Octadecaneuropeptide, ODN, Promotes Cell Survival against 6-OHDA-Induced Oxidative Stress and Apoptosis by Modulating the Expression of miR-34b, miR-29a, and miR-21in Cultured Astrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Cell Culture
2.4. Cell Treatment
2.5. Crystal Violet Assay for Viability
2.6. Measurement of Cell Survival
2.7. Measurement of Cellular Cytotoxicity
2.8. Measurement of Intracellular Formation of Reactive Oxygen Species and Nitric Oxide
2.9. Flow Cytometric Detection of Apoptosis and Necrosis
2.10. Total RNA Extraction and Quantitative RT-PCR
2.11. MicroRNA Transfection and 6-OHDA Treatment
2.12. Western Blot Analysis for Caspase-3 Protein Expression
2.13. Rat Cytokine Antibody Array
2.14. Detection of Cytokine Release by ELISA
2.15. Bioinformatics Analysis
2.16. Statistical Analysis
3. Results
3.1. Delayed Administration of ODN Protects Astroglial Cells from 6-OHDA Toxicity
3.2. ODN Exerts Its Protective Action against 6-OHDA Toxicity by Attenuating Apoptotic Signaling Pathway
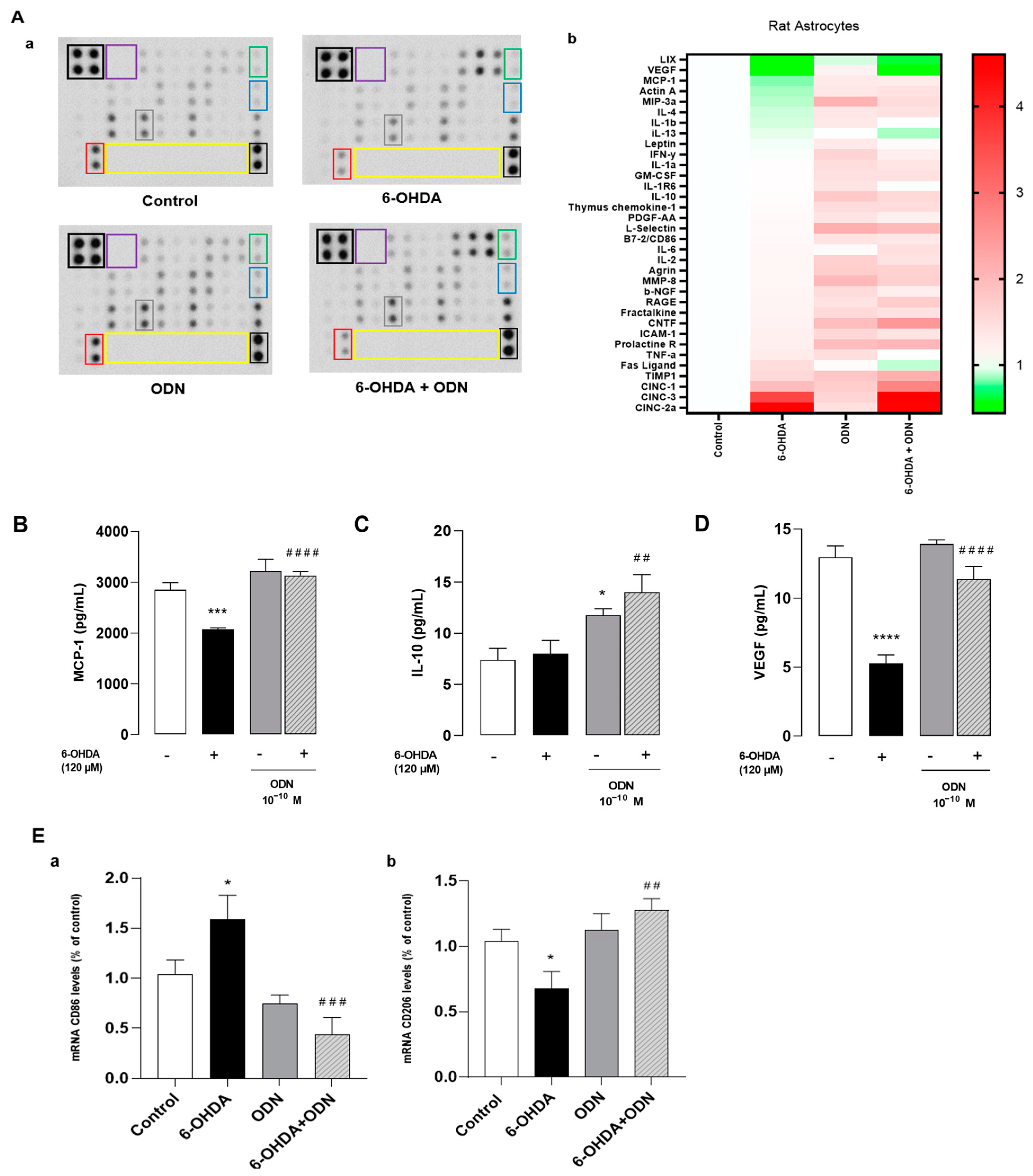
3.3. Effect of ODN on 6-OHDA-Induced Production of Cytokines
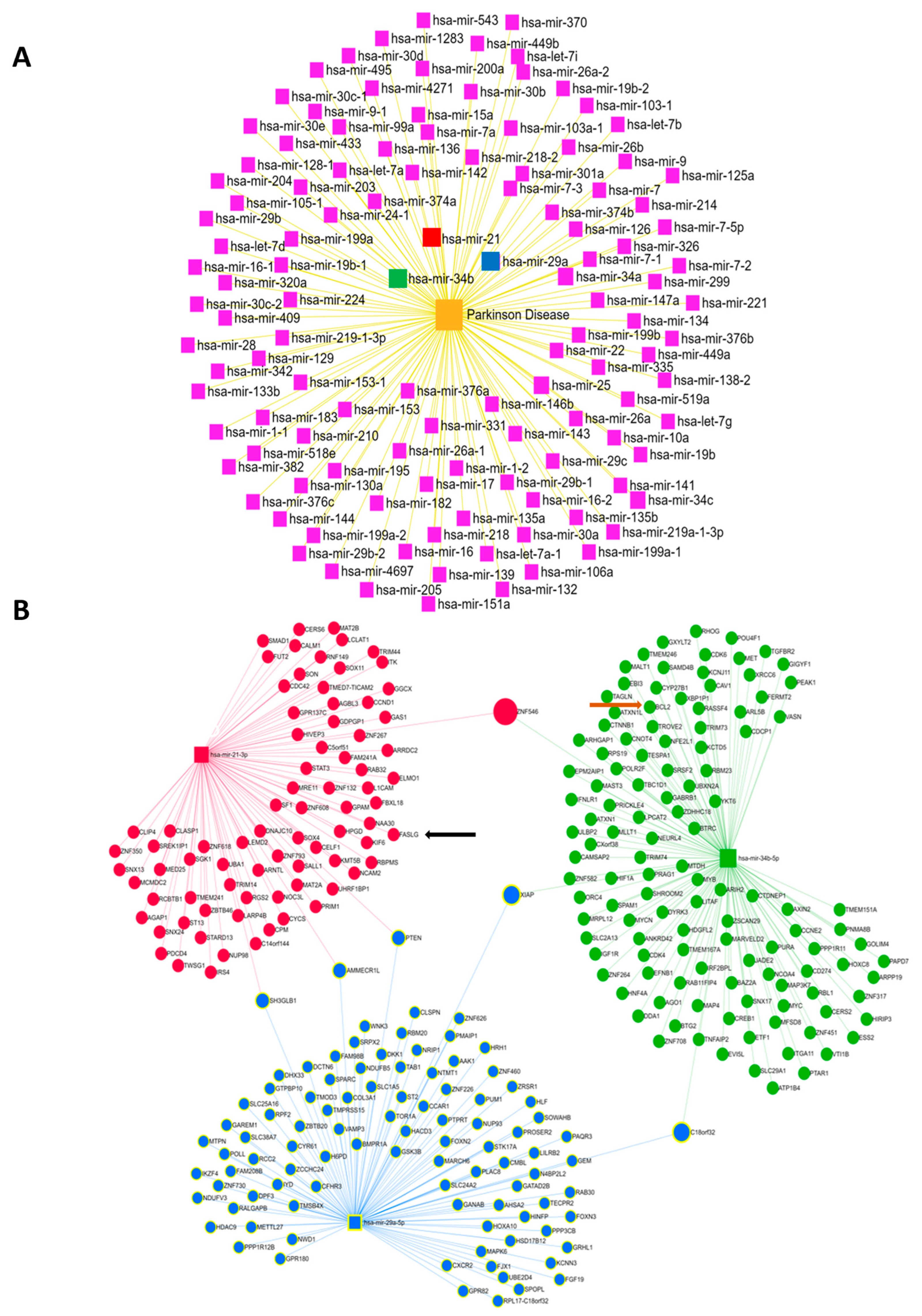
3.4. Bioinformatics Analysis of the microRNAs Implicated in Parkinson’s Disease
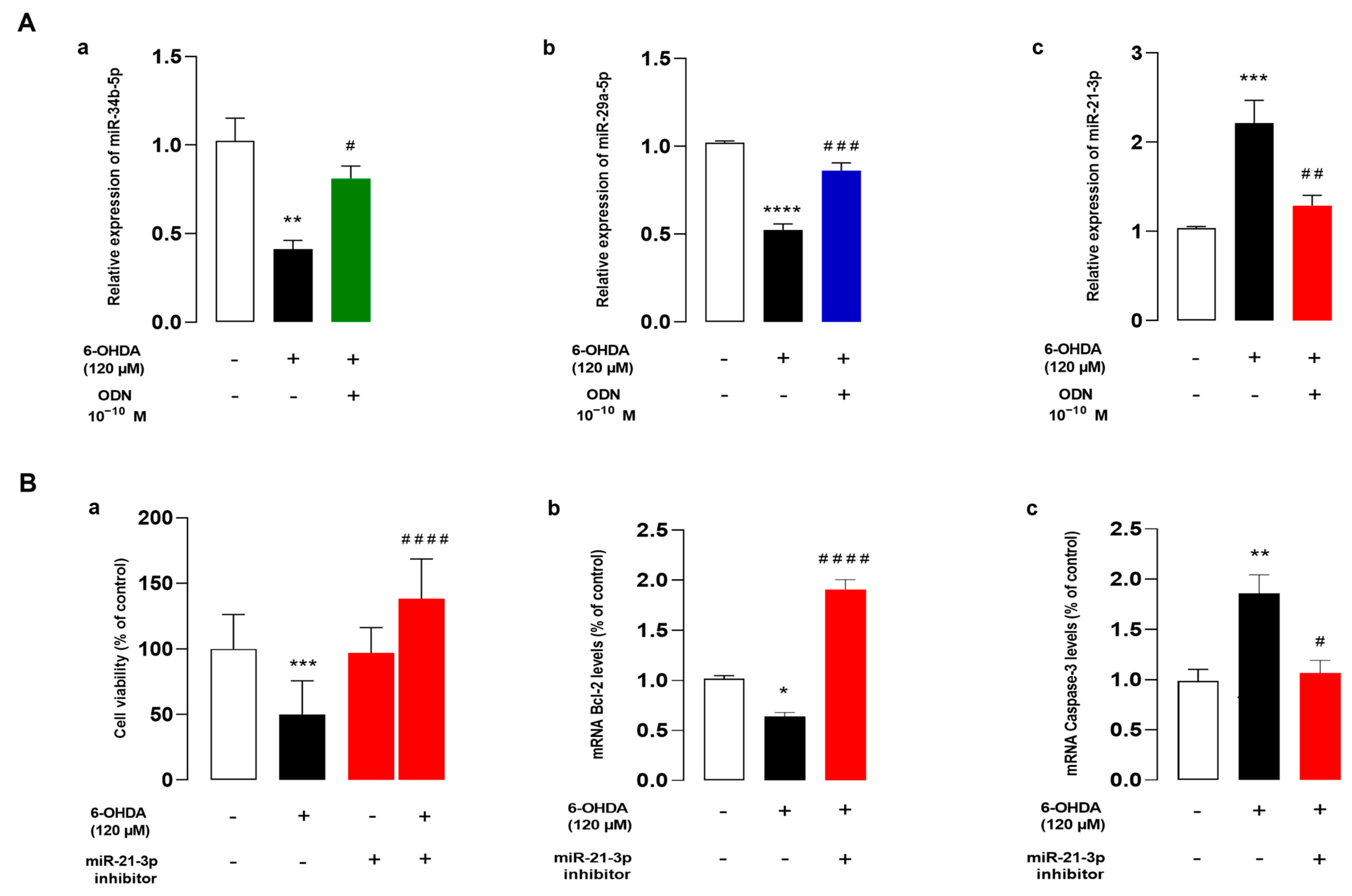
3.5. ODN Regulates the Expression of miRNAs, and miR-21 Inhibitor Reduces Cell Death against 6-OHDA on Cultured Astrocytes
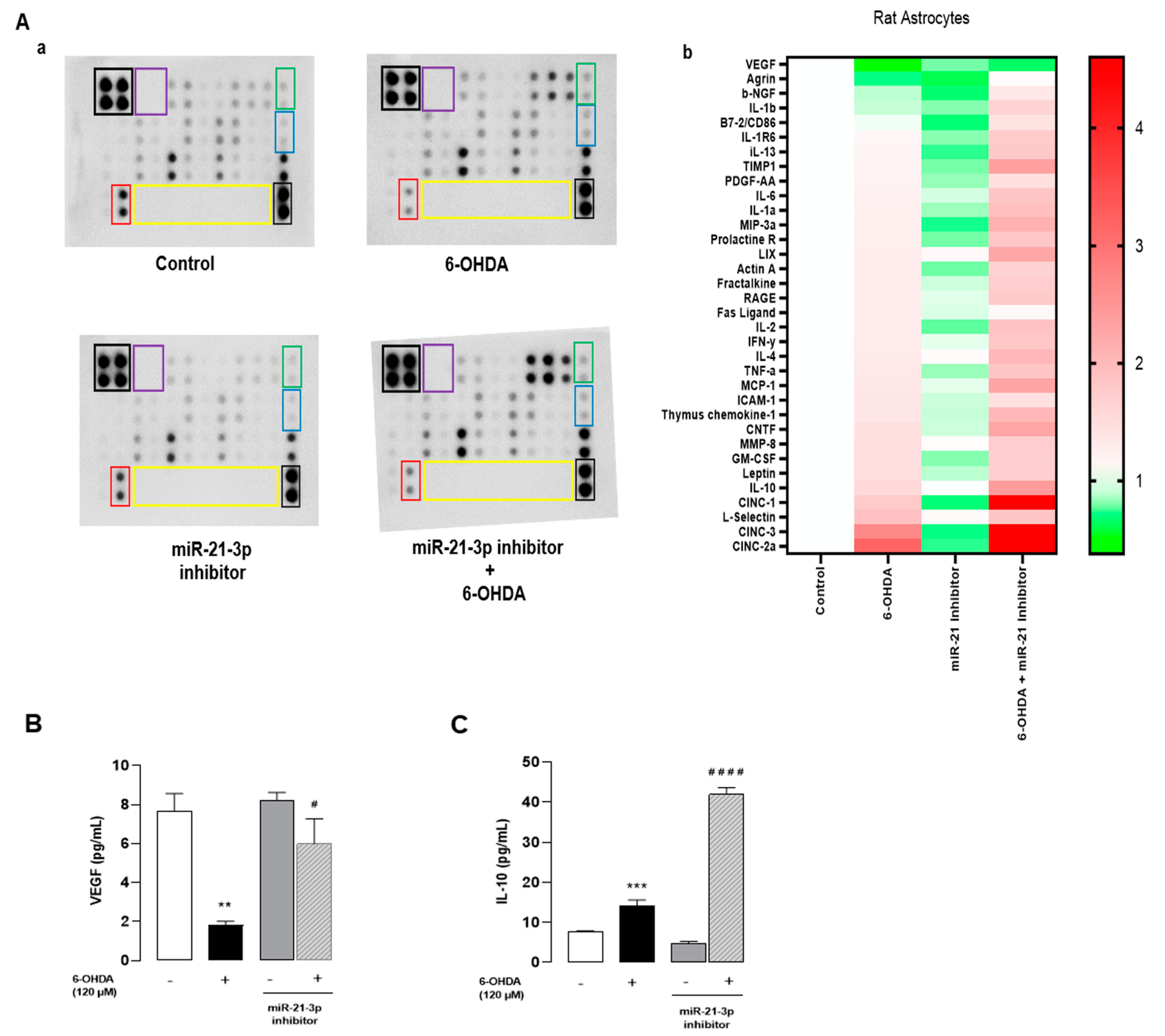
3.6. Inhibition of miR-21-3p Reduces Cytokines and Activates Chemotaxis on Cultured Astrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| ADNF | Activity-dependent neurotrophic factor |
| CNTF | Ciliary neurotrophic factor |
| DBI | Diazepam-binding inhibitor |
| FDA | Fluorescein diacetate |
| GPCR | G protein-coupled receptor |
| H2O2 | hydrogen peroxide |
| LDH | Lactate dehydrogenase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| miRNA | microRNA |
| MPTP | -methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| ODN | Octadecaneuropeptide |
| PD | Parkinson’s disease |
| ROS | Reactive oxygen species |
| SNpc | Substantia nigra pars compacta |
| VEGF | Vascular endothelial growth factor |
References
- Singleton, A.B.; Farrer, M.J.; Bonifati, V. The genetics of Parkinson’s disease: Progress and therapeutic implications. Mov. Disord. 2013, 28, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Nalls, M.A.; Shi, M.; Joubert, B.R.; Hernandez, D.G.; Huang, X.; Hollenbeck, A.; Singleton, A.B.; Chen, H. An exploratory analysis on gene-environment interactions for Parkinson disease. Neurobiol. Aging 2012, 33, 2528.e1. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Airavaara, M.; Voutilainen, M.H.; Wang, Y.; Hoffer, B. Neurorestoration. Park. Relat. Disord. 2012, 18 (Suppl. S1), S143–S146. [Google Scholar] [CrossRef] [PubMed]
- Altarche-Xifro, W.; di Vicino, U.; Muñoz-Martin, M.I.; Bortolozzi, A.; Bové, J.; Vila, M.; Cosma, M.P. Functional Rescue of Dopaminergic Neuron Loss in Parkinson’s Disease Mice After Transplantation of Hematopoietic Stem and Progenitor Cells. eBioMedicine 2016, 8, 83–95. [Google Scholar] [CrossRef]
- Voutilainen, M.H.; Arumäe, U.; Airavaara, M.; Saarma, M. Therapeutic potential of the endoplasmic reticulum located and secreted CDNF/MANF family of neurotrophic factors in Parkinson’s disease. FEBS Lett. 2015, 589 Pt A, 3739–3748. [Google Scholar] [CrossRef]
- Lindholm, P.; Voutilainen, M.H.; Laurén, J.; Peränen, J.; Leppänen, V.M.; Andressoo, J.O.; Lindahl, M.; Janhunen, S.; Kalkkinen, N.; Timmusk, T.; et al. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 2007, 448, 73–77. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Taylor, D.C. Protection against Parkinson’s disease progression: Clinical experience. Neurotherapeutics 2008, 5, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Raymick, J.; Imam, S. Neuroprotective and Therapeutic Strategies against Parkinson’s Disease: Recent Perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef]
- Lindholm, P.; Saarma, M. Cerebral dopamine neurotrophic factor protects and repairs dopamine neurons by novel mechanism. Mol. Psychiatry 2022, 27, 1310–1321. [Google Scholar] [CrossRef]
- Lee, H.G.; Wheeler, M.A.; Quintana, F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Tonon, M.C.; Vaudry, H.; Chuquet, J.; Guillebaud, F.; Fan, J.; Masmoudi-Kouki, O.; Vaudry, D.; Lanfray, D.; Morin, F.; Prevot, V.; et al. Endozepines and their receptors: Structure, functions and pathophysiological significance. Pharmacol. Ther. 2020, 208, 107386. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, Y.; Kaddour, H.; Vaudry, D.; Leprince, J.; Zarrouk, A.; Hammami, M.; Vaudry, H.; Tonon, M.C.; Amri, M.; Masmoudi-Kouki, O. Octadecaneuropeptide ODN prevents hydrogen peroxide-induced oxidative damage of biomolecules in cultured rat astrocytes. Peptides 2015, 71, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, H.; Hamdi, Y.; Vaudry, D.; Basille, M.; Desrues, L.; Leprince, J.; Castel, H.; Vaudry, H.; Tonon, M.C.; Amri, M.; et al. The octadecaneuropeptide ODN prevents 6-hydroxydopamine-induced apoptosis of cerebellar granule neurons through a PKC-MAPK-dependent pathway. J. Neurochem. 2013, 125, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, H.; Hamdi, Y.; Amri, F.; Bahdoudi, S.; Bouannee, I.; Leprince, J.; Zekri, S.; Vaudry, H.; Tonon, M.C.; Vaudry, D.; et al. Antioxidant and Anti-Apoptotic Activity of Octadecaneuropeptide against 6-OHDA Toxicity in Cultured Rat Astrocytes. J. Mol. Neurosci. 2019, 69, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bahdoudi, S.; Ghouili, I.; Hmiden, M.; Rego, J.L.D.; Lefranc, B.; Leprince, J.; Chuquet, J.; Rego, J.C.D.; Marcher, A.B.; Mandrup, S.; et al. Neuroprotective effects of the gliopeptide ODN in an in vivo model of Parkinson’s disease. Cell Mol. Life Sci. 2018, 75, 2075–2091. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, A.; Forchetti, C.M.; Corda, M.G.; Konkel, D.; Bennett, C.D.; Costa, E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc. Natl. Acad. Sci. USA 1983, 80, 3531–3535. [Google Scholar] [CrossRef] [PubMed]
- Farzampour, Z.; Reimer, R.J.; Huguenard, J. Endozepines. Adv. Pharmacol. 2015, 72, 147–164. [Google Scholar]
- Dumitru, I.; Neitz, A.; Alfonso, J.; Monyer, H. Diazepam Binding Inhibitor Promotes Stem Cell Expansion Controlling Environment-Dependent Neurogenesis. Neuron 2017, 94, 125–137. [Google Scholar] [CrossRef]
- Montégut, L.; Abdellatif, M.; Motiño, O.; Madeo, F.; Martins, I.; Quesada, V.; López-Otín, C.; Kroemer, G. Acyl coenzyme A binding protein (ACBP): An aging- and disease-relevant “autophagy checkpoint”. Aging Cell 2023, 22, e13910. [Google Scholar] [CrossRef]
- Bouyakdan, K.; Martin, H.; Liénard, F.; Budry, L.; Taib, B.; Rodaros, D.; Chrétien, C.; Biron, É.; Husson, Z.; Cota, D.; et al. The gliotransmitter ACBP controls feeding and energy homeostasis via the melanocortin system. J. Clin. Investig. 2019, 129, 2417–2430. [Google Scholar] [CrossRef]
- Roy, S.C.; Sapkota, S.; Pasula, M.B.; Bheemanapally, K.; Briski, K.P. Diazepam Binding Inhibitor Control of Eu- and Hypoglycemic Patterns of Ventromedial Hypothalamic Nucleus Glucose-Regulatory Signaling. ASN Neuro 2023, 15, 17590914231214116. [Google Scholar] [CrossRef]
- Hamdi, Y.; Kaddour, H.; Vaudry, D.; Douiri, S.; Bahdoudi, S.; Leprince, J.; Castel, H.; Vaudry, H.; Amri, M.; Tonon, M.C.; et al. The stimulatory effect of the octadecaneuropeptide ODN on astroglial antioxidant enzyme systems is mediated through a GPCR. Front. Endocrinol. 2012, 3, 138. [Google Scholar] [CrossRef]
- Hamdi, Y.; Kaddour, H.; Vaudry, D.; Bahdoudi, S.; Douiri, S.; Leprince, J.; Castel, H.; Vaudry, H.; Tonon, M.C.; Amri, M.; et al. The octadecaneuropeptide ODN protects astrocytes against hydrogen peroxide-induced apoptosis via a PKA/MAPK-dependent mechanism. PLoS ONE 2012, 7, e42498. [Google Scholar] [CrossRef]
- Ferrarese, C.; Appollonio, I.; Frigo, M.; Meregalli, S.; Piolti, R.; Tamma, F.; Frattola, L. Cerebrospinal fluid levels of diazepam-binding inhibitor in neurodegenerative disorders with dementia. Neurology 1990, 40, 632–635. [Google Scholar] [CrossRef]
- Ghouili, I.; Bahdoudi, S.; Morin, F.; Amri, F.; Hamdi, Y.; Coly, P.M.; Walet-Balieu, M.L.; Leprince, J.; Zekri, S.; Vaudry, H.; et al. Endogenous Expression of ODN-Related Peptides in Astrocytes Contributes to Cell Protection against Oxidative Stress: Astrocyte-Neuron Crosstalk Relevance for Neuronal Survival. Mol. Neurobiol. 2018, 55, 4596–4611. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Tsujimura, K.; Shiohama, T.; Takahashi, E. microRNA Biology on Brain Development and Neuroimaging Approach. Brain Sci. 2022, 12, 1366. [Google Scholar] [CrossRef]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.N.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef]
- Sonntag, K.C. MicroRNAs and deregulated gene expression networks in neurodegeneration. Brain Res. 2010, 1338, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Su, X.; Piao, L.; Jin, Z.; Jin, R. Involvement of Astrocytes and microRNA Dysregulation in Neurodegenerative Diseases: From Pathogenesis to Therapeutic Potential. Front. Mol. Neurosci. 2021, 14, 556215. [Google Scholar] [CrossRef] [PubMed]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Mouradian, M.M.; Junn, E. Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett. 2015, 589, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.; Dionísio, P.A.; Guedes, L.C.; Gonçalves, N.; Coelho, M.; Rosa, M.M.; Amaral, J.D.; Ferreira, J.J.; Rodrigues, C.M.P. Circulating Inflammatory miRNAs Associated with Parkinson’s Disease Pathophysiology. Biomolecules 2020, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tang, Y.; Yu, M.; Wu, L.; Liu, F.; Ni, J.; Wang, Z.; Wang, J.; Fei, J.; Wang, W.; et al. Downregulation of blood serum microRNA 29 family in patients with Parkinson’s disease. Sci. Rep. 2017, 7, 5411. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Ding, L. Downregulation of miR-21 suppresses 1-methyl-4-phenylpyridinium-induced neuronal damage in MES23.5 cells. Exp. Ther. Med. 2019, 18, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Buscaglia, L.E.B.; Barker, J.R.; Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef]
- Zarei-Kheirabadi, M.; Mirsadeghi, S.; Vaccaro, A.R.; Rahimi-Movaghar, V.; Kiani, S. Protocol for purification and culture of astrocytes: Useful not only in 2 days postnatal but also in adult rat brain. Mol. Biol. Rep. 2020, 47, 1783–1794. [Google Scholar] [CrossRef]
- Buonfiglioli, A.; Efe, I.E.; Guneykaya, D.; Ivanov, A.; Huang, Y.; Orlowski, E.; Krüger, C.; Deisz, R.A.; Markovic, D.; Flüh, C.; et al. let-7 MicroRNAs Regulate Microglial Function and Suppress Glioma Growth through Toll-like Receptor 7. Cell Rep. 2019, 29, 3460–3471. [Google Scholar] [CrossRef]
- Lamacz, M.; Tonon, M.C.; Smih-Rouet, F.; Patte, C.; Gasque, P.; Fontaine, M.; Vaudry, H. The endogenous benzodiazepine receptor ligand ODN increases cytosolic calcium in cultured rat astrocytes. Brain Res. Mol. Brain Res. 1996, 37, 290–296. [Google Scholar] [CrossRef]
- Douiri, S.; Bahdoudi, S.; Hamdi, Y.; Cubì, R.; Basille, M.; Fournier, A.; Vaudry, H.; Tonon, M.C.; Amri, M.; Vaudry, D.; et al. Involvement of endogenous antioxidant systems in the protective activity of pituitary adenylate cyclase-activating polypeptide against hydrogen peroxide-induced oxidative damages in cultured rat astrocytes. J. Neurochem. 2016, 137, 913–930. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, I.A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, A.E.; Garcia-Bueno, B.; Leza, J.C.; Madrigal, J.L. CCL2/MCP-1 modulation of microglial activation and proliferation. J. Neuroinflamm. 2011, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef] [PubMed]
- Marega, L.F.; Teocchi, M.A.; Dos Santos Vilela, M.M. Differential regulation of miR-146a/FAS and miR-21/FASLG axes in autoimmune lymphoproliferative syndrome due to FAS mutation (ALPS-FAS). Clin. Exp. Immunol. 2016, 185, 148–153. [Google Scholar] [CrossRef]
- Manna, I.; Quattrone, A.; De Benedittis, S.; Vescio, B.; Iaccino, E.; Quattrone, A. Exosomal miRNA as peripheral biomarkers in Parkinson’s disease and progressive supranuclear palsy: A pilot study. Park. Relat. Disord. 2021, 93, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, Q.; Zhou, M.; Chen, Y.; Jiang, C.; Jiang, Y.; Lin, Y.; He, Q.; Zhao, L.; Dong, Y.; et al. Plasma miR-153 and miR-223 Levels as Potential Biomarkers in Parkinson’s Disease. Front. Neurosci. 2022, 16, 865139. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, D.; Huang, X.; Huang, H. Astragaloside IV Protects 6-Hydroxydopamine-Induced SH-SY5Y Cell Model of Parkinson’s Disease via Activating the JAK2/STAT3 Pathway. Front. Neurosci. 2021, 15, 631501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Zhou, L.; Long, T.; Qin, D.L.; Wang, Y.L.; Ye, Y.; Zhou, X.G.; Wu, J.M.; Wu, A.G. Galangin Exhibits Neuroprotective Effects in 6-OHDA-Induced Models of Parkinson’s Disease via the Nrf2/Keap1 Pathway. Pharmaceuticals 2022, 15, 1014. [Google Scholar] [CrossRef]
- Masmoudi-Kouki, O.; Namsi, A.; Hamdi, Y.; Bahdoudi, S.; Ghouili, I.; Chuquet, J.; Leprince, J.; Lefranc, B.; Ghrairi, T.; Tonon, M.C.; et al. Cytoprotective and Neurotrophic Effects of Octadecaneuropeptide (ODN) in in vitro and in vivo Models of Neurodegenerative Diseases. Front. Endocrinol. 2020, 11, 566026. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Yi, L.; Li, X.; Luo, M.; Pang, Y.; Wang, M.; Li, Z.; Xu, M.; Dong, Z.; et al. Octadecaneuropeptide Ameliorates Cognitive Impairments Through Inhibiting Oxidative Stress in Alzheimer’s Disease Models. J. Alzheimers Dis. 2023, 92, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, P.; Patte, C.; Thoumas, J.L.; Leprince, J.; Vaudry, H.; Tonon, M.C. The endozepine ODN stimulates [3H]thymidine incorporation in cultured rat astrocytes. Neuropharmacology 1999, 38, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Beautrait, A.; Paradis, J.S.; Zimmerman, B.; Giubilaro, J.; Nikolajev, L.; Armando, S.; Kobayashi, H.; Yamani, L.; Namkung, Y.; Heydenreich, F.M.; et al. A new inhibitor of the β-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling. Nat. Commun. 2017, 8, 15054. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, M.; Holdfeldt, A.; Wright, S.C.; Møller, T.C.; Siaw, E.; Jennbacken, K.; Franzyk, H.; Bouvier, M.; Dahlgren, C.; Forsman, H. Barbadin selectively modulates FPR2-mediated neutrophil functions independent of receptor endocytosis. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118849. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Mendoza, A.; Robichaux, D.J.; Wang, M.C.; Wehrens, X.H.T.; Karch, J. Inhibition of the Anti-Apoptotic Bcl-2 Family by BH3 Mimetics Sensitize the Mitochondrial Permeability Transition Pore Through Bax and Bak. Front. Cell Dev. Biol. 2021, 9, 765973. [Google Scholar] [CrossRef] [PubMed]
- Brauchle, E.; Thude, S.; Brucker, S.Y.; Schenke-Layland, K. Cell death stages in single apoptotic and necrotic cells monitored by Raman microspectroscopy. Sci. Rep. 2014, 4, 4698. [Google Scholar] [PubMed]
- Namsi, A.; Nury, T.; Khan, A.S.; Leprince, J.; Vaudry, D.; Caccia, C.; Leoni, V.; Atanasov, A.G.; Tonon, M.C.; Masmoudi-Kouki, O.; et al. Octadecaneuropeptide (ODN) Induces N2a Cells Differentiation through a PKA/PLC/PKC/MEK/ERK-Dependent Pathway: Incidence on Peroxisome, Mitochondria, and Lipid Profiles. Molecules 2019, 24, 3310. [Google Scholar] [CrossRef] [PubMed]
- Barnum, C.J.; Chen, X.; Chung, J.; Chang, J.; Williams, M.; Grigoryan, N.; Tesi, R.J.; Tansey, M.G. Peripheral administration of the selective inhibitor of soluble tumor necrosis factor (TNF) XPro®1595 attenuates nigral cell loss and glial activation in 6-OHDA hemiparkinsonian rats. J. Park. Dis. 2014, 4, 349–360. [Google Scholar] [CrossRef]
- Parra, I.; Martínez, I.; Ramírez-García, G.; Tizabi, Y.; Mendieta, L. Differential Effects of LPS and 6-OHDA on Microglia’s Morphology in Rats: Implications for Inflammatory Model of Parkinson’s Disease. Neurotox. Res. 2020, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Rothhammer, V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front. Immunol. 2020, 11, 573256. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Sun, X.; Zhou, C.; Yan, J.; Cheng, C. Neuroblasts migration under control of reactive astrocyte-derived BDNF: A promising therapy in late neurogenesis after traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Zhao, Z.; Luo, Y.; Hou, Y.; Li, H.; He, L.; Zhou, L.; Wu, W. Effect of VEGF on Inflammatory Regulation, Neural Survival, and Functional Improvement in Rats following a Complete Spinal Cord Transection. Front. Cell Neurosci. 2017, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.M.; Obuchowicz, E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: A new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides 2012, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Mashima, K. Neuroprotection and Disease Modification by Astrocytes and Microglia in Parkinson Disease. Antioxidants 2022, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- AJurga, M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell Neurosci. 2020, 14, 198. [Google Scholar]
- Zhang, Y.; Park, Y.S.; Kim, I.B. A Distinct Microglial Cell Population Expressing Both CD86 and CD206 Constitutes a Dominant Type and Executes Phagocytosis in Two Mouse Models of Retinal Degeneration. Int. J. Mol. Sci. 2023, 24, 14236. [Google Scholar] [CrossRef]
- Du, Y.; Ma, Z.; Lin, S.; Dodel, R.C.; Gao, F.; Bales, K.R.; Triarhou, L.C.; Chernet, E.; Perry, K.W.; Nelson, D.L.; et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2001, 98, 14669–14674. [Google Scholar] [CrossRef]
- He, Y.; Appel, S.; Le, W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001, 909, 187–193. [Google Scholar] [CrossRef]
- Wu, D.C.; Jackson-Lewis, V.; Vila, M.; Tieu, K.; Teismann, P.; Vadseth, C.; Choi, D.K.; Ischiropoulos, H.; Przedborski, S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002, 22, 1763–1771. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Huang, R.; Ma, J.; Niu, B.; Li, J.; Chang, J.; Zhang, Y.; Liu, P.; Luan, X. MiR-34b Protects against Focal Cerebral Ischemia-Reperfusion (I/R) Injury in Rat by Targeting Keap1. J. Stroke Cerebrovasc. Dis. 2019, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, M.; Gao, S.; Zhu, L.; Yu, L.; Hu, D.; Zhu, L.; Huang, W.; Zhang, W.; Deng, J.; et al. miR-29a Promotes the Neurite Outgrowth of Rat Neural Stem Cells by Targeting Extracellular Matrix to Repair Brain Injury. Stem Cells Dev. 2020, 29, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lu, Y.; Yuan, L.; Zhang, P.; Zou, D.; Wei, F.; Chen, X. miR-29a-5p Alleviates Traumatic Brain Injury- (TBI-) Induced Permeability Disruption via Regulating NLRP3 Pathway. Dis. Markers 2021, 2021, 9556513. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Lakhter, A.J.; Anderson-Baucum, E.; Kono, T.; Tong, X.; Evans-Molina, C. MicroRNA 21 targets BCL2 mRNA to increase apoptosis in rat and human beta cells. Diabetologia 2017, 60, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Matsuoka, I.; Wetmore, C.; Olson, L.; Thoenen, H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: Different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992, 119, 45–54. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, N.; Liu, K.; Zhou, N.; Sun, Y.; Zeng, W. CNTF-STAT3-IL-6 Axis Mediates Neuroinflammatory Cascade across Schwann Cell-Neuron-Microglia. Cell Rep. 2020, 31, 107657. [Google Scholar] [CrossRef]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef] [PubMed]
- Waring, P.; Müllbacher, A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 1999, 77, 312–317. [Google Scholar] [CrossRef] [PubMed]


| Gene | GenBank Accession Number | Sequence |
|---|---|---|
| Caspase-3 | NM_012922.2 | |
| Forward | 5-AATTCAAGGGACGGGTCATG-3 | |
| Reverse | 5-GCTTGTGCCGTACAGTTTC-3 | |
| Bax | NM_017059.2 | |
| Forward | 5-TGCAGAGGATGATTGCTGATGT-3 | |
| Reverse | 5-CAGCTGCCACACGGAAGAA-3 | |
| Bcl-2 | NM_016993.2 | |
| Forward | 5-GGCTGGGATGCCTTTGTG-3 | |
| Reverse | 5-CAGCCAGGAGAAATCAAACAGA-3 | |
| CD 86 | NM_020081.2 | |
| Forward | 5-GTGGAAAGGGGCTGTTGATTGG-3 | |
| Reverse | 5-TTCTGCCTCTCAGCCAGTTACC-3 | |
| CD 206 | NM_001106123.2 | |
| Forward | 5-GAACGAGAGGTCACAGAGCAGT | |
| Reverse | 5-TACCCCTCACATCTCCCTCACA | |
| GAPDH | NM_017008.4 | |
| Forward | 5-CAGCCTCGTCTCATAGACAAGATG-3 | |
| Reverse | 5-CAATGTCCAACTTTGTCACAAGAGAAA-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourzam, A.; Hamdi, Y.; Bahdoudi, S.; Duraisamy, K.; El Mehdi, M.; Basille-Dugay, M.; Dlimi, O.; Kharrat, M.; Vejux, A.; Lizard, G.; et al. Octadecaneuropeptide, ODN, Promotes Cell Survival against 6-OHDA-Induced Oxidative Stress and Apoptosis by Modulating the Expression of miR-34b, miR-29a, and miR-21in Cultured Astrocytes. Cells 2024, 13, 1188. https://doi.org/10.3390/cells13141188
Bourzam A, Hamdi Y, Bahdoudi S, Duraisamy K, El Mehdi M, Basille-Dugay M, Dlimi O, Kharrat M, Vejux A, Lizard G, et al. Octadecaneuropeptide, ODN, Promotes Cell Survival against 6-OHDA-Induced Oxidative Stress and Apoptosis by Modulating the Expression of miR-34b, miR-29a, and miR-21in Cultured Astrocytes. Cells. 2024; 13(14):1188. https://doi.org/10.3390/cells13141188
Chicago/Turabian StyleBourzam, Amine, Yosra Hamdi, Seyma Bahdoudi, Karthi Duraisamy, Mouna El Mehdi, Magali Basille-Dugay, Omayma Dlimi, Maher Kharrat, Anne Vejux, Gérard Lizard, and et al. 2024. "Octadecaneuropeptide, ODN, Promotes Cell Survival against 6-OHDA-Induced Oxidative Stress and Apoptosis by Modulating the Expression of miR-34b, miR-29a, and miR-21in Cultured Astrocytes" Cells 13, no. 14: 1188. https://doi.org/10.3390/cells13141188
APA StyleBourzam, A., Hamdi, Y., Bahdoudi, S., Duraisamy, K., El Mehdi, M., Basille-Dugay, M., Dlimi, O., Kharrat, M., Vejux, A., Lizard, G., Ghrairi, T., Lefranc, B., Vaudry, D., Boutin, J. A., Leprince, J., & Masmoudi-Kouki, O. (2024). Octadecaneuropeptide, ODN, Promotes Cell Survival against 6-OHDA-Induced Oxidative Stress and Apoptosis by Modulating the Expression of miR-34b, miR-29a, and miR-21in Cultured Astrocytes. Cells, 13(14), 1188. https://doi.org/10.3390/cells13141188











