6C3F1 Mice. Cells. 2024; 13(17):1423. https://doi.org/10.3390/cells13171423
Chicago/Turabian StyleTang, Feng Ru, Ignacia Braga Tanaka, III, Hong Wang, Salihah Lau, Satoshi Tanaka, Amanda Tan, Daisaku Takai, and Akiko Abe. 2024. "Effects of Continuous Prenatal Low Dose Rate Irradiation on Neurobehavior, Hippocampal Cellularity, Messenger RNA and MicroRNA Expression on B6C3F1 Mice" Cells 13, no. 17: 1423. https://doi.org/10.3390/cells13171423
APA StyleTang, F. R., Tanaka, I. B., III, Wang, H., Lau, S., Tanaka, S., Tan, A., Takai, D., & Abe, A. (2024). Effects of Continuous Prenatal Low Dose Rate Irradiation on Neurobehavior, Hippocampal Cellularity, Messenger RNA and MicroRNA Expression on B6C3F1 Mice. Cells, 13(17), 1423. https://doi.org/10.3390/cells13171423








