Abstract
Pediatric low-grade gliomas (PLGGs) comprise a heterogeneous set of low-grade glial and glioneuronal tumors, collectively representing the most frequent CNS tumors of childhood and adolescence. Despite excellent overall survival rates, the chronic nature of the disease bears a high risk of long-term disease- and therapy-related morbidity in affected patients. Recent in-depth molecular profiling and studies of the genetic landscape of PLGGs led to the discovery of the paramount role of frequent upregulation of RAS/MAPK and mTOR signaling in tumorigenesis and progression of these tumors. Beyond, the subsequent unveiling of RAS/MAPK-driven oncogene-induced senescence in these tumors may shape the understanding of the molecular mechanisms determining the versatile progression patterns of PLGGs, potentially providing a promising target for novel therapies. Recent in vitro and in vivo studies moreover indicate a strong dependence of PLGG formation and growth on the tumor microenvironment. In this work, we provide an overview of the current understanding of the multilayered cellular mechanisms and clinical factors determining the natural progression patterns and the characteristic biological behavior of these tumors, aiming to provide a foundation for advanced stratification for the management of these tumors within a multimodal treatment approach.
1. Pediatric Low-Grade Glioma: Epidemiology, Classification and Contemporary Treatment Patterns
Pediatric low-grade gliomas (PLGGs) comprise several brain tumor entities of glial and glioneuronal histology assigned to WHO CNS grade 1 and 2 [1,2,3]. These tumors collectively represent the most common CNS tumors of the pediatric population, comprising approximately 30% to 40% of newly diagnosed brain tumor cases in children and adolescents. In Western populations, the cross-entity incidence rate is currently estimated at 2–3 per 100,000 children [4,5]. Occurring in all age groups from infancy to adolescence, PLGGs show a peak incidence in children 5 to 9 years of age [6]. In the current WHO classification of central nervous system tumors published in 2021, PLGGs are categorized into six categories under the umbrella term “glioma, glioneuronal and neuronal tumors” [1,2,3]. Common histological entities include pilocytic astrocytoma and pleomorphic xanthoastrocytoma, which are characterized as circumscribed astrocytic gliomas, alongside several entities categorized as pediatric-type diffuse low-grade gliomas, including the less common MAPK pathway-altered diffuse low-grade glioma or MYB-/MYBL1-altered diffuse astrocytoma [1,2,3]. Among glioneural and neuronal tumors, gangliogliomas and dysembryoplastic neuroepithelial tumors (DNETs) are also considered PLGGs [1,2,3]. PLGGs occur at all sites within the central nervous system, while predominant regions include the cerebellum and the supratentorial midline structures, and entity-specific distribution of predominant tumor locations is observed [6].
A key characteristic of PLGGs is their frequent association with tumor predisposition syndromes including neurofibromatosis type 1 (NF1) and tuberous sclerosis complex (TSC) [7]. In particular, tumors of the visual pathway are associated with NF1 and occur in approximately 20% of these patients within the first decade of life, while almost exclusively pilocytic astrocytomas are found [8,9,10]. Conversely, NF1 can be detected in about 40% of all patients with OPG [10]. Although these patients, specifically in the case of tumors involving the chiasmatic/hypothalamic region, showed superior progression-free survival rates compared to their non-NF1 counterparts, they often present a significant challenge for their caregivers due to increased toxicity to most conventional treatments [11,12].
Despite promising recent advances in the implementation of molecular targeted therapies, surgery remains the mainstay of therapy of PLGGs. The progression-free survival (PFS) is substantially determined by the extent of surgical resection [11,13,14,15,16,17]. In many cases, however, resectability can be severely compromised by surrounding highly eloquent brain tissue, and extensive resection may cause substantial morbidity. Recent population-based cohort studies consistently report high rates of incomplete resection (IR) ranging between 65% and 73%, despite recent advancements in neurosurgical technology [11,14,17,18]. In cases where resection is not feasible, surgical biopsy is recommended, whereas in patients with confirmed NF1 and characteristic MRI findings of an optic pathway glioma, this is not considered obligatory [19].
In the case of limited resectability and progressive disease, chemotherapy has been considered the treatment of choice in recent years. Approximately four decades ago, initial chemotherapy protocols for pediatric low-grade gliomas (PLGGs) were introduced and assessed, with the purpose of postponing radiotherapy and offering an effective substitute treatment for NF1 patients, who inherently face a notably elevated risk of secondary malignancies following radiation therapy [20]. Currently applied chemotherapy regimens include either carboplatin and vincristine, vinblastine monotherapy, or a combination of thioguanine, procarbazine, CCNU, and vincristine [21,22,23,24]. Further second-line protocols include irinotecan and bevacizumab [25]. Notably, however, 5-year PFS rates of established first-line chemotherapy protocols of approximately 50% have been reported, and response rates of subsequent second-line treatments in most cases significantly decrease, causing significant long-term morbidity in affected patients [22,23,24,26].
Given its efficacy in tumor control, radiation therapy has been the primary choice for salvage treatment in PLGGs in previous decades [20]. Considering the substantial age-related long-term sequelae such as cognitive decline, endocrine disorders, and secondary malignancies, conventional photon radiation should currently be reserved for a certain subset of older non-NF1 patients following careful consideration [20,27,28]. This shift has prompted the advancement of alternative radiation applications, including proton beam therapy and stereotactic radiation, as these methods aim to enhance local tumor control while reducing the risk of long-term side effects [20,29].
In recent years, in-depth molecular profiling and incremental decoding of the molecular mechanisms of tumorigenesis in PLGGs has led to clinical evaluation of the efficacy of molecular therapies targeting the RAS/MAPK and mTOR pathways of these tumors [21,30]. In previous phase I/II studies, several agents, including MEK inhibitors (selumetinib, trametinib, and binimetinib), a pan-RAF inhibitor (tovorafenib), first-generation BRAF inhibitors (vemurafenib and dabrafenib), an mTOR inhibitor (everolimus), and an FGFR inhibitor (erdafitinib), have shown promising results, leading to the implementation of randomized controlled studies comparing several agents, including selumetinib, trametinib, and tovorafenib, to the first-line chemotherapy regimen in newly diagnosed, previously untreated PLGGs [31,32,33,34,35,36,37]. It was recently shown that a combination therapy of trametinib and dabrafenib achieved significantly higher response rates compared to carboplatin and vincristine in PLGGs bearing a BRAF V600E mutation and therefore is currently being considered the first-line treatment in these patients [38]. Crucial questions, however, which include the optimal duration of treatment, have not yet been addressed in studies and are the subject of current debate. In a significant subset of patients, rapid rebound growth after discontinuation of therapy has been observed, while potential risk factors have not yet been identified [39]. Remarkably, renewed response to subsequent treatment periods indicates an insignificant role of acquired resistance in these therapies. Significant data regarding potential long-term toxicities, especially in the pediatric demographic, are as yet missing [30]. Apart from MEK inhibitors, other molecular therapies targeting the RAS/MAPK pathway are currently not used in NF1 patients due to concerns involving therapy-related paradoxical ERK activation due to RAF homodimerization, which has previously been observed in vivo and in vitro [40,41].
Recurrent progressions and the necessity for multiple different lines of therapies in many cases emphasize the chronic nature of this disease, which bears a high risk of long-term disease- and therapy-related morbidity in affected patients.
2. Multi-Layered Clinical Factors Determine the Natural Progression Patterns of PLGGs
The clinical course of pediatric low-grade gliomas is commonly characterized by an indolent growth behavior. In a previously reported cohort, it was shown that almost half of patients diagnosed with PLGG exhibited symptoms related to their disease more than six months prior to diagnosis. Again, this emphasizes the indolent growth patterns of these tumors [14]. After incomplete resection, growth deceleration and senescence are frequently observed, as long-term progression-free survival (PFS) rates of approximately 50% were correspondingly reported from previous population-based cohort studies [11,14,42]. Both spontaneous regression as well as regrowth of senescent tumors up to 12 years after initial diagnosis and malignant transformation to secondary high-grade lesions have occasionally been reported [7,14,43,44,45,46,47,48,49,50]. The versatile postoperative progression patterns of pediatric low-grade gliomas after incomplete resection (IR) is illustrated in Figure 1 based on five cases of distinct histological entities and tumor locations.
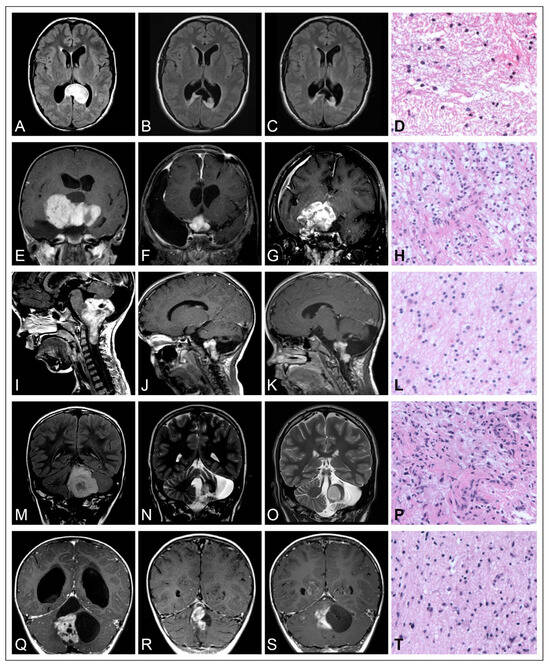
Figure 1.
Illustration of the versatile postoperative progression patterns of pediatric low-grade gliomas after incomplete resection (IR). (A–D) Diffuse astrocytoma WHO grade 2 of the posterior corpus callosum in a 15-year-old girl. (A) Preoperative MRI image, (B) postoperative MRI image after IR, (C) follow-up MRI image 9 years after IR, showing stable tumor remnants. (D) H&E stain of the tumor, 200× magnification. (E–H) Pilocytic astrocytoma (PA) WHO grade 1 of the chiasm and hypothalamus in a 7-year-old girl. (E) Preoperative image, (F) postoperative image after IR, (G) follow-up MRI image 7 years after IR, showing tumor progression under adjuvant treatment. (H) H&E stain of the tumor, 200× magnification. (E–H) Pilocytic astrocytoma (PA) WHO grade 1 of the chiasm and hypothalamus in a 3-year-old girl. (E) Preoperative MRI image, (F) postoperative MRI image after IR, (G) follow-up MRI image 7 years after IR, showing tumor progression under adjuvant treatment. (H) H&E stain of the tumor, 200× magnification. (I–L) Pilocytic astrocytoma (PA) WHO grade 1 of the medulla oblongata in a 4-year-old boy. (I) Preoperative MRI image, (J) postoperative MRI image after IR, (K) follow-up MRI image 2 years after IR, showing stable tumor remnants. (L) H&E stain of the hypocellular tumor showing a predominantly fibrillary matrix, 200× magnification. (M–P) Pilocytic astrocytoma (PA) WHO grade 1 of the cerebellum in a 7-year-old boy. (I) Preoperative MRI image, (O) postoperative MRI image after IR, (N) follow-up MRI image 6 years after IR showing tumor progression. (P) H&E stain of the tumor, 200× magnification. (Q–T) Pilocytic astrocytoma (PA) WHO grade 1 of the cerebellum in a 2-year-old boy. (Q) Preoperative MRI image, (R) postoperative MRI image after IR, (S) follow-up MRI image 4 years after IR, showing stable tumor remnants. (T) H&E stain of the solid tumor portions resembling a diffuse growth pattern, 200× magnification.
While the growth behavior and progression patterns of PLGGs are repeatedly described as mostly unpredictable at first glance, the results of a multi-state analysis dissecting the natural course of the disease indicate that future progressiveness may be predicted by the tumor growth behavior during the first two years after initial diagnosis [51]. The comprehensive analysis of more than 1500 patients during multiple disease states identified various levels of disease progressiveness, which were determined by age, tumor location, and histologic grade [51]. Previous population-based cohort studies have identified several risk factors potentially determining progression patterns of these tumors, mainly including the extent of surgical resection, tumor location, age at diagnosis, and histological and molecular features [11,13,14,15,18,51,52].
2.1. Extent of Resection
The extent of surgical resection has emerged as the predominant predictor of the progression patterns throughout previous population-based cohort studies of PLGG [11,13,14,15,16,17]. Five- and 10-year PFS rates of completely resected PLGGs have been reported to be around 94 and 85%, respectively [11,14]. In incompletely resected PLGGs, progression-free survival was substantially determined by the extent of surgical resection, as 10-year PFS rates of 48% after near-total resection, 18% after partial resection, and 16% after biopsy were reported. Above, a recently published analysis of the pre- and postoperative tumor growth velocity of a cohort of 171 pediatric low-grade gliomas reported a persistent growth deceleration of incompletely resected tumors, while a clear linear negative correlation of percentual extent of resection and postoperative tumor growth velocity was shown [42]. The same study also identified a residual cut-off tumor volume >2.0 cm3 associated with a higher risk of radiologically detectable progression post incomplete resection, while the residual tumor mass after initial surgical resection was moreover reported as a risk factor for progression by a further study [23,42]. These data indicate the crucial role of surgical treatment as the mainstay of therapy alongside emerging targeted molecular therapy approaches.
2.2. Tumor Localization
The influence of tumor localization on the progression behavior of these tumors has repeatedly been described. Multiple tumor sites or extensive tumor spread at time of diagnosis bearing a significantly higher risk of tumor progression during various disease states have been identified by previous population-based cohort studies. In contrast, supratentorial midline location has been found to be the most prominent tumor site associated with highly progressive disease and disease-related morbidity [11,14,51]. Further tumor locations associated with a higher risk of tumor progression involve the brainstem, spinal cord, and diencephalon [13]. The impact of the tumor location as a prognostic factor, however, may naturally be confounded with the extent of resection of these tumors, as resectability can be severely compromised by surrounding highly eloquent brain tissue, where extensive resection may cause substantial morbidity. In a large multi-institutional analysis involving 798 patients, hypothalamic/chiasmatic tumors demonstrated the most sustained tendency to progress, while a multivariate analysis did not confirm tumor site as an independent risk factor for highly progressive disease, and a strong correlation of tumor site and resection extent was shown [14]. Consistent with these findings, an analysis of the preoperative tumor growth velocity revealed no significant differences comparing tumor growth rates in PLGGs at various tumor locations, while mean postoperative tumor growth rates were highest in PLGGs located in the supratentorial midline, where the lowest mean resection extent was achieved [42]. In contrast, however, a prospective multivariate analysis of two large population-based cohorts involving 1031 patients identified supratentorial midline location as an independent risk factor for highly progressive disease [15]. Comparing the outcome of supra- and infratentorial PLGGs, it should be considered, however, that a reasonable proportion of circumscribed glial and glioneuronal tumors at distinct locations bear additional histone H3 mutations or overexpression of EZHIP, which had not been assessed earlier yet potentially may have influenced the reported outcomes of previously published series [53,54].
2.3. Age at Diagnosis
Earlier reports demonstrate a substantial dependence of the clinical progression patterns of PLGGs on the patient’s age at diagnosis. While younger patients are at a higher risk of recurrent treatment progression and inferior treatment outcome, treatment-related sequelae, and tumor-related death, the highest risk of progression has correspondingly been observed in patients < 1 year of age [14,15,18,52,55,56,57,58,59]. Although occasional preselecting treatment studies may point in various directions, age dependency of progression patterns has consistently been reported from major population-based cohort analyses [11,14,15,18,55,56,57,58,59,60,61]. Considering a further distinct predominance of distinct tumor locations among different age groups, various age-dependent progression patterns are currently considered an expression of an evolving tumor microenvironment and probably also some sort of maturation of glial cells across early stages of childhood, promoting differential risks of tumorigenesis and progression of these tumors [11,62]. The underlying mechanistic causes, however, are barely understood, and further investigations are hampered by difficulties in generating representative preclinical models of PLGG, as mentioned above.
2.4. Histology
Moreover, previous reports indicate that histology may independently predict progression, as nonpilocytic and diffuse PLGGs have repeatedly been associated with a higher risk of highly progressive disease. Several studies and population-based analyses indicate a significantly higher progression rate in nonpilocytic tumors, diffuse fibrillary histology, and low-grade gliomas assigned to WHO grade 2 [6,11,14,15,58,63,64,65,66]. While distinct tumor location-specific distributions of various histological types of PLGG have been shown, the impact of tumor histology on PFS, however, may be confounded with differences in resectability. It was shown that patients with pilocytic astrocytoma had higher chances of final complete resection [67]. Nonetheless, several large population-based studies confirmed diffuse fibrillary histology as an independent risk factor for a worse PFS in multivariate analyses [11,14,15].
3. Aberrant RAS/MAPK Pathway Signaling Drives Tumorigenesis and Tumor Progression in PLGGs
Within the past two decades, in-depth molecular profiling and studies of the genetic landscape of PLGGs identified underlying genetic alterations leading to a frequent activation of the RAS–mitogen-activated protein kinase (RAS/MAPK) pathway as a nearly universal biological feature of these tumors. PLGGs are therefore often referred to as a “single-pathway disease” [68,69,70,71,72,73]. The RAS/MAPK pathway represents one of the best characterized signaling pathways in cell biology. It is involved in regulating essential mechanisms of cell cycle control, cell migration, and angiogenesis, which play a crucial role in tumorigenesis and tumor progression in several malignancies [74,75,76,77]. MAPK/mTOR signaling and its downstream activation of transcriptional factors resulting in tumorigenesis and oncogene-induced senescence (OIS) is illustrated in Figure 2. The first indications of the crucial role of the RAS/MAPK pathway in PLGGs were found in patients with neurofibromatosis type I (NF1). In these patients, germline mutations involving the NF1 tumor suppressor gene cause a loss of function of neurofibromin, a GTPase-activating protein functioning as a negative regulator of RAS [78,79]. This alteration consecutively leads to the development of low-grade gliomas, mainly involving the optic pathway, in up to 20% of cases during childhood [80,81]. Distinct alterations in non-NF1 patients all converging on consecutive RAS/MAPK activation were subsequently discovered. Key mutations driving these tumors include the KIAA1549-BRAF fusion, prevalent in pilocytic astrocytoma (approx. 70%) and rosette-forming glioneural tumors (approx. 30%) in non-NF1 patients, as well as BRAF V600E mutations, commonly found in pleomorphic xanthoastrocytoma (approx. 80%), ganglioglioma (approx. 45%), and pediatric-type diffuse low-grade gliomas (approx. 40%) [69,70,79,82,83,84]. Other less frequent mutations activating the RAS/MAPK pathway include FGFR1/2 alterations, ALK fusions, KRAS mutations, and less frequent mutation or fusions of BRAF with the removal of BRAF’s N-regulatory domain [68,69,79,85,86]. Less common types of PLGGs contain recurrent genetic alterations, including NTRK and the MYB family of transcription factors and fusions involving RAF1 [68,69,87,88]. Protooncogenes of the MYB family are known for their crucial role as transcriptional regulators of proliferation and differentiation, while oncogenic alterations are repeatedly found in PLGGs with diffuse histological features, including angiocentric gliomas [69,79,87,89]. Notably, IDH1/2 mutations, frequently seen in adult gliomas, are rare occurrences in PLGGs and cluster among adolescent patients [90]. Previous observations indicate similar clinical characteristics and progression patterns of IDH1/2-mutant glioma in pediatric and adolescent patients compared to those in adults [90]. Therefore, astrocytoma, IDH-mutant and oligodendroglioma, and IDH-mutant and 1p/19q co-deleted are not covered in this review. An illustration of the frequent and less common genomic alterations of distinct histological types of PLGG is provided in Supplementary Table S1. Beyond contributing to a more profound understanding of tumorigenesis and the driving forces of tumor progression in PLGGs, dissecting the molecular landscape of PLGGs facilitated precise diagnosis and advanced molecular stratification, while also representing a promising avenue for emerging molecular therapies [91].
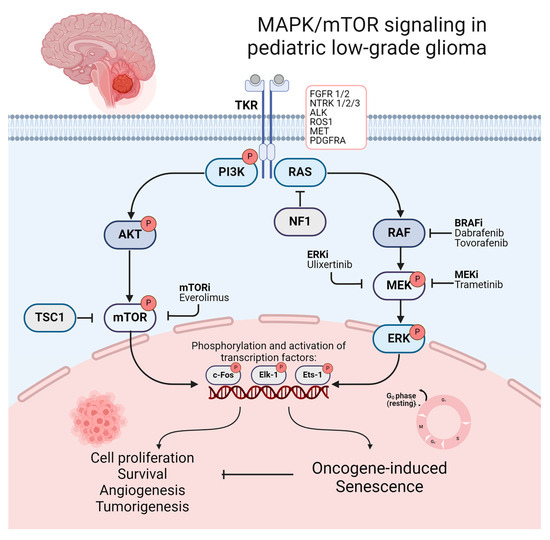
Figure 2.
Illustration of MAPK/mTOR signaling and its downstream activation of transcriptional factors resulting in tumorigenesis OIS. Promising molecular therapeutics and their target points in the mTOR/MAPK pathway are additionally illustrated. Created with BioRender.com (accessed on 8 July 2024).
4. OIS as a Potent Tumor-Suppressive Mechanism in RAS/MAPK-Driven Cancers
After first being described in vivo more than 25 years ago, the discovery of a potential induction of cellular senescence by a constitutive activation of oncogenic pathways has shaped an increasingly profound understanding of cellular protective mechanisms against tumorigenesis, beyond potentially providing a promising target for novel combination therapies for various cancers [92,93]. Within the past two decades, similar mechanisms of cell cycle arrest, potentially resulting in growth deceleration and senescence, have been described in several cancers driven by activation of the RAS–RAF–mitogen-activated protein kinase (MAPK) pathway, MYC activation, hyperactivated WNT-β-catenin signaling, activation of the INK4A-RB pathway, or loss of PTEN [93,94,95,96,97,98,99]. In most cases, OIS is characterized by the accumulation of p53 and/or p16INK4a following the activation of oncogenic pathways, whereas inactivation of p53 or p16INK4a following additional genetic lesions has been associated with an escape of senescence and malignant transformation of premalignant lesions in several cancers [93,100,101,102,103,104].
OIS in RAS/MAPK-activated neoplasms was among the first to be discovered, and its incremental unravelling has led to an increasing understanding of cellular mechanisms of intrinsic tumor suppression following oncogenic genetic alterations [92,100]. While aberrant RAS/MAPK pathway signaling is attributed a critical role in oncogenesis, cancer cell survival, dissemination, and drug resistance in a variety of human cancers, in vitro studies have repeatedly shown an induction of cell cycle arrest in G1 due to the accumulation of cell cycle inhibitors p53 and p16INK4a and a decreased expression of cyclin A following persistent oncogenic RAS/MAPK signaling in murine and human cells [92,100,105,106,107,108,109,110,111]. Previous in vitro analyses implicate a dependency of RAS/MAPK-driven tumorigenesis on additional genetic alterations, resulting in an inactivation of p53 or p16INK4a rendering senescence escape [100,112]. Further investigations of cellular responses to oncogenic RAS signaling in human fibroblasts, however, suggest a correlation of senescence response to the intensity of MAPK activation without dependency on additional genetic lesions, while moderate MAPK activation promoted cell growth, and hyperactivation induced senescence via p16INK4a expression [109].
OIS following MAPK signaling has been associated with various mutations in KRAS and BRAF or upstream growth factor receptors as EGFR and have been translated to multiple cancerogenesis models involving hematologic malignancies, melanoma, and lung cancer [113,114,115,116,117]. Patient-derived cellular PLGG models replicating OIS are therefore useful for further studies in the transcriptional role of the MAPK pathway [31,118].
5. Regulation of OIS and in PLGGs: Recent Discoveries and Current Understanding
In pediatric low-grade glial tumors, the pivotal role of OIS has previously been studied in various preclinical MAPK-activated pilocytic astrocytoma models. It was shown that the insertion of a constitutively active BRAFV600E allele into human neural stem cells promoted clonogenic growth, albeit followed by subsequent proliferation arrest and expression of markers of OIS, including acidic β-galactosidase and p16INK4a [119]. Similar observations were reported from BRAF wild-type and mutant astrocytic cell lines characterized by aberrant MAPK signaling, whereas abrogation of OIS could be induced by additional loss of p16INK4a [120].
OIS and the resulting growth arrest due to constitutive upregulation of MAPK signaling has previously moreover been described as the underlying cause of difficulties in the establishment of representative low-grade glioma models, including primary cultures and patient-derived xenograft (PDX) models [119,120,121,122,123]. SV40 large T antigen workflows are usually applied to circumvent OIS in such models [123].
Variable inactivation of OIS, however, enabled the establishment of representative in vitro models for further investigation of OIS in pilocytic astrocytoma. In a patient-derived KIAA1549::BRAF fusion positive pilocytic astrocytoma model, a doxycycline-inducible switching between a proliferative state and OIS by doxycycline-inducible inhibition of TP53/CDKN1A and CDKN2A/RB1 pathways was implemented, while the induction of CDKN1A and an accumulation of TP53 with subsequent G1 growth arrest was shown during a senescent state [122,124].
Similar to preclinical models of pilocytic astrocytoma, enhanced expression of hallmarks of senescence including p16INK4a and acidic β-galactosidase, as well as a significantly upregulated mRNA expression of senescence-associated secretory phenotype (SASP) factors, was shown in human pilocytic astrocytoma samples, possibly indicating the significance and translational potential of the observations in preclinical in vitro models to clinical routine [120,122].
Consequently, the indolent growth behavior and versatile progression pattern of pediatric low-grade gliomas has repeatedly been linked with RAS/MAPK-driven OIS in pilocytic astrocytoma by previous authors [122,125,126]. This hypothesis is supported by the observation of Buhl et al. indicating a clear dependency of progression-free survival on SASP factor mRNA expression, which showed a favorable outcome of patients with higher SASP expression in a multi-institutional ICGC PedBrain pilocytic astrocytoma cohort including 110 patients [122]. Other potentially interfering factors, including the extent of resection and postoperative radiotherapy, were excluded in a multivariate analysis.
As the accumulation of p53 or p16INK4a following the activation of the MAPK pathway plays a paramount role in MAPK-driven OIS, additional CDKN2A alterations are described to facilitate a senescence escape, potentially defining a particularly high-risk, low-grade glioma phenotype [79,119,120]. CDKN2A is known for its endogenous function in G1 cell cycle regulation [79,127,128]. The prognostic impact of a concomitant loss of CDKN2A, which is described in a frequency range of 6–20% in PLGGs, has previously been studied in several pediatric low-grade glioma cohorts [79,83,129,130,131]. It was shown that additional homozygous CDKN2A deletion in MAPK-activated BRAF V600E mutant PLGGs contributed independently to poor outcome, therefore constituting a distinct entity with a significantly worse prognosis [83,130,131]. Moreover, the interplay between these cofounding alterations has been shown to foster progression to secondary high-grade glioma (sHGG) in pediatric patients, as a comprehensive molecular analysis of a population-based sHGG cohort showed a significant enrichment of MAPK-activating BRAF mutations and a homozygous loss of CDKN2A in these tumors [132,133]. Notably, as a retrospective longitudinal assessment revealed, these alterations could be traced back to their PLGG counterparts before malignant transformation [132]. Notably, co-occurrence of BRAF V600E mutation and CDKN2A co-deletion moreover showed a significant enrichment in pleomorphic xanthoastrocytoma, a PLGG type known for an increased tendency of malignant transformation to sHGG [69,134,135]. Another example is the recently discovered entity of high-grade glioma with piloid features (HGAP) [136]. Such cases may occur in the setting of NF1, and these tumors frequently harbor co-occurring CDKN2A/B homozygous deletion and/or ATRX stop gain mutations [137]. A significant number of cases show a low-grade precursor lesion originating from the PLGG spectrum [138]. Beyond providing significant aspects for risk stratification, these data moreover support the transferability of the mechanistic model of MAPK-driven PLGGs to clinical practice.
In contrast, additional TP53 mutations appear to play a minor role in the context of OIS escape in the treatment of PLGGs. These DNA repair pathway mutations show a significant accumulation in pediatric high-grade gliomas, particularly in H3K27-altered diffuse midline gliomas, and have shown a notable enrichment in pediatric HGG of the cerebellum in a recently published series [139,140]. Previous data suggest a negative impact of this mutation on the prognosis of pediatric HGGs [141]. However, in PLGGs, TP53 mutations have only been sporadically described, and their detection failed to show a negative prognostic value in a previously published series, indicating that the observation of their crucial impact on OIS escape in preclinical models does not seem to reflect in clinical practice [6,58].
The identification of mediating factors of OIS in pediatric low-grade gliomas was the subject of a previous work by Buhl et al., who analyzed gene and protein expression during a state of proliferation and senescence in a previously mentioned patient-derived KIAA1549::BRAF fusion positive pilocytic astrocytoma (PA) model, which allows for doxycycline-inducible switching between a proliferative state and OIS [122,124]. It was shown that SASP factors, including the pro-inflammatory cytokines IL1B and IL6, were upregulated in pilocytic astrocytoma cells during OIS. This furthermore led to the transcription of SASP factors in an autocrine manner due to downstream activation of NFκB, therefore sustaining the senescent state and resulting a reduced growth of PA cells [122]. Incubation with the anti-inflammatory agent dexamethasone conversely induced regrowth of senescent cells and inhibited the SASP [122]. A schematic illustration of OIS fostering the SASP in PLGGs is provided in Figure 3. These findings may indicate a paramount impact of inflammation on OIS and therefore the growth behavior of these tumors. Considering recently published data of an analysis of pre- and postoperative tumor growth velocity of PLGGs, showing a significant radiologically detectable growth deceleration after subtotal resection, it may be hypothesized that a local inflammatory response to surgical therapy may foster OIS and therefore growth deceleration in residual tumor tissue [42]. Moreover, the in vitro observation of a regrowth of senescent PA cells and suppression of OIS following the incubation with dexamethasone raises the question regarding the safety of the use of anti-inflammatory agents such as dexamethasone in PLGGs, which are frequently applied for symptom control in brain tumor patients with raised intracranial pressure [142]. A retrospective analysis, however, did not show any significant impact of short-term dexamethasone use on tumor growth velocity or PFS in a PLGG cohort [142].
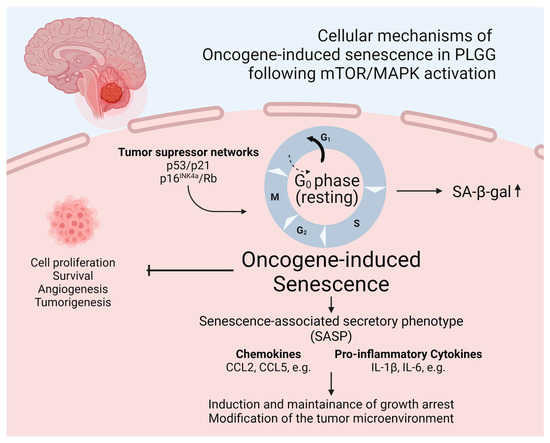
Figure 3.
Schematic illustration of OIS fostering the SASP in PLGGs. Created with BioRender.com (accessed on 8 July 2024).
Notably, previous studies of OIS in pediatric low-grade gliomas are confined to preclinical models and tumor samples of pilocytic astrocytoma, while correspondent analyses in distinct histological types of low-grade gliomas are yet missing. Unrestrained translation to distinct histological LGG types should be challenged, paying regard to biological and molecular differences.
6. OIS Represents a Promising Target for Senolytic Treatment in PLGGs
The intratumoral heterogeneity with a significantly increased, varying population of senescent cells in pediatric low-grade gliomas may not only serve as an explanation for the indolent growth behavior of these tumors but moreover bears the challenge of potential resistance of senescent compartments to conventional chemotherapy-based treatment regimens [66,125]. In this setting, the potential implementation of senolytic agents may appear as a promising complementary treatment approach.
The evaluation of the therapeutic potential of clearing senescent cells by senolytic drugs through the induction of apoptosis has been the subject of basic research and clinical investigations in a variety of cancers [143,144,145,146,147]. In glial tumors, the potential of Bcl-2 inhibition in overcoming therapy resistance and the clearance of senescent tumor stem cells has previously been demonstrated in vitro and in vivo in glioblastoma [148,149,150,151]. Evidence of increased Bcl-xL levels in pilocytic astrocytoma, indicating a significant fraction of senescent cells and providing a potential target for senolytic BH3-mimetics, underline the potential of this treatment approach in pediatric low-grade gliomas [152]. In a comprehensive in vitro analysis including several patient-derived MAPK-activated pilocytic astrocytoma cell lines, including KIAA1549-BRAF fusions or a BRAF V600E mutation, Selt et al. demonstrated the upregulation of Bcl-xL during a state of cellular senescence, while inhibition of BCL-xL induced mitochondrial apoptosis [152]. These significant findings highlight the potential of BCL-xL inhibition as a promising treatment approach in targeting the senescent compartment in low-grade gliomas, which may be difficult to treat with conventional anti-proliferative agents. While upfront clinical trials are required, limited penetration of the blood–brain barrier, resulting in relatively low plasma:CSF ratios in clinically available BCL-2 inhibitors, provides a challenge that may potentially affect the translation of these promising findings into clinical practice [153].
7. The Role of the Tumor Microenvironment in PLGG Tumor Formation and Growth
The crucial role of the tumor microenvironment (TME) as a sophisticated ecosystem in oncogenesis, potential evasion of immune surveillance, and tumor advancement has been studied extensively in a variety of tumor entities and carcinogenesis models within the past decades [154,155,156,157,158,159]. In pediatric CNS tumors—in particular, PLGGs—major questions involving the interaction between the TME and tumor cells, as well the spatial distribution and impact of various infiltrating immune cells on senescence and tumor progression patterns, mostly remain unanswered [160].
The composition of infiltrating immune cells in pediatric gliomas has been the subject of previous analyses using a variety of methods ranging from basic immunohistochemistry to single-cell RNA sequencing. It was shown that primary immune cells found in pediatric gliomas include macrophages, microglia, and T cells, while MAPK-driven low-grade gliomas showed a significantly stronger immune cell infiltration in contrast to their high-grade counterparts, and the composition of infiltrating immune cells showed significant differences to distinct brain tumor entities [160,161,162]. Several previous studies have identified microglia and T cells as the predominant infiltrating cell type in PLGGs, while a significant proportion of resident microglial tumor-associated macrophages (TAMs) has moreover been identified [161,162,163]. More detailed analyses have observed a notably variable extent of infiltrating T cells, with the density of infiltrating T cells being described as highest in ganglioglioma, pleomorphic xanthoastrocytoma, and hypermutated tumors [164]. However, a more recent study describes the detection of a more immunogenic, immune-activating infiltrating cell population in pilocytic astrocytoma compared to ganglioglioma. [165]. The underlying MAPK-activating genetic alteration has been shown to impact the composition of infiltrating immune cells, as a stronger immune infiltration including a higher proportion of pro-inflammatory, activated macrophages and microglia was shown in KIAA1549::BRAF fusion positive compared to BRAF wild-type PLGGs [166,167]. Another study using spatial transcriptomics that has not yet been published found a higher concentration of immune cell-mobilizing chemokines in KIAA1549::BRAF fusion-driven tumors compared to BRAF V600E-mutated tumors [165]. Across various types of pediatric gliomas, previous data suggest a correlation between a strong immune infiltration and significantly prolonged overall survival [168]. Albeit the composition and grade of immune cell infiltration has shown significant variability in pediatric low-grade gliomas, data addressing its prognostic utility and a possible impact on progression patterns are missing [164,169].
While the potential modulation of immunosurveillance by RAS/MAPK-driven OIS in PLGGs has not yet been the subject of detailed investigation, there are clear indications derived from preclinical models of distinct tumor entities that MAPK-driven OIS significantly affects the TME and could thus influence immunological clearance. In a mosaic mouse model of liver carcinoma, it was shown that the secretion of inflammatory cytokines in a senescence state triggered an innate immune response and fostered tumor clearance [170]. In contrast, in a fibroblast model of OIS, it was shown that the expression of inhibitory immune checkpoint molecules such as PD-L1 correlates with gene expression signatures of SASP [171]. In liver tumors, it was shown that senescent tumor cells can alter their surface proteome to evade clearance by the immune system [172]. A previously published work examined the expression of ligands for PD1 (PD-L1 and PD-L2) and CTLA4 (CD80 and CD86), which could potentially influence T-cell-mediated immune clearance, on cells of pilocytic astrocytoma. This study showed a very low expression of these proteins on tumor cells, although a robust expression of MHC class 1 antigens (HLA-A, HLA-B, HLA-C) suggests intact antigen expression. Although there have been no dedicated studies on the modulation of cellular immune response and clearance by the SASP in PLGGs, there are indications that MAPK activation in PLGGs has a crucial impact on immune infiltration. For instance, it has been shown that the likelihood of responding to MAPK inhibitor therapy correlates with the extent of immune infiltration, and rebound growth after MAPK inhibitor withdrawal is associated with the secretion of microglia-recruiting cytokines [39,169]. The potentially crucial interaction of RAS/MAP-driven OIS and immunosurveillance is characterized by many open questions and represents the subject of current research [173].
Remarkably, previous data derived from murine models of PLGGs indicate a strong dependence of tumorigenesis and tumor progression on infiltrating immune cells in these tumors. In a previously published work investigating the impact of BRAF fusion-expressing neural stem cells and microglia in mice, it was shown that increased microglia infiltration induced glioma-like formation of BRAF fusion-expressing neural stem cells, while intercepting chemokine receptor type 2 (CCR2)-mediated microglia recruitment prevented tumor formation [174]. In a genetically engineered neurofibromatosis 1 mouse model, multi-potent low-grade glioma stem cells failed to generate glioma-like lesions in athymic mice lacking T cells or chemotactic receptors such as CCR2 and chemokine c-motif ligand 5 (CCL5), which mediate the interaction of T cells and microglia and moreover were shown to promote low-grade glioma growth [175,176]. It was also shown that various patterns of chemokine expression of both low-grade glioma stem cells and infiltrating immune cells resulted in various growth patterns of these tumors [177,178].
However, both the translation of these findings and further mechanistic studies pursuing a deeper understanding of the impact of the TME on oncogenesis, potential evasion of immune surveillance, and tumor advancement in humanized models of pediatric low-grade gliomas require the development of preclinical in vivo models containing humanized immune systems, as current preclinical models involving xenotransplantation are commonly characterized by impaired immunity [121]. While the multi-faceted mechanisms of interaction between the tumor microenvironment and tumor cells remain mostly unknown, further investigations may crucially impact the future understanding of tumor formation and progression in these tumors.
8. Spontaneous Regression and Malignant Transformation in PLGGs: Characteristics of Two Rare Phenomena
Malignant transformation of low-grade gliomas is observed habitually in adult patients, showing a prevalence of up to 72% in previously reported adult LGG cohorts [168,179,180]. In the pediatric demographic, transformation towards high-grade lesions is a rare phenomenon, with reported incidences ranging around 5% [46,47,48,49,50]. This observation moreover underscores the vast biological differences between pediatric and adult LGGs [7]. While the molecular and biological basis has not yet been deciphered, individual factors associated with malignant transformation of PLGGs could be identified. As previously mentioned, a co-occurrence of BRAF V600E mutation and CDKN2A co-deletion has been associated with progression to sHGG, as a significant enrichment in a cohort of sHGG was shown, and these mutations could be traced back to the PLGG counterparts of sHGG bearing these molecular alterations [132]. Notably, co-occurrence of BRAF V600E mutation and CDKN2A co-deletion moreover showed a significant enrichment in pleomorphic xanthoastrocytoma, a PLGG type known for an increased tendency towards malignant transformation [69,134,181,182,183]. Moreover, previous administration of radiotherapy and older age have been suggested as risk factors for a progression towards sHGG, while the latter has shown no statistical significance due to the small sample size in a series of sHGG [16,46,63,184].
Beyond its characteristically indolent growth behavior and a tendency towards growth deceleration and senescence after incomplete resection, occasional spontaneous regression of these tumors is observed in childhood LGGs, representing another distinguishing feature as compared to adult LGGs [7,43,44,45]. Occasional series of incompletely resected cerebellar pilocytic astrocytoma, including long-term follow-up periods of up to 18 years, report high rates of spontaneous regression in 14% to 38% of cases, while solely the extent of surgical resection could be identified as a significant prognostic factor [185,186,187]. Comparable studies including distinct histological types of PLGGs and a comprehensive molecular characterization of tumors showing spontaneous regression are lacking.
9. Conclusions and Outlook
Recent in-depth molecular profiling and studies of the genetic landscape of pediatric low-grade gliomas led to the discovery of the paramount role of frequent upregulation of RAS/MAPK and mTOR signaling in the tumorigenesis and progression of PLGGs. The subsequent clinical evaluation of molecular therapies targeting these pathways shows promising results, partly indicating superior response rates compared to established chemotherapy regimens in randomized controlled trials [38]. Rapid implementation into the multimodal treatment approach of these tumors may substantially improve quality of life, particularly in patients suffering from unresectable tumors, multiple progressions, and a high risk of disease-related morbidity and treatment sequelae. A comprehensive understanding of the multilayered clinical and molecular factors determining the natural progression patterns of these tumors may form the foundation for advanced stratification and provide guidance for the management of these tumors within a multimodal treatment approach.
The discovery of the significant role of RAS/MAPK-driven OIS in these tumors may provide an in-depth look into the molecular mechanisms of the indolent growth behavior, versatile progression patterns, and a potential resistance of senescent compartments to conventional chemotherapy-based treatment regimens. In this setting, the potential implementation of senolytic agents may appear as a promising complementary treatment approach. A recently published comprehensive analysis provides proof of principle for the induction of apoptosis in senescent pilocytic astrocytoma cells by Bcl-xL inhibition [152].
The incremental discovery of the multi-layered cellular mechanisms, including molecular alterations promoting aberrant RAS/MAPK and mTOR signaling, reversible complexly regulated OIS, and a bidirectional dependence of these tumors on their tumor microenvironment, will shape an integrated mechanistic model of the underlying biology of these tumors. This model needs to be incorporated into in vivo and in vitro models for preclinical drug development and future studies of the biology of PLGGs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells13141215/s1, Table S1: Illustration of the frequent and less common genomic alterations of distinct histological types of pLGG, as well as the state-of-the-art treatment based on the current European standard clinical practice recommendations for primary pediatric low-grade gliomas. Reference [188] is cited in Supplementary Materials.
Author Contributions
Conceptualization, D.G. and J.S.; methodology, J.S. and D.G.; investigation, D.G., J.S., M.E. and M.U.S.; writing—original draft preparation, D.G. and J.S.; writing—review and editing, J.S., M.E., M.U.S. and D.G.; visualization, D.G.; supervision, J.S.; project administration, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours of the Central Nervous System, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.A.; Rosenblum, M.K. The 2021 WHO Classification of Tumors of the Central Nervous System: An update on pediatric low-grade gliomas and glioneuronal tumors. Brain Pathol. 2022, 32, e13060. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Ryan, K.; Edelson, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2022, 24 (Suppl. S3), iii1–iii38. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, P.; Rickert, C.H.; Kühl, J.; Schüz, J.; Michaelis, J. Population-based epidemiologic data on brain tumors in German children. Cancer 2001, 92, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Sievert, A.J.; Fisher, M.J. Pediatric low-grade gliomas. J. Child. Neurol. 2009, 24, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Greuter, L.; Guzman, R.; Soleman, J. Pediatric and Adult Low-Grade Gliomas: Where Do the Differences Lie? Children 2021, 8, 1075. [Google Scholar] [CrossRef] [PubMed]
- Shofty, B.; Ben Sira, L.; Constantini, S. Neurofibromatosis 1-associated optic pathway gliomas. Childs Nerv. Syst. 2020, 36, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Campen, C.J.; Gutmann, D.H. Optic Pathway Gliomas in Neurofibromatosis Type 1. J. Child Neurol. 2018, 33, 73–81. [Google Scholar] [CrossRef]
- Evans, D.G.R.; Salvador, H.; Chang, V.Y.; Erez, A.; Voss, S.D.; Schneider, K.W.; Scott, H.S.; Plon, S.E.; Tabori, U. Cancer and Central Nervous System Tumor Surveillance in Pediatric Neurofibromatosis 1. Clin. Cancer Res. 2017, 23, e46–e53. [Google Scholar] [CrossRef]
- Stokland, T.; Liu, J.F.; Ironside, J.W.; Ellison, D.W.; Taylor, R.; Robinson, K.J.; Picton, S.V.; Walker, D.A. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: A population-based cohort study (CCLG CNS9702). Neuro-Oncology 2010, 12, 1257–1268. [Google Scholar] [CrossRef]
- Guerreiro Stücklin, A.S.; Mueller, S. Opportunities for the treatment of NF1-associated low-grade gliomas: How to decide on the best treatment options for patients? Neuro-Oncology 2020, 22, 1415–1416. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Conklin, H.M.; Huang, S.; Srivastava, D.; Sanford, R.; Ellison, D.W.; Merchant, T.E.; Hudson, M.M.; Hoehn, M.E.; Robison, L.L.; et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro-Oncology 2011, 13, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Gnekow, A.K.; Falkenstein, F.; von Hornstein, S.; Zwiener, I.; Berkefeld, S.; Bison, B.; Warmuth-Metz, M.; Driever, P.H.; Soerensen, N.; Kortmann, R.D.; et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro-Oncology 2012, 14, 1265–1284. [Google Scholar] [CrossRef]
- Fisher, P.G.; Tihan, T.; Goldthwaite, P.T.; Wharam, M.D.; Carson, B.S.; Weingart, J.D.; Repka, M.X.; Cohen, K.J.; Burger, P.C. Outcome analysis of childhood low-grade astrocytomas. Pediatr. Blood Cancer 2008, 51, 245–250. [Google Scholar] [CrossRef]
- Collins, K.L.; Pollack, I.F. Pediatric Low-Grade Gliomas. Cancers 2020, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
- Wisoff, J.H.; Sanford, R.A.; Heier, L.A.; Sposto, R.; Burger, P.C.; Yates, A.J.; Holmes, E.J.; Kun, L.E. Primary neurosurgery for pediatric low-grade gliomas: A prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery 2011, 68, 1548–1554; discussion 1554–1555. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhayay, P.; Bergthold, G.; London, W.B.; Goumnerova, L.C.; La Madrid, A.M.; Marcus, K.J.; Guo, D.; Ullrich, N.J.; Robison, N.J.; Chi, S.N.; et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: An analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr. Blood Cancer. 2014, 61, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.R.; Perry, A.; Rubin, J.B.; King, A.A.; Chicoine, M.R.; Gutmann, D.H. The role of surgical biopsy in the diagnosis of glioma in individuals with neurofibromatosis-1. Neurology 2006, 67, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- de Blank, P.; Bandopadhayay, P.; Haas-Kogan, D.; Fouladi, M.; Fangusaro, J. Management of pediatric low-grade glioma. Curr. Opin. Pediatr. 2019, 31, 21–27. [Google Scholar] [CrossRef]
- Ater, J.L.; Xia, C.; Mazewski, C.M.; Booth, T.N.; Freyer, D.R.; Packer, R.J.; Sposto, R.; Vezina, G.; Pollack, I.F. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children’s Oncology Group. Cancer 2016, 122, 1928–1936. [Google Scholar] [CrossRef]
- Lassaletta, A.; Scheinemann, K.; Zelcer, S.M.; Hukin, J.; Wilson, B.A.; Jabado, N.; Carret, A.S.; Lafay-Cousin, L.; Larouche, V.; Hawkins, C.E.; et al. Phase II Weekly Vinblastine for Chemotherapy-Naïve Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J. Clin. Oncol. 2016, 34, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Ater, J.L.; Zhou, T.; Holmes, E.; Mazewski, C.M.; Booth, T.N.; Freyer, D.R.; Lazarus, K.H.; Packer, R.J.; Prados, M.; Sposto, R.; et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Gnekow, A.K.; Walker, D.A.; Kandels, D.; Picton, S.; Giorgio Perilongo Grill, J.; Stokland, T.; Sandstrom, P.E.; Warmuth-Metz, M.; Pietsch, T.; Giangaspero, F.; et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma—A final report. Eur. J. Cancer 2017, 81, 206–225. [Google Scholar] [CrossRef]
- Gururangan, S.; Fangusaro, J.; Poussaint, T.Y.; McLendon, R.E.; Onar-Thomas, A.; Wu, S.; Packer, R.J.; Banerjee, A.; Gilbertson, R.J.; Fahey, F.; et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—A Pediatric Brain Tumor Consortium study. Neuro-Oncology 2014, 16, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kandels, D.; Pietsch, T.; Bison, B.; Warmuth-Metz, M.; Thomale, U.W.; Kortmann, R.D.; Timmermann, B.; Hernáiz Driever, P.; Witt, O.; Schmidt, R.; et al. Loss of efficacy of subsequent nonsurgical therapy after primary treatment failure in pediatric low-grade glioma patients-Report from the German SIOP-LGG 2004 cohort. Int. J. Cancer 2020, 147, 3471–3489. [Google Scholar] [CrossRef]
- Merchant, T.E.; Conklin, H.M.; Wu, S.; Lustig, R.H.; Xiong, X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 2009, 27, 3691–3697. [Google Scholar] [CrossRef]
- Greenberger, B.A.; Pulsifer, M.B.; Ebb, D.H.; MacDonald, S.M.; Jones, R.M.; Butler, W.E.; Huang, M.S.; Marcus, K.J.; Oberg, J.A.; Tarbell, N.J.; et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1060–1068. [Google Scholar] [CrossRef]
- Marcus, K.J.; Goumnerova, L.; Billett, A.L.; Lavally, B.; Scott, R.M.; Bishop, K.; Xu, R.; Young Poussaint, T.; Kieran, M.; Kooy, H.; et al. Stereotactic radiotherapy for localized low-grade gliomas in children: Final results of a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 374–379. [Google Scholar] [CrossRef]
- Manoharan, N.; Liu, K.X.; Mueller, S.; Haas-Kogan, D.A.; Bandopadhayay, P. Pediatric low-grade glioma: Targeted therapeutics and clinical trials in the molecular era. Neoplasia 2023, 36, 100857. [Google Scholar] [CrossRef]
- Fangusaro, J.; Onar-Thomas, A.; Poussaint, T.Y.; Wu, S.; Ligon, A.H.; Lindeman, N.; Campagne, O.; Banerjee, A.; Gururangan, S.; Kilburn, L.B.; et al. A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: A Pediatric Brain Tumor Consortium study. Neuro-Oncology 2021, 23, 1777–1788. [Google Scholar] [CrossRef]
- Bouffet, E.; Geoerger, B.; Moertel, C.; Whitlock, J.A.; Aerts, I.; Hargrave, D.; Osterloh, L.; Tan, E.; Choi, J.; Russo, M.; et al. Efficacy and Safety of Trametinib Monotherapy or in Combination With Dabrafenib in Pediatric BRAF V600-Mutant Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, L.B.; Khuong-Quang, D.A.; Hansford, J.R.; Landi, D.; van der Lugt, J.; Leary, S.E.S.; Driever, P.H.; Bailey, S.; Perreault, S.; McCowage, G.; et al. The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: The phase 2 FIREFLY-1 trial. Nat. Med. 2024, 30, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, T.; Nazemi, K.J.; Crawford, J.; Kilburn, L.; Minturn, J.; Gajjar, A.; Gauvain, K.; Leary, S.; Dhall, G.; Aboian, M.; et al. Phase I study of vemurafenib in children with recurrent or progressive BRAFV600E mutant brain tumors: Pacific Pediatric Neuro-Oncology Consortium study (PNOC-002). Oncotarget 2020, 11, 1942–1952. [Google Scholar] [CrossRef]
- Hargrave, D.R.; Bouffet, E.; Tabori, U.; Broniscer, A.; Cohen, K.J.; Hansford, J.R.; Geoerger, B.; Hingorani, P.; Dunkel, I.J.; Russo, M.W.; et al. Efficacy and Safety of Dabrafenib in Pediatric Patients with BRAF V600 Mutation-Positive Relapsed or Refractory Low-Grade Glioma: Results from a Phase I/IIa Study. Clin. Cancer Res. 2019, 25, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.D.; Yao, X.; London, W.B.; Kao, P.C.; Gore, L.; Hunger, S.; Geyer, R.; Cohen, K.J.; Allen, J.C.; Katzenstein, H.M.; et al. A POETIC Phase II study of continuous oral everolimus in recurrent, radiographically progressive pediatric low-grade glioma. Pediatr. Blood Cancer 2021, 68, e28787. [Google Scholar] [CrossRef] [PubMed]
- Fangusaro, J.; Jones, D.T.; Packer, R.J.; Gutmann, D.H.; Milde, T.; Witt, O.; Mueller, S.; Fisher, M.J.; Hansford, J.R.; Tabori, U.; et al. Pediatric low-grade glioma: State-of-the-art and ongoing challenges. Neuro-Oncology 2024, 26, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Hansford, J.R.; Garrè, M.L.; Hara, J.; Plant-Fox, A.; Aerts, I.; Locatelli, F.; van der Lugt, J.; Papusha, L.; Sahm, F.; et al. Dabrafenib plus Trametinib in Pediatric Glioma with BRAF V600 Mutations. N. Engl. J. Med. 2023, 389, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Kocher, D.; Cao, L.; Guiho, R.; Langhammer, M.; Lai, Y.L.; Becker, P.; Hamdi, H.; Friedel, D.; Selt, F.; Vonhören, D.; et al. Rebound growth of BRAF mutant pediatric glioma cells after MAPKi withdrawal is associated with MAPK reactivation and secretion of microglia-recruiting cytokines. J. Neuro-Oncol. 2024, 168, 317–332. [Google Scholar] [CrossRef]
- Talloa, D.; Triarico, S.; Agresti, P.; Mastrangelo, S.; Attinà, G.; Romano, A.; Maurizi, P.; Ruggiero, A. BRAF and MEK Targeted Therapies in Pediatric Central Nervous System Tumors. Cancers 2022, 14, 4264. [Google Scholar] [CrossRef]
- Karajannis, M.A.; Legault, G.; Fisher, M.J.; Milla, S.S.; Cohen, K.J.; Wisoff, J.H.; Harter, D.H.; Goldberg, J.D.; Hochman, T.; Merkelson, A.; et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro-Oncology 2014, 16, 1408–1416. [Google Scholar] [CrossRef]
- Gorodezki, D.; Zipfel, J.; Queudeville, M.; Sosa, J.; Holzer, U.; Kern, J.; Bevot, A.; Schittenhelm, J.; Nägele, T.; Ebinger, M.; et al. Resection extent and BRAF V600E mutation status determine postoperative tumor growth velocity in pediatric low-grade glioma: Results from a single-center cohort analysis. J. Neuro-Oncol. 2022, 160, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Rozen, W.M.; Joseph, S.; Lo, P.A. Spontaneous regression of low-grade gliomas in pediatric patients without neurofibromatosis. Pediatr. Neurosurg. 2008, 44, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Parsa, C.F.; Hoyt, C.S.; Lesser, R.L.; Weinstein, J.M.; Strother, C.M.; Muci-Mendoza, R.; Ramella, M.; Manor, R.S.; Fletcher, W.A.; Repka, M.X.; et al. Spontaneous regression of optic gliomas: Thirteen cases documented by serial neuroimaging. Arch. Ophthalmol. 2001, 119, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Perilongo, G.; Moras, P.; Carollo, C.; Battistella, A.; Clementi, M.; Laverda, A.; Murgia, A. Spontaneous partial regression of low-grade glioma in children with neurofibromatosis-1: A real possibility. J. Child. Neurol. 1999, 14, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Broniscer, A.; Baker, S.J.; West, A.N.; Fraser, M.M.; Proko, E.; Kocak, M.; Dalton, J.; Zambetti, G.P.; Ellison, D.W.; Kun, L.E.; et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J. Clin. Oncol. 2007, 25, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Soleman, J.; Roth, J.; Ram, Z.; Yalon, M.; Constantini, S. Malignant transformation of a conservatively managed incidental childhood cerebral mass lesion: Controversy regarding management paradigm. Child’s Nerv. Syst. 2017, 33, 2169–2175. [Google Scholar] [CrossRef]
- Winograd, E.; Pencovich, N.; Yalon, M.; Soffer, D.; Beni-Adani, L.; Constantini, S. Malignant transformation in pediatric spinal intramedullary tumors: Case-based update. Child’s Nerv. Syst. 2012, 28, 1679–1686. [Google Scholar] [CrossRef]
- Ünal, E.; Koksal, Y.; Çimen, O.; Paksoy, Y.; Tavli, L. Malignant glioblastomatous transformation of a low-grade glioma in a child. Child’s Nerv. Syst. 2008, 24, 1385–1389. [Google Scholar] [CrossRef]
- Van Der Wal, E.P.; Azzarelli, B.; Edwards-Brown, M. Malignant transformation of a chiasmatic pilocytic astrocytoma in a patient with diencephalic syndrome. Pediatr. Radiol. 2003, 33, 207–210. [Google Scholar] [CrossRef]
- Goebel, A.M.; Gnekow, A.K.; Kandels, D.; Witt, O.; Schmidt, R.; Hernáiz Driever, P. Natural History of Pediatric Low-Grade Glioma Disease—First Multi-State Model Analysis. J. Cancer 2019, 10, 6314–6326. [Google Scholar] [CrossRef]
- Gajjar, A.; Sanford, R.A.; Heideman, R.; Jenkins, J.J.; Walter, A.; Li, Y.; Langston, J.W.; Muhlbauer, M.; Boyett, J.M.; Kun, L.E. Low-grade astrocytoma: A decade of experience at St. Jude Children’s Research Hospital. J. Clin. Oncol. 1997, 15, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
- Ryall, S.; Krishnatry, R.; Arnoldo, A.; Buczkowicz, P.; Mistry, M.; Siddaway, R.; Ling, C.; Pajovic, S.; Yu, M.; Rubin, J.B.; et al. Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol. Commun. 2016, 4, 93. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Skardelly, M.; Schuhmann, M.U.; Ebinger, M.; Reuss, D.; Neumann, M.; Tabatabai, G.; Kohlhof-Meinecke, P.; Schittenhelm, J. High frequency of H3 K27M mutations in adult midline gliomas. J. Cancer Res. Clin. Oncol. 2019, 145, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Mirow, C.; Pietsch, T.; Berkefeld, S.; Kwiecien, R.; Warmuth-Metz, M.; Falkenstein, F.; Diehl, B.; von Hornstein, S.; Gnekow, A.K. Children <1 year show an inferior outcome when treated according to the traditional LGG treatment strategy: A report from the German multicenter trial HIT-LGG 1996 for children with low grade glioma (LGG). Pediatr. Blood Cancer 2014, 61, 457–463. [Google Scholar] [CrossRef]
- Janss, A.J.; Grundy, R.; Cnaan, A.; Savino, P.J.; Packer, R.J.; Zackai, E.H.; Goldwein, J.W.; Sutton, L.N.; Radcliffe, J.; Molloy, P.T.; et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer 1995, 75, 1051–1059. [Google Scholar] [CrossRef]
- Opocher, E.; Kremer, L.C.; Da Dalt, L.; van de Wetering, M.D.; Viscardi, E.; Caron, H.N.; Perilongo, G. Prognostic factors for progression of childhood optic pathway glioma: A systematic review. Eur. J. Cancer 2006, 42, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, M.; Hunt, D.L.; Pollack, I.F.; Dueckers, G.; Burger, P.C.; Becker, L.E.; Yates, A.J.; Gilles, F.H.; Davis, R.L.; Boyett, J.M.; et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer 2003, 98, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Laithier, V.; Grill, J.; Le Deley, M.-C.; Ruchoux, M.-M.; Couanet, D.; Doz, F.; Pichon, F.; Rubie, H.; Frappaz, D.; Vannier, J.-P.; et al. Progression-free survival in children with optic pathway tumors: Dependence on age and the quality of the response to chemotherapy—Results of the first French prospective study for the French Society of Pediatric Oncology. J. Clin. Oncol. 2003, 21, 4572–4578. [Google Scholar] [CrossRef]
- Gururangan, S.; Cavazos, C.M.; Ashley, D.; Herndon, J.E., 2nd; Bruggers, C.S.; Moghrabi, A.; Scarcella, D.L.; Watral, M.; Tourt-Uhlig, S.; Reardon, D.; et al. Phase II study of carboplatin in children with progressive low-grade gliomas. J. Clin. Oncol. 2002, 20, 2951–2958. [Google Scholar] [CrossRef]
- Packer, R.J.; Ater, J.; Allen, J.; Phillips, P.; Geyer, R.; Nicholson, H.S.; Jakacki, R.; Kurczynski, E.; Needle, M.; Finlay, J.; et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J. Neurosurg. 1997, 86, 747–754. [Google Scholar] [CrossRef]
- Scotting, P.J.; Walker, D.A.; Perilongo, G. Childhood solid tumours: A developmental disorder. Nat. Rev. Cancer 2005, 5, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Claassen, D.; al-Shboul, Q.; Janosky, J.E.; Deutsch, M. Low-grade gliomas of the cerebral hemispheres in children: An analysis of 71 cases. J. Neurosurg. 1995, 82, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Smoots, D.W.; Geyer, J.R.; Lieberman, D.M.; Berger, M.S. Predicting disease progression in childhood cerebellar astrocytoma. Childs Nerv. Syst. 1998, 14, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.I.; Nadkarni, T.D.; Muzumdar, D.P.; Goel, A. Prognostic factors for cerebellar astrocytomas in children: A study of 102 cases. Pediatr. Neurosurg. 2001, 35, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Carreno, G.; Guiho, R.; Martinez-Barbera, J.P. Cell senescence in neuropathology: A focus on neurodegeneration and tumours. Neuropathol. Appl. Neurobiol. 2021, 47, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Thomale, U.W.; Gnekow, A.K.; Kandels, D.; Bison, B.; Hernáiz Driever, P.; Witt, O.; Pietsch, T.; Koch, A.; Capper, D.; Kortmann, R.D.; et al. Long-term follow-up of surgical intervention pattern in pediatric low-grade gliomas: Report from the German SIOP-LGG 2004 cohort. J. Neurosurg. Pediatr. 2022, 45, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Hutter, B.; Jäger, N.; Korshunov, A.; Kool, M.; Warnatz, H.J.; Zichner, T.; Lambert, S.R.; Ryzhova, M.; Quang, D.A.; et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 2013, 45, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, G.; Miller, C.P.; Tatevossian, R.G.; Dalton, J.D.; Tang, B.; Orisme, W.; Punchihewa, C.; Parker, M.; Qaddoumi, I.; et al. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 2013, 45, 602–612. [Google Scholar] [CrossRef]
- Ryall, S.; Zapotocky, M.; Fukuoka, K.; Nobre, L.; Guerreiro Stucklin, A.; Bennett, J.; Siddaway, R.; Li, C.; Pajovic, S.; Arnoldo, A.; et al. Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas. Cancer Cell 2020, 37, 569–583.e5. [Google Scholar] [CrossRef]
- Jones, D.T.; Kocialkowski, S.; Liu, L.; Pearson, D.M.; Bäcklund, L.M.; Ichimura, K.; Collins, V.P. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008, 68, 8673–8677. [Google Scholar] [CrossRef]
- Jones, D.T.; Kocialkowski, S.; Liu, L.; Pearson, D.M.; Ichimura, K.; Collins, V.P. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene 2009, 28, 2119–2123. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.; Janzarik, W.G.; Remke, M.; Ernst, A.; Werft, W.; Becker, N.; Toedt, G.; Wittmann, A.; Kratz, C.; Olbrich, H.; et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J. Clin. Investig. 2008, 118, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Adjei, A.A. The Ras/Raf/MAPK pathway. J. Thorac. Oncol. 2006, 1, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, O.; Gebbink, M.F.; Voest, E.E. Stimulation of angiogenesis by Ras proteins. Biochim. Biophys. Acta 2004, 1654, 23–37. [Google Scholar] [CrossRef]
- Stacey, D.W. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr. Opin. Cell Biol. 2003, 15, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert. Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Friedman, J.M. NF1 gene and neurofibromatosis 1. Am. J. Epidemiol. 2000, 151, 33–40. [Google Scholar] [CrossRef]
- Ryall, S.; Tabori, U.; Hawkins, C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 2020, 8, 30. [Google Scholar] [CrossRef]
- Uusitalo, E.; Rantanen, M.; Kallionpää, R.A.; Pöyhönen, M.; Leppävirta, J.; Ylä-Outinen, H.; Riccardi, V.M.; Pukkala, E.; Pitkäniemi, J.; Peltonen, S.; et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J. Clin. Oncol. 2016, 34, 1978–1986. [Google Scholar] [CrossRef]
- Seminog, O.O.; Goldacre, M.J. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: Population-based record-linkage study. Br. J. Cancer. 2013, 108, 193–198. [Google Scholar] [CrossRef]
- Schindler, G.; Capper, D.; Meyer, J.; Janzarik, W.; Omran, H.; Herold-Mende, C.; Schmieder, K.; Wesseling, P.; Mawrin, C.; Hasselblatt, M.; et al. Analysis of BRAF V600E mutation in 1320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011, 121, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, A.; Zapotocky, M.; Mistry, M.; Ramaswamy, V.; Honnorat, M.; Krishnatry, R.; Stucklin, A.G.; Zhukova, N.; Arnoldo, A.; Ryall, S.; et al. Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J. Clin. Oncol. 2017, 35, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Villanueva-Meyer, J.E.; Goode, B.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Bastian, B.C.; Chamyan, G.; Maher, O.M.; Khatib, Z.; et al. The genetic landscape of ganglioglioma. Acta Neuropathol. Commun. 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Behling, F.; Beschorner, R.; Eckert, F.; Kohlhof, P.; Tatagiba, M.; Tabatabai, G.; Schuhmann, M.U.; Ebinger, M.; Schittenhelm, J. Frequent FGFR1 hotspot alterations in driver-unknown low-grade glioma and mixed neuronal-glial tumors. J. Cancer Res. Clin. Oncol. 2022, 148, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, I.; Orisme, W.; Wen, J.; Santiago, T.; Gupta, K.; Dalton, J.D.; Tang, B.; Haupfear, K.; Punchihewa, C.; Easton, J.; et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathologica. 2016, 131, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, L.A.; Horowitz, P.M.; Craig, J.M.; Ramkissoon, S.H.; Rich, B.E.; Schumacher, S.E.; McKenna, A.; Lawrence, M.S.; Bergthold, G.; Brastianos, P.K.; et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc. Natl. Acad. Sci. USA 2013, 110, 8188–8193. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.W.; Gronych, J.; Lichter, P.; Witt, O.; Pfister, S.M. MAPK pathway activation in pilocytic astrocytoma. Cell Mol. Life Sci. 2012, 69, 1799–1811. [Google Scholar] [CrossRef]
- Bandopadhayay, P.; Ramkissoon, A.L.; Jain, P.; Bergthold, G.; Wala, J.; Zeid, R.; Schumacher, E.S.; Urbanski, L.; O’Rourke, R.; Gibson, W.J.; et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat. Genet. 2016, 48, 273–282. [Google Scholar] [CrossRef]
- Yeo, K.K.; Alexandrescu, S.; Cotter, J.A.; Vogelzang, J.; Bhave, V.; Li, M.M.; Ji, J.; Benhamida, J.K.; Rosenblum, M.K.; Bale, T.A.; et al. Multi-institutional study of the frequency, genomic landscape, and outcome of IDH-mutant glioma in pediatrics. Neuro-Oncology 2023, 25, 199–210. [Google Scholar] [CrossRef]
- Northcott, P.A.; Pfister, S.M.; Jones, D.T. Next-generation (epi)genetic drivers of childhood brain tumours and the outlook for targeted therapies. Lancet Oncol. 2015, 16, e293–e302. [Google Scholar] [CrossRef]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Lee, S.; Schmitt, C.A.; Reimann, M. The Myc/macrophage tango: Oncogene-induced senescence, Myc style. Semin. Cancer Biol. 2011, 21, 377–384. [Google Scholar] [CrossRef]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Coppé, J.P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.Y.; Campisi, J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef]
- Wu, C.-H.; van Riggelen, J.; Yetil, A.; Fan, A.C.; Bachireddy, P.; Felsher, D.W. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc. Natl. Acad. Sci. USA 2007, 104, 13028–13033. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wang, H.J.; Tan, Y.Z. Wnt/β-catenin signaling induces the aging of Mesenchymal stem cells through the DNA damage response and the P53/P21 pathway. PLoS ONE 2011, 6, e21397. [Google Scholar] [CrossRef]
- Lin, A.W.; Barradas, M.; Stone, J.C.; van Aelst, L.; Serrano, M.; Lowe, S.W. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes. Dev. 1998, 12, 3008–3019. [Google Scholar] [CrossRef]
- Sarkisian, C.J.; Keister, B.A.; Stairs, D.B.; Boxer, R.B.; Moody, S.E.; Chodosh, L.A. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 2007, 9, 493–505. [Google Scholar] [CrossRef]
- Carrière, C.; Gore, A.J.; Norris, A.M.; Gunn, J.R.; Young, A.L.; Longnecker, D.S.; Korc, M. Deletion of Rb accelerates pancreatic carcinogenesis by oncogenic Kras and impairs senescence in premalignant lesions. Gastroenterology 2011, 141, 1091–1101. [Google Scholar] [CrossRef]
- Qiu, W.; Sahin, F.; Iacobuzio-Donahue, C.A.; Garcia-Carracedo, D.; Wang, W.M.; Kuo, C.Y.; Chen, D.; Arking, D.E.; Lowy, A.M.; Hruban, R.H.; et al. Disruption of p16 and activation of Kras in pancreas increase ductal adenocarcinoma formation and metastasis in vivo. Oncotarget 2011, 2, 862–873. [Google Scholar] [CrossRef]
- Morton, J.P.; Timpson, P.; Karim, S.A.; Ridgway, R.A.; Athineos, D.; Doyle, B.; Jamieson, N.B.; Oien, K.A.; Lowy, A.M.; Brunton, V.G.; et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 246–251. [Google Scholar] [CrossRef]
- Burotto, M.; Chiou, V.L.; Lee, J.M.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef]
- De Luca, A.; Maiello, M.R.; D’Alessio, A.; Pergameno, M.; Normanno, N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert. Opin. Ther. Targets 2012, 16 (Suppl. S2), S17–S27. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef]
- Deng, Q.; Liao, R.; Wu, B.L.; Sun, P. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J. Biol. Chem. 2004, 279, 1050–1059. [Google Scholar] [CrossRef]
- Zhu, J.; Woods, D.; McMahon, M.; Bishop, J.M. Senescence of human fibroblasts induced by oncogenic Raf. Genes. Dev. 1998, 12, 2997–3007. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.X.; Liao, R.; Deng, Q.; Zhou, J.J.; Huang, S.; Sun, P. Sequential Activation of the MEK-Extracellular Signal-Regulated Kinase and MKK3/6-p38 Mitogen-Activated Protein Kinase Pathways Mediates Oncogenic ras-Induced Premature Senescence. Mol. Cell. Biol. 2002, 22, 3389–3403. [Google Scholar] [CrossRef]
- Dankort, D.; Filenova, E.; Collado, M.; Serrano, M.; Jones, K.; McMahon, M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes. Dev. 2007, 21, 379–384. [Google Scholar] [CrossRef]
- Cisowski, J.; Sayin, V.I.; Liu, M.; Karlsson, C.; Bergo, M.O. Oncogene-induced senescence underlies the mutual exclusive nature of oncogenic KRAS and BRAF. Oncogene 2016, 35, 1328–1333. [Google Scholar] [CrossRef]
- Petti, C.; Molla, A.; Vegetti, C.; Ferrone, S.; Anichini, A.; Sensi, M. Coexpression of NRASQ61R and BRAFV600E in human melanoma cells activates senescence and increases susceptibility to cell-mediated cytotoxicity. Cancer Res. 2006, 66, 6503–6511. [Google Scholar] [CrossRef]
- Michaloglou, C.; Vredeveld, L.C.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; van der Horst, C.M.; Majoor, D.M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef]
- Bigenwald, C.; Bigenwald, C.; Le Berichel, J.; Le Berichel, J.; Wilk, C.M.; Wilk, C.M.; Chakraborty, R.; Chakraborty, R.; Chen, S.T.; Chen, S.T.; et al. BRAFV600E-induced senescence drives Langerhans cell histiocytosis pathophysiology. Nat. Med. 2021, 27, 851–861. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, J.; Zhang, C.; Li, P.; Tan, C.; Yang, M.; Zhao, G. Cellular Senescence in Non-Small Cell Lung Cancer. Front. Biosci. 2023, 28, 357. [Google Scholar] [CrossRef]
- Chiacchiarini, M.; Besharat, Z.M.; Carai, A.; Miele, E.; Del Baldo, G.; Mastronuzzi, A.; Catanzaro, G.; Ferretti, E. Pediatric low-grade gliomas: Molecular characterization of patient-derived cellular models. Childs Nerv. Syst. 2021, 37, 771–778. [Google Scholar] [CrossRef]
- Raabe, E.H.; Lim, K.S.; Kim, J.M.; Meeker, A.; Mao, X.-G.; Nikkhah, G.; Maciaczyk, J.; Kahlert, U.; Jain, D.; Bar, E.; et al. BRAF activation induces transformation and then senescence in human neural stem cells: A pilocytic astrocytoma model. Clin. Cancer Res. 2011, 17, 3590–3599. [Google Scholar] [CrossRef]
- Jacob, K.; Quang-Khuong, D.A.; Jones, D.T.; Witt, H.; Lambert, S.; Albrecht, S.; Witt, O.; Vezina, C.; Shirinian, M.; Faury, D.; et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin. Cancer Res. 2011, 17, 4650–4660. [Google Scholar] [CrossRef]
- Milde, T.; Fangusaro, J.; Fisher, M.J.; Hawkins, C.; Rodriguez, F.J.; Tabori, U.; Witt, O.; Zhu, Y.; Gutmann, D.H. Optimizing preclinical pediatric low-grade glioma models for meaningful clinical translation. Neuro-Oncology 2023, 25, 1920–1931. [Google Scholar] [CrossRef]
- Buhl, J.L.; Selt, F.; Hielscher, T.; Guiho, R.; Ecker, J.; Sahm, F.; Ridinger, J.; Riehl, D.; Usta, D.; Ismer, B.; et al. The Senescence-associated Secretory Phenotype Mediates Oncogene-induced Senescence in Pediatric Pilocytic Astrocytoma. Clin. Cancer Res. 2019, 25, 1851–1866. [Google Scholar] [CrossRef]
- Selt, F.; El Damaty, A.; Schuhmann, M.U.; Sigaud, R.; Ecker, J.; Sievers, P.; Kocher, D.; Herold-Mende, C.; Oehme, I.; von Deimling, A.; et al. Generation of patient-derived pediatric pilocytic astrocytoma in-vitro models using SV40 large T: Evaluation of a modeling workflow. J. Neuro-Oncol. 2023, 165, 467–478. [Google Scholar] [CrossRef]
- Selt, F.; Hohloch, J.; Hielscher, T.; Sahm, F.; Capper, D.; Korshunov, A.; Usta, D.; Brabetz, S.; Ridinger, J.; Ecker, J.; et al. Establishment and application of a novel patient-derived KIAA1549:BRAF-driven pediatric pilocytic astrocytoma model for preclinical drug testing. Oncotarget 2017, 8, 11460–11479. [Google Scholar] [CrossRef]
- Packer, R.J.; Pfister, S.; Bouffet, E.; Avery, R.; Bandopadhayay, P.; Bornhorst, M.; Bowers, D.C.; Ellison, D.; Fangusaro, J.; Foreman, N.; et al. Pediatric low-grade gliomas: Implications of the biologic era. Neuro-Oncology 2017, 19, 750–761. [Google Scholar] [CrossRef]
- Sturm, D.; Pfister, S.M.; Jones, D.T.W. Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management. J. Clin. Oncol. 2017, 35, 2370–2377. [Google Scholar] [CrossRef]
- Liggett, W.H., Jr.; Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef]
- Ruas, M.; Peters, G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1998, 1378, F115–F177. [Google Scholar] [CrossRef]
- Horbinski, C.; Hamilton, R.L.; Nikiforov, Y.; Pollack, I.F. Association of molecular alterations, including BRAF, with biology and outcome in pilocytic astrocytomas. Acta Neuropathol. 2010, 119, 641–649. [Google Scholar] [CrossRef]
- Jones, D.T.W.; Witt, O.; Pfister, S.M. BRAF V600E Status Alone Is Not Sufficient as a Prognostic Biomarker in Pediatric Low-Grade Glioma. J. Clin. Oncol. 2018, 36, 96. [Google Scholar] [CrossRef]
- Horbinski, C.; Nikiforova, M.N.; Hagenkord, J.M.; Hamilton, R.L.; Pollack, I.F. Interplay among BRAF, p16, p53, and MIB1 in pediatric low-grade gliomas. Neuro-Oncology 2012, 14, 777–789. [Google Scholar] [CrossRef]
- Mistry, M.; Zhukova, N.; Merico, D.; Rakopoulos, P.; Krishnatry, R.; Shago, M.; Stavropoulos, J.; Alon, N.; Pole, J.D.; Ray, P.N.; et al. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J. Clin. Oncol. 2015, 33, 1015–1022. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Hodgson, J.G.; VandenBerg, S.R.; Flaherty, P.; Polley, M.Y.; Yu, M.; Fisher, P.G.; Rowitch, D.H.; Ford, J.M.; Berger, M.S.; et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010, 70, 512–519. [Google Scholar] [CrossRef]
- Binesh, F.; Akhavan, A.; Navabii, H. Pleomorphic xanthoastrocytoma with malignant transformation and multiple recurrences in an Iranian girl. BMJ Case Rep. 2012, 2012, bcr1220115372. [Google Scholar] [CrossRef]
- Rodríguez-Mena, R.; Joanes-Alepuz, V.; Barbella-Aponte, R.; Pérez-Valles, A. Xantoastrocitoma pleomórfico con extensión intraventricular y transformación anaplásica en paciente adulto: Caso clínico [Pleomorphic xanthoastrocytoma with intraventricular extension and anaplastic transformation in an adult patient: Case report]. Neurocirugia 2012, 23, 203–210. (In Spanish) [Google Scholar] [CrossRef]
- Bender, K.; Perez, E.; Chirica, M.; Onken, J.; Kahn, J.; Brenner, W.; Ehret, F.; Euskirchen, P.; Koch, A.; Capper, D.; et al. High-grade astrocytoma with piloid features (HGAP): The Charité experience with a new central nervous system tumor entity. J. Neurooncol. 2021, 153, 109–120. [Google Scholar] [CrossRef]
- Cimino, P.J.; Ketchum, C.; Turakulov, R.; Singh, O.; Abdullaev, Z.; Giannini, C.; Pytel, P.; Lopez, G.Y.; Colman, H.; Nasrallah, M.P.; et al. Expanded analysis of high-grade astrocytoma with piloid features identifies an epigenetically and clinically distinct subtype associated with neurofibromatosis type 1. Acta Neuropathol. 2023, 145, 71–82. [Google Scholar] [CrossRef]
- Reinhardt, A.; Stichel, D.; Schrimpf, D.; Sahm, F.; Korshunov, A.; Reuss, D.E.; Koelsche, C.; Huang, K.; Wefers, A.K.; Hovestadt, V.; et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018, 136, 273–291. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537.e5. [Google Scholar] [CrossRef]
- Shi, Z.F.; Li, K.K.; Liu, A.P.; Chung, N.Y.; Chow, C.; Chen, H.; Kan, N.A.; Zhu, X.L.; Chan, D.T.; Mao, Y.; et al. Rare Pediatric Cerebellar High-Grade Gliomas Mimic Medulloblastomas Histologically and Transcriptomically and Show p53 Mutations. Cancers 2024, 16, 232. [Google Scholar] [CrossRef]
- Pollack, I.F.; Hamilton, R.L.; Finkelstein, S.D.; Campbell, J.W.; Martinez, A.J.; Sherwin, R.N.; Bozik, M.E.; Gollin, S.M. The relationship between TP53 mutations and overexpression of p53 and prognosis in malignant gliomas of childhood. Cancer Res. 1997, 57, 304–309. [Google Scholar] [PubMed]
- Gorodezki, D.; Zipfel, J.; Queudeville, M.; Holzer, U.; Bevot, A.; Schittenhelm, J.; Nägele, T.; Schuhmann, M.U.; Ebinger, M. Evaluating the safety of perioperative dexamethasone treatment: A retrospective analysis of a single center pediatric low-grade glioma cohort. Int. J. Cancer 2023, 152, 1875–1883. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Xu, J.; Dong, X.; Huang, D.C.S.; Xu, P.; Zhao, Q.; Chen, B. Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers. Cancers 2023, 15, 4957. [Google Scholar] [CrossRef]
- Hafezi, S.; Rahmani, M. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers 2021, 13, 1292. [Google Scholar] [CrossRef]
- Calis, S.; Dogan, B.; Durdagi, S.; Celebi, A.; Yapicier, O.; Kilic, T.; Turanli, E.T.; Avsar, T. A novel BH3 mimetic Bcl-2 inhibitor promotes autophagic cell death and reduces in vivo Glioblastoma tumor growth. Cell Death Discov. 2022, 8, 433. [Google Scholar] [CrossRef]
- Rahman, M.; Olson, I.; Mansour, M.; Carlstrom, L.P.; Sutiwisesak, R.; Saber, R.; Rajani, K.; Warrington, A.E.; Howard, A.; Schroeder, M.; et al. Selective Vulnerability of Senescent Glioblastoma Cells to BCL-XL Inhibition. Mol. Cancer Res. 2022, 20, 938–948. [Google Scholar] [CrossRef]
- Fanfone, D.; Idbaih, A.; Mammi, J.; Gabut, M.; Ichim, G. Profiling Anti-Apoptotic BCL-xL Protein Expression in Glioblastoma Tumorspheres. Cancers 2020, 12, 2853. [Google Scholar] [CrossRef]
- Koessinger, A.L.; Cloix, C.; Koessinger, D.; Heiland, D.H.; Bock, F.J.; Strathdee, K.; Kinch, K.; Martínez-Escardó, L.; Paul, N.R.; Nixon, C.; et al. Increased apoptotic sensitivity of glioblastoma enables therapeutic targeting by BH3-mimetics. Cell Death Differ. 2022, 29, 2089–2104. [Google Scholar] [CrossRef]
- Selt, F.; Sigaud, R.; Valinciute, G.; Sievers, P.; Zaman, J.; Alcon, C.; Schmid, S.; Peterziel, H.; Tsai, J.W.; Guiho, R.; et al. BH3 mimetics targeting BCL-XL impact the senescent compartment of pilocytic astrocytoma. Neuro-Oncolgy 2023, 25, 735–747. [Google Scholar] [CrossRef]
- Badawi, M.; Menon, R.; Place, A.E.; Palenski, T.; Sunkersett, G.; Arrendale, R.; Deng, R.; Federico, S.M.; Cooper, T.M.; Salem, A.H. Venetoclax Penetrates the Blood Brain Barrier: A Pharmacokinetic Analysis in Pediatric Leukemia Patients. J. Cancer 2023, 14, 1151–1156. [Google Scholar] [CrossRef]
- Chew, V.; Toh, H.C.; Abastado, J.P. Immune microenvironment in tumor progression: Characteristics and challenges for therapy. J. Oncol. 2012, 2012, 608406. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Kienzl, M.; Maitz, K.; Sarsembayeva, A.; Valadez-Cosmes, P.; Gruden, E.; Ristic, D.; Herceg, K.; Kargl, J.; Schicho, R. Comparative Study of the Immune Microenvironment in Heterotopic Tumor Models. Cancers 2024, 16, 295. [Google Scholar] [CrossRef]
- Dzobo, K.; Senthebane, D.A.; Dandara, C. The Tumor Microenvironment in Tumorigenesis and Therapy Resistance Revisited. Cancers 2023, 15, 376. [Google Scholar] [CrossRef]
- Du, Q.; An, Q.; Zhang, J.; Liu, C.; Hu, Q. Unravelling immune microenvironment features underlying tumor progression in the single-cell era. Cancer Cell Int. 2024, 24, 143. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Messiaen, J.; Jacobs, S.A.; De Smet, F. The tumor micro-environment in pediatric glioma: Friend or foe? Front. Immunol. 2023, 14, 1227126. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Gao, L.; Wang, Y.; Guo, Y.; Xing, B.; Ma, W. Classification of pediatric gliomas based on immunological profiling: Implications for immunotherapy strategies. Mol. Ther. Oncolytics 2020, 20, 34–47. [Google Scholar] [CrossRef]
- Grabovska, Y.; Mackay, A.; O’Hare, P.; Crosier, S.; Finetti, M.; Schwalbe, E.C.; Pickles, J.C.; Fairchild, A.R.; Avery, A.; Cockle, J.; et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 2020, 11, 4324. [Google Scholar] [CrossRef]
- Venteicher, A.S.; Tirosh, I.; Hebert, C.; Yizhak, K.; Neftel, C.; Filbin, M.G.; Hovestadt, V.; Escalante, L.E.; Shaw, M.L.; Rodman, C.; et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 2017, 355, eaai8478. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.H.; Vasquez, J.; Kaushal, A.; MacDonald, T.J.; Velázquez Vega, J.E.; Schniederjan, M.; Dhodapkar, K. Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma. J. Immunother. Cancer 2020, 8, e001066. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.; Riemondy, K.; Griesinger, A.M.; Donson, A.M.; Fu, R.; Crespo, M.; DeSisto, J.; Groat, M.M.; Bratbak, E.; Green, A.; et al. Multi-pronged analysis of pediatric low-grade glioma reveals a unique tumor microenvironment associated with BRAF alterations. bioRxiv 2024, 183, 1962–1985.e31. [Google Scholar] [CrossRef]
- Petralia, F.; Tignor, N.; Reva, B.; Koptyra, M.; Chowdhury, S.; Rykunov, D.; Krek, A.; Ma, W.; Zhu, Y.; Ji, J.; et al. Integrated Proteogenomic Characterization across Major Histological Types of Pediatric Brain Cancer. Cell 2020, 183, 1962–1985.e31. [Google Scholar] [CrossRef] [PubMed]
- Panwalkar, P.; Pratt, D.; Chung, C.; Dang, D.; Le, P.; Martinez, D.; Bayliss, J.M.; Smith, K.S.; Adam, M.; Potter, S.; et al. SWI/SNF complex heterogeneity is related to polyphenotypic differentiation, prognosis, and immune response in rhabdoid tumors. Neuro-Oncology 2020, 22, 785–796. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Afra, D.; de Witte, O.; Ben Hassel, M.; Schraub, S.; Hoang-Xuan, K.; Malmström, P.-O.; Collette, L.; Piérart, M.; Mirimanoff, R.; et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet 2005, 366, 985–990. [Google Scholar] [CrossRef]
- Sigaud, R.; Albert, T.K.; Hess, C.; Hielscher, T.; Winkler, N.; Kocher, D.; Walter, C.; Münter, D.; Selt, F.; Usta, D.; et al. MAPK inhibitor sensitivity scores predict sensitivity driven by the immune infiltration in pediatric low-grade gliomas. Nat. Commun. 2023, 14, 4533. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef]
- Wang, T.W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef]
- Chen, H.A.; Ho, Y.J.; Mezzadra, R.; Adrover, J.M.; Smolkin, R.; Zhu, C.; Woess, K.; Bernstein, N.; Schmitt, G.; Fong, L.; et al. Senescence Rewires Microenvironment Sensing to Facilitate Antitumor Immunity. Cancer Discov. 2023, 13, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Sigaud, R.; Brummer, T.; Kocher, D.; Milde, T.; Selt, F. MOST wanted: Navigating the MAPK-OIS-SASP-tumor microenvironment axis in primary pediatric low-grade glioma and preclinical models. Childs Nerv. Syst. 2024, 21, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Keoni, C.; Waker, C.A.; Lober, R.M.; Chen, Y.H.; Gutmann, D.H. KIAA1549-BRAF Expression Establishes a Permissive Tumor Microenvironment Through NFκB-Mediated CCL2 Production. Neoplasia 2019, 21, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; McGowan, L.D.; Cimino, P.J.; Dahiya, S.; Leonard, J.R.; Lee, D.Y.; Gutmann, D.H. Mouse low-grade gliomas contain cancer stem cells with unique molecular and functional properties. Cell Rep. 2015, 10, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xiong, M.; Chen, R.; Ma, Y.; Corman, C.; Maricos, M.; Kindler, U.; Semtner, M.; Chen, Y.H.; Dahiya, S.; et al. Athymic mice reveal a requirement for T-cell-microglia interactions in establishing a microenvironment supportive of Nf1 low-grade glioma growth. Genes. Dev. 2018, 32, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Pan, Y.; Gutmann, D.H. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell-specific chemokine recruitment of T cells and microglia. Neuro-Oncology 2019, 21, 1250–1262. [Google Scholar] [CrossRef]
- Guo, X.; Pan, Y.; Xiong, M.; Sanapala, S.; Anastasaki, C.; Cobb, O.; Dahiya, S.; Gutmann, D.H. Midkine activation of CD8+ T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat. Commun. 2020, 11, 2177. [Google Scholar] [CrossRef]
- Tom, M.C.; Park, D.Y.J.; Yang, K.; Leyrer, C.M.; Wei, W.; Jia, X.; Varra, V.; Yu, J.S.; Chao, S.T.; Balagamwala, E.H.; et al. Malignant Transformation of Molecularly Classified Adult Low-Grade Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1106–1112. [Google Scholar] [CrossRef]
- Murphy, E.S.; Leyrer, C.M.; Parsons, M.; Suh, J.H.; Chao, S.T.; Yu, J.S.; Kotecha, R.; Jia, X.; Peereboom, D.M.; Prayson, R.A.; et al. Risk Factors for Malignant Transformation of Low-Grade Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 965–971. [Google Scholar] [CrossRef]
- Rao, A.A.; Laack, N.N.; Giannini, C.; Wetmore, C. Pleomorphic xanthoastrocytoma in children and adolescents. Pediatr. Blood Cancer 2010, 55, 290–294. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Moschovi, M.; Stefanaki, K.; Prodromou, C.; Sfakianos, G.; Prodromou, N. Malignant progression of a pleomorphic xanthoastrocytoma in a child. Neuropediatrics 2010, 41, 69–71. [Google Scholar] [CrossRef]
- Marton, E.; Feletti, A.; Orvieto, E.; Longatti, P. Malignant progression in pleomorphic xanthoastrocytoma: Personal experience and review of the literature. J. Neurol. Sci. 2007, 252, 144–153. [Google Scholar] [CrossRef]
- Dirks, P.B.; Jay, V.; Becker, L.E.; Drake, J.M.; Humphreys, R.P.; Hoffman, H.J.; Rutka, J.T. Development of anaplastic changes in low-grade astrocytomas of childhood. Neurosurgery 1994, 34, 68–78. [Google Scholar]
- Ogiwara, H.; Bowman, R.M.; Tomita, T. Long-term follow-up of pediatric benign cerebellar astrocytomas. Neurosurgery 2012, 70, 40–47; discussion 47–48. [Google Scholar] [CrossRef]
- Palma, L.; Celli, P.; Mariottini, A. Long-term follow-up of childhood cerebellar astrocytomas after incomplete resection with particular reference to arrested growth or spontaneous tumour regression. Acta Neurochir. 2004, 146, 581–588; discussion 588. [Google Scholar] [CrossRef]
- Gunny, R.S.; Hayward, R.D.; Phipps, K.P.; Harding, B.N.; Saunders, D.E. Spontaneous regression of residual low-grade cerebellar pilocytic astrocytomas in children. Pediatr. Radiol. 2005, 35, 1086–1091. [Google Scholar] [CrossRef]
- Roka, K.; Scheinemann, K.; Avula, S.; Maduro, J.H.; Thomale, U.W.; Shested, A.; Meeteren, A.Y.N.S. European standard clinical practice recommendations for primary pediatric low-grade gliomas. EJC Paeditri. Oncol. 2024, 100169, 2772-610X. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).