hTERT-Immortalized Mesenchymal Stem Cell-Derived Extracellular Vesicles: Large-Scale Manufacturing, Cargo Profiling, and Functional Effects in Retinal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. EV Isolation and Nanoparticle Tracking Analysis
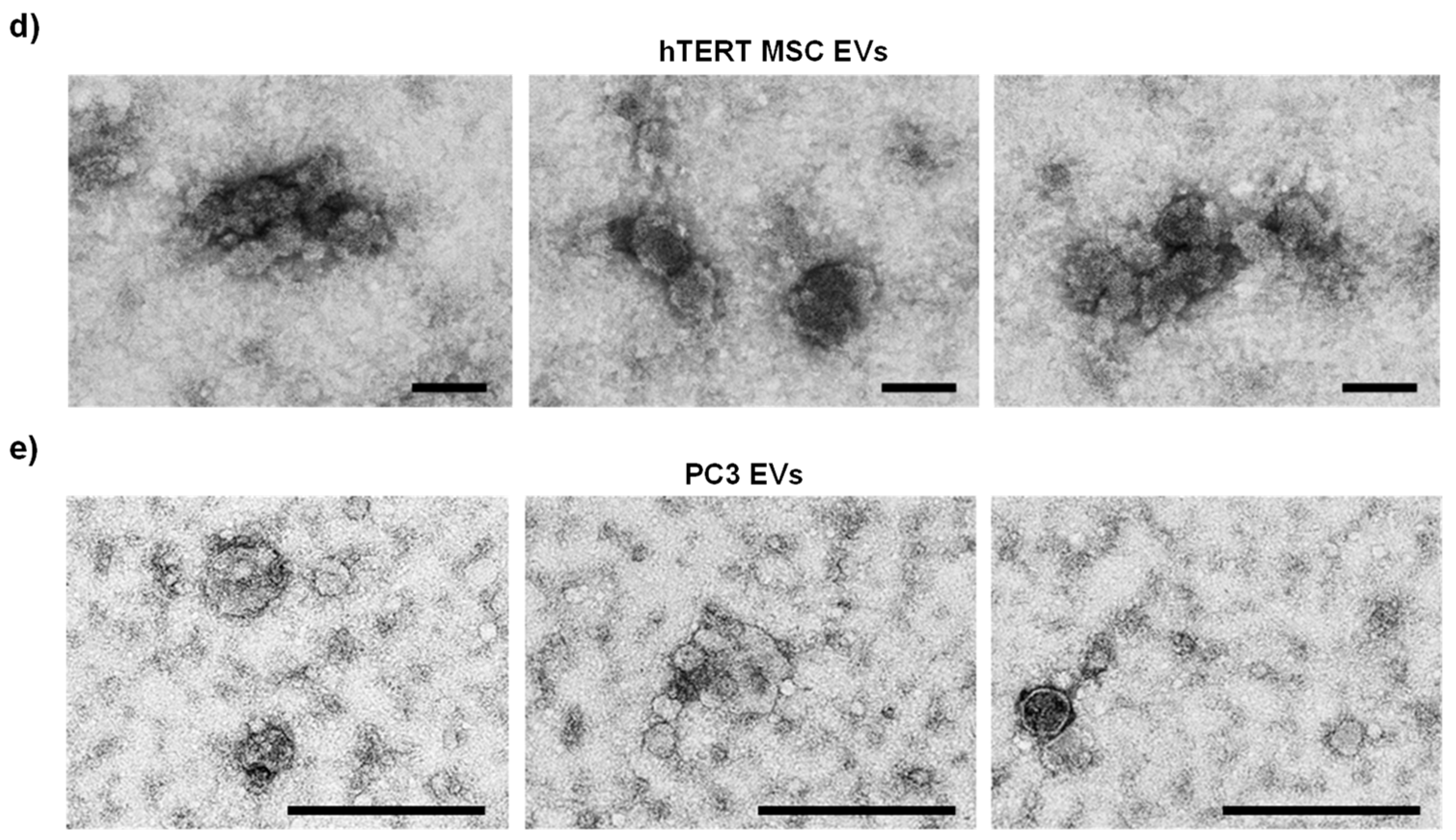
2.3. Transmission Electron Microscopy
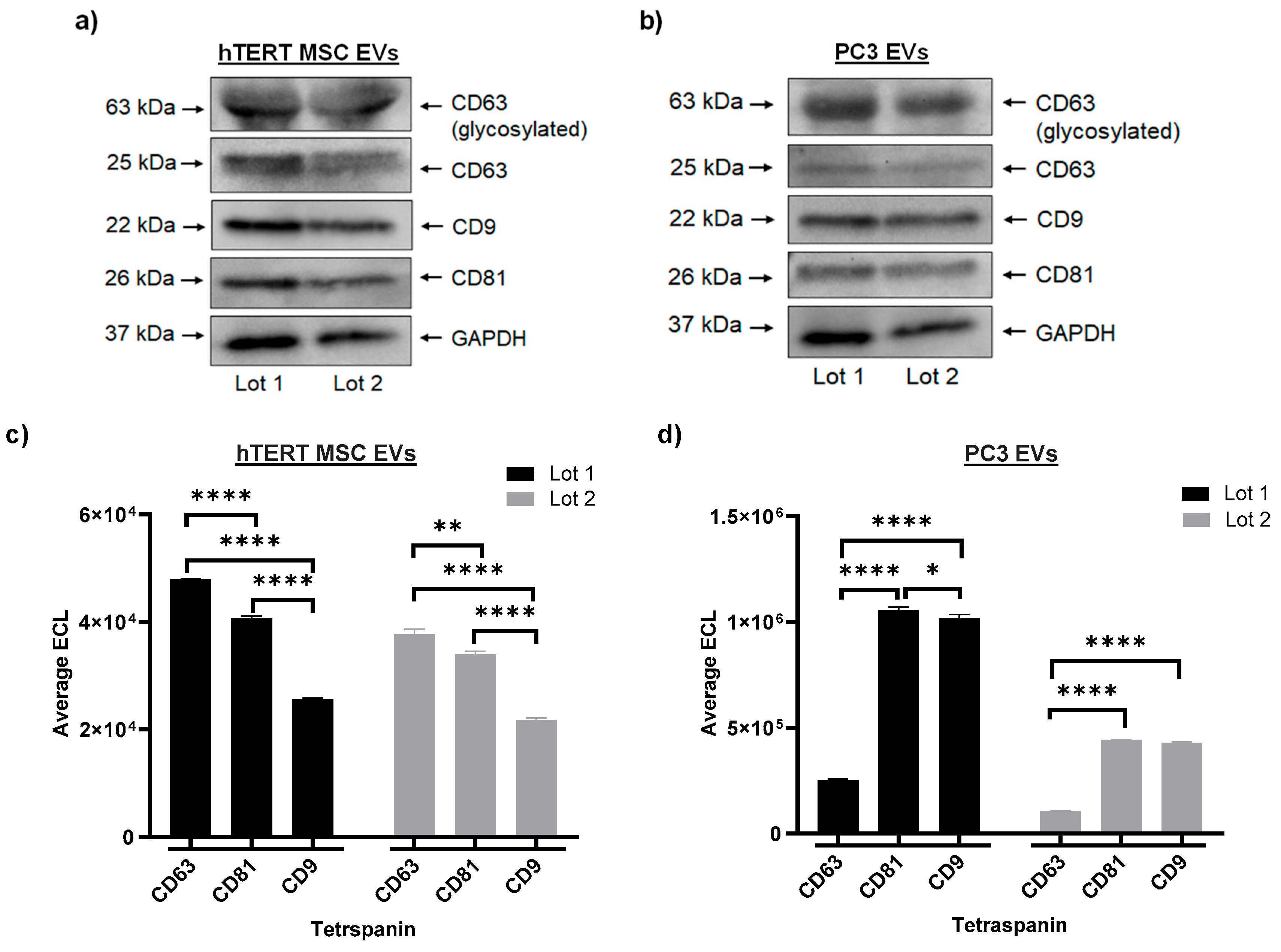
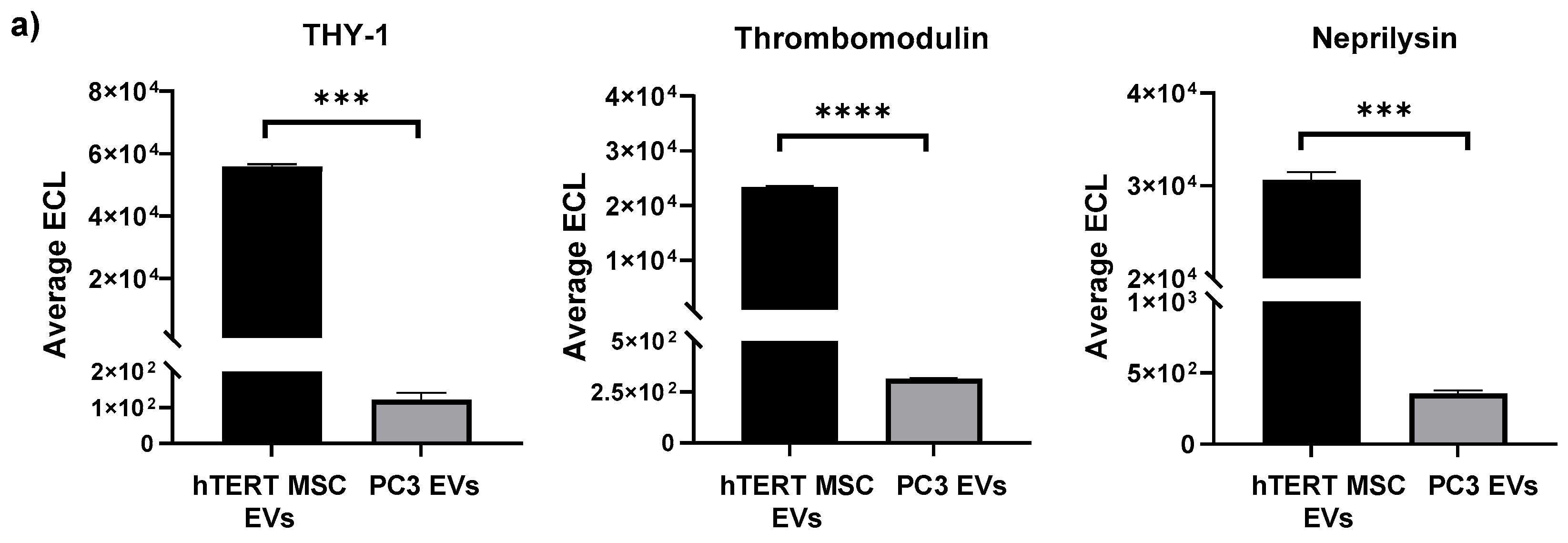
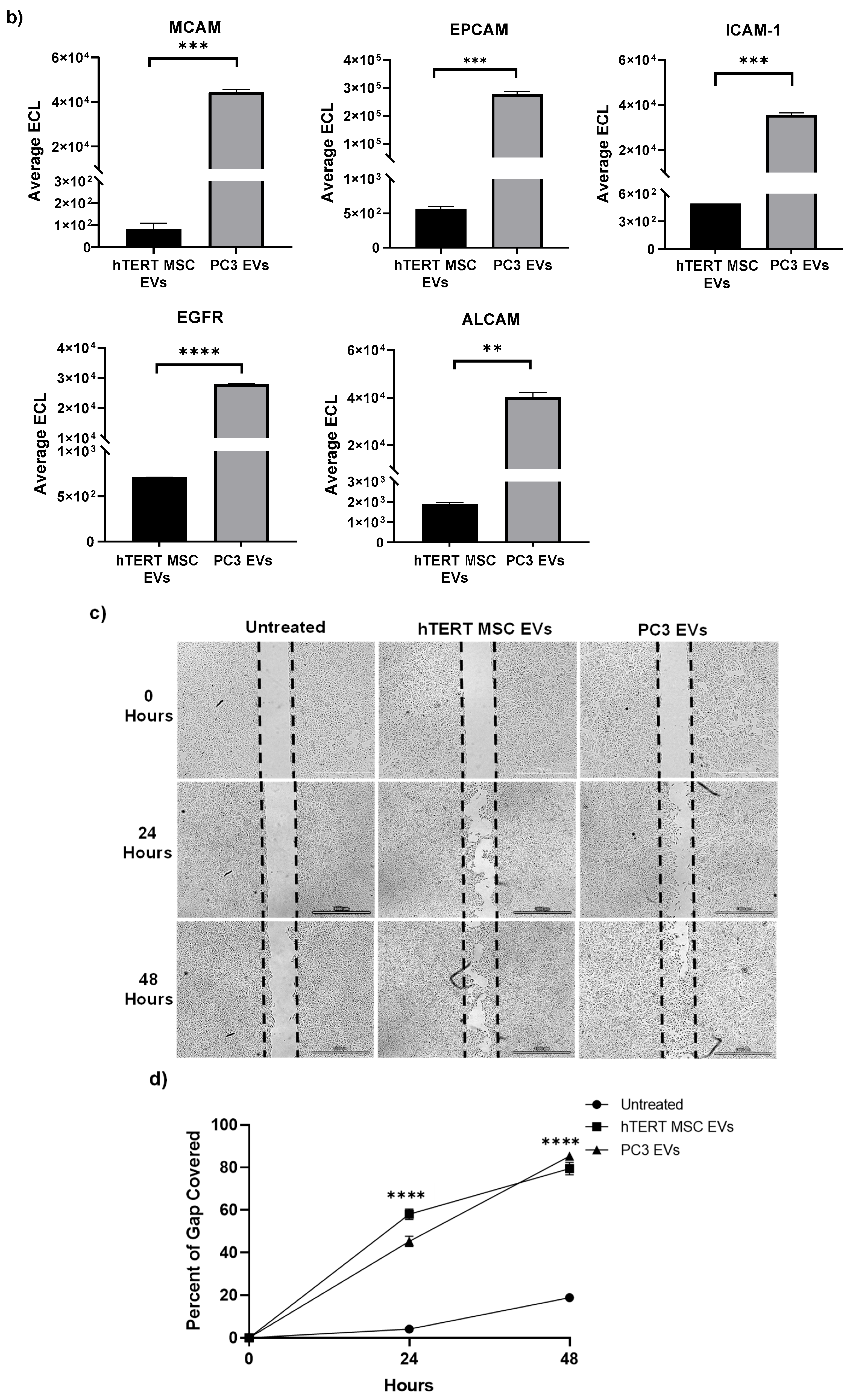
2.4. Multiplex Immunoassays
2.5. Western Blot
2.6. Proteomics
2.7. RNA Extraction and qPCR
2.8. Cell Migration Assay
2.9. In Vivo Wound Healing Assay
2.10. Ionizing Radiation (IR)
2.11. Cell Viability and Repair Assays
2.12. Statistical Analysis
3. Results
3.1. Large-Scale EV Isolation and Characterization
3.2. Profiling of EV Surface Proteins and EV-Mediated Effects on Cell Migration
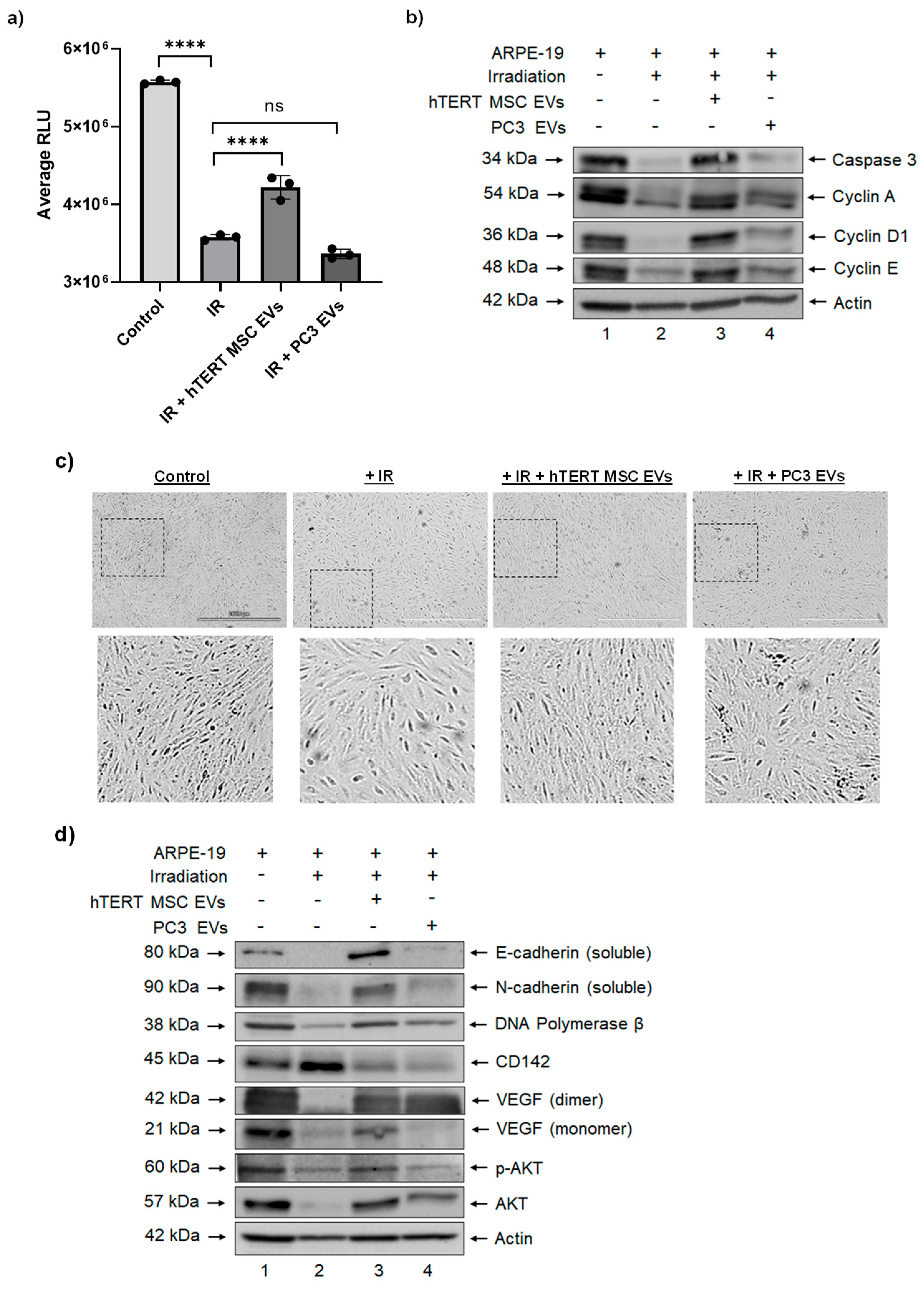
3.3. Effects of hTERT-Immortalized MSC EVs on Cell Viability and Apoptosis in RPE Cells after Ionizing Radiation
3.4. hTERT-Immortalized MSC EVs Rescue the Expression of Cell Migration and Repair Proteins in RPE Cells after Ionizing Radiation
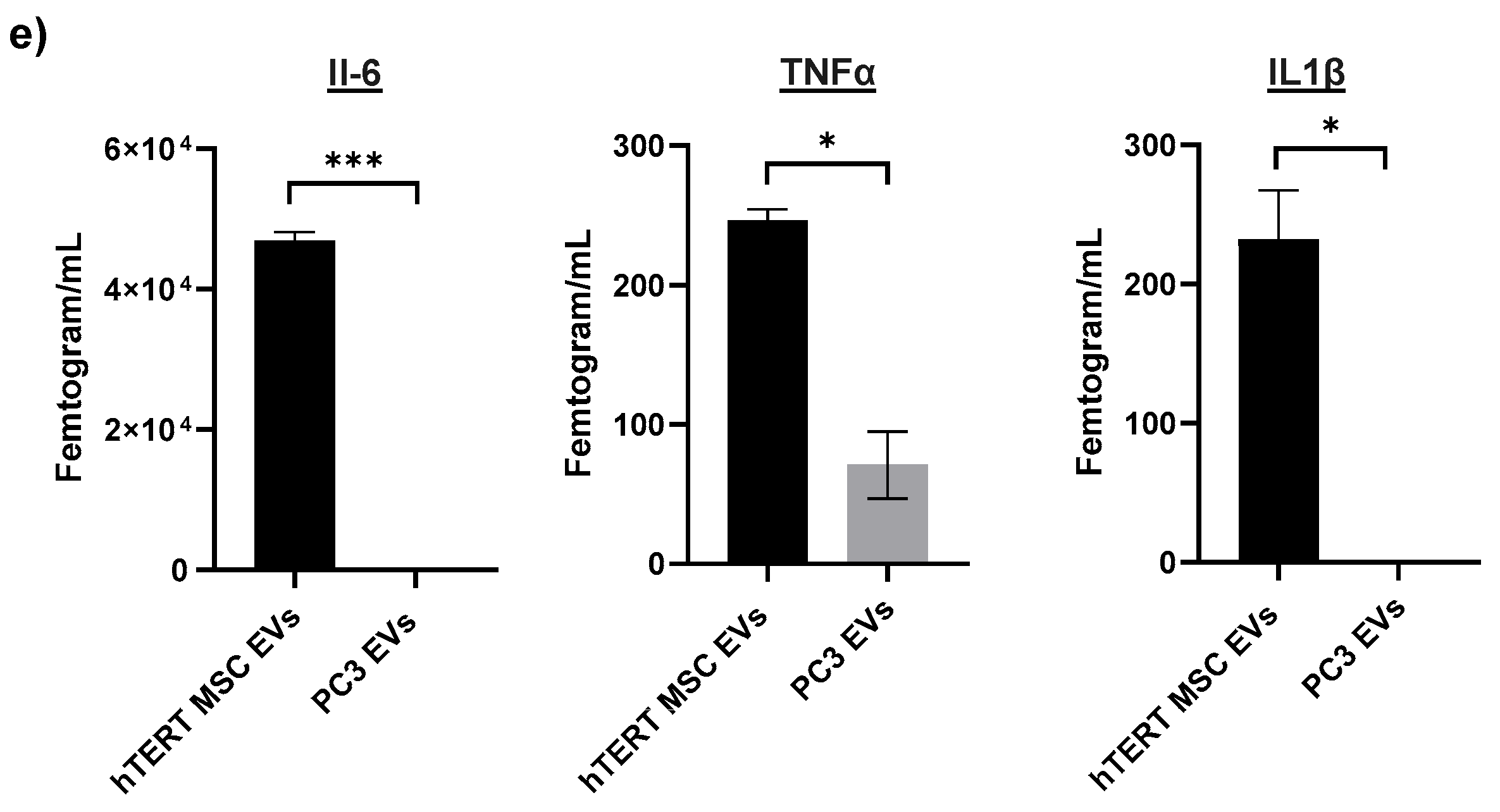
3.5. Cargo Profiling of hTERT-Immortalized MSC EVs Reveals Proteins, Cytokines, and mRNAs Involved in Cellular Repair
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowsk, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Saburi, E.; Abazari, M.F.; Hassannia, H.; Mansour, R.N.; Eshaghi-Gorji, R.; Gheibi, M.; Rahmati, M.; Enderami, S.E. The use of mesenchymal stem cells in the process of treatment and tissue regeneration after recovery in patients with COVID-19. Gene 2021, 777, 145471. [Google Scholar] [CrossRef]
- Shi, L.; Wang, L.; Xu, R.; Zhang, C.; Xie, Y.; Liu, K.; Li, T.; Hu, W.; Zhen, C.; Wang, F.S. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct. Target. Ther. 2021, 6, 339. [Google Scholar] [CrossRef]
- Zhu, R.; Yan, T.; Feng, Y.; Liu, Y.; Cao, H.; Peng, G.; Yang, Y.; Xu, Z.; Liu, J.; Hou, W.; et al. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021, 31, 1244–1262. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Hur, Y.H.; Cerione, R.A.; Antonyak, M.A. Extracellular vesicles and their roles in stem cell biology. Stem Cells 2020, 38, 469–476. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, 2. [Google Scholar] [CrossRef]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef]
- Branscome, H.; Paul, S.; Yin, D.; El-Hage, N.; Agbottah, E.T.; Zadeh, M.A.; Liotta, L.A.; Kashanchi, F. Use of Stem Cell Extracellular Vesicles as a “Holistic” Approach to CNS Repair. Front. Cell Dev. Biol. 2020, 8, 455. [Google Scholar] [CrossRef]
- Roefs, M.T.; Sluijter, J.P.G.; Vader, P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol. 2020, 30, 12. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery after Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Liu, L.; Jin, X.; Hu, C.F.; Li, R.; Zhou, Z.; Shen, C.X. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell Physiol Biochem 2017, 43, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pei, S.; Han, L.; Guo, B.; Li, Y.; Duan, R.; Yao, Y.; Xue, B.; Chen, X.; Jia, Y. Mesenchymal Stem Cell-Derived Exosomes Reduce A1 Astrocytes via Downregulation of Phosphorylated NFκB P65 Subunit in Spinal Cord Injury. Cell Physiol. Biochem. 2018, 50, 1535–1559. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Jia, Y.; Huang, X. Exosomes from adipose-derived stem cells alleviate neural injury caused by microglia activation via suppressing NF-kB and MAPK pathway. J. Neuroimmunol. 2019, 334, 576996. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2021, 11, 591065. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Marques, A.P.; Ramke, J.; Cairns, J.; Butt, T.; Zhang, J.H.; Jones, I.; Jovic, M.; Nandakumar, A.; Faal, H.; Taylor, H.; et al. The Economics of Vision Impairment and it’s Leading Causes: A Systematic Review. EClinicalMedicine 2022, 46, 101354. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Zhang, P.; Sublett, F.; Lamuda, P.A.; Lundeen, E.A.; Saaddine, J. The Economic Burden of Vision Loss and Blindness in the United States. Ophthalmology 2022, 129, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Sia, R.K.; Ryan, D.S.; Brooks, D.I.; Kagemann, J.M.; Bower, K.S.; French, L.M.; Justin, G.A.; Colyer, M.H. The Impact of Combat Ocular Trauma and Traumatic Brain Injury on Vision- and Health-Related Quality of Life among U.S. Military Casualties. Mil. Med. 2022, 187, 209–215. [Google Scholar] [CrossRef]
- Joyce, N.C.; Harris, D.L.; Markov, V.; Zhang, Z.; Saitta, B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol. Vis. 2012, 18, 547–564. [Google Scholar] [PubMed]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef]
- Adak, S.; Magdalene, D.; Deshmukh, S.; Das, D.; Jaganathan, B.G. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev. Rep. 2021, 17, 1154–1173. [Google Scholar] [CrossRef]
- Harrell, C.R.; Simovic, M.B.; Fellabaum, C.; Arsenijevic, A.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Eye Diseases. Adv. Exp. Med. Biol. 2018, 1089, 47–57. [Google Scholar] [PubMed]
- Nuzzi, R.; Caselgrandi, P.; Vercelli, A. Effect of Mesenchymal Stem Cell-Derived Exosomes on Retinal Injury: A Review of Current Findings. Stem Cells Int. 2020, 2020, 8883616. [Google Scholar] [CrossRef] [PubMed]
- Bonnell, E.; Pasquier, E.; Wellinger, R.J. Telomere Replication: Solving Multiple End Replication Problems. Front. Cell Dev. Biol. 2021, 9, 668171. [Google Scholar] [CrossRef] [PubMed]
- Parsch, D.; Fellenberg, J.; Brümmendorf, T.H.; Eschlbeck, A.M.; Richter, W. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J. Mol. Med. 2004, 82, 49–55. [Google Scholar] [PubMed]
- Bernardo, M.E.; Zaffaroni, N.; Novara, F.; Cometa, A.M.; Avanzini, M.A.; Moretta, A.; Montagna, D.; Maccario, R.; Villa, R.; Daidone, M.G.; et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007, 67, 9142–9149. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Voss, M.; Kaiser, S.; Kapp, U.; Waller, C.F.; Martens, U.M. Lack of telomerase activity in human mesenchymal stem cells. Leukemia 2003, 17, 1146–1149. [Google Scholar] [CrossRef]
- Ouellette, M.M.; McDaniel, L.D.; Wright, W.E.; Shay, J.W.; Schultz, R.A. The establishment of telomerase-immortalized cell lines representing human chromosome instability syndromes. Hum. Mol. Genet. 2000, 9, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.; Abdallah, B.M.; Yu, Z.; Ditzel, N.; Burns, J.S. The use of hTERT-immortalized cells in tissue engineering. Cytotechnology 2004, 45, 39–46. [Google Scholar] [CrossRef]
- Belair, C.D.; Yeager, T.R.; Lopez, P.M.; Reznikoff, C.A. Telomerase activity: A biomarker of cell proliferation, not malignant transformation. Proc. Natl. Acad. Sci. USA 1997, 94, 13677–13682. [Google Scholar] [CrossRef]
- Lee, K.M.; Choi, K.H.; Ouellette, M.M. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology 2004, 45, 33–38. [Google Scholar] [CrossRef]
- Liu, T.M.; Ng, W.M.; Tan, H.S.; Vinitha, D.; Yang, Z.; Fan, J.B.; Zou, Y.; Hui, J.H.; Lee, E.H.; Lim, B. Molecular basis of immortalization of human mesenchymal stem cells by combination of p53 knockdown and human telomerase reverse transcriptase overexpression. Stem Cells Dev. 2013, 22, 268–278. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Fox, J.; Afsari, F.; Lee, J.; Clough, S.; Knight, C.; Ashmore, J.; Ashton, P.; Preham, O.; Hoogduijn, M.; et al. Multiparameter Analysis of Human Bone Marrow Stromal Cells Identifies Distinct Immunomodulatory and Differentiation-Competent Subtypes. Stem Cell Rep. 2015, 4, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Siska, E.K.; Weisman, I.; Romano, J.; Ivics, Z.; Izsvák, Z.; Barkai, U.; Petrakis, S.; Koliakos, G. Generation of an immortalized mesenchymal stem cell line producing a secreted biosensor protein for glucose monitoring. PLoS ONE 2017, 12, e0185498. [Google Scholar] [CrossRef] [PubMed]
- Milyavsky, M.; Shats, I.; Erez, N.; Tang, X.; Senderovich, S.; Meerson, A.; Tabach, Y.; Goldfinger, N.; Ginsberg, D.; Harris, C.C.; et al. Prolonged culture of telomerase-immortalized human fibroblasts leads to a premalignant phenotype. Cancer Res. 2003, 63, 7147–7157. [Google Scholar] [PubMed]
- Park, Y.J.; Kim, E.K.; Bae, J.Y.; Moon, S.; Kim, J. Human telomerase reverse transcriptase (hTERT) promotes cancer invasion by modulating cathepsin D via early growth response (EGR)-1. Cancer Lett. 2016, 370, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bikkul, M.U.; Faragher, R.G.A.; Worthington, G.; Meinke, P.; Kerr, A.R.W.; Sammy, A.; Riyahi, K.; Horton, D.; Schirmer, E.C.; Hubank, M.; et al. Telomere elongation through hTERT immortalization leads to chromosome repositioning in control cells and genomic instability in Hutchinson-Gilford progeria syndrome fibroblasts, expressing a novel SUN1 isoform. Genes Chromosomes Cancer 2019, 58, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Branscome, H.; Paul, S.; Khatkar, P.; Kim, Y.; Barclay, R.A.; Pinto, D.O.; Yin, D.; Zhou, W.; Liotta, L.A.; El-Hage, N.; et al. Stem Cell Extracellular Vesicles and their Potential to Contribute to the Repair of Damaged CNS Cells. J. Neuroimmune Pharmacol. 2020, 15, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Erickson, J.; Cuba, Z.; Kim, S.; Zhou, W.; Gade, P.; Carter, R.; Mitchell, K.; Branscome, H.; Siddhi, D.; et al. A secretory form of Parkin-independent mitophagy contributes to the repertoire of extracellular vesicles released into the tumour interstitial fluid in vivo. J. Extracell. Vesicles 2022, 11, e12244. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, N.; Griffin, L.D.; Keser, A.; Macown, R.J.; Super, A.; Veraitch, F.S.; Szita, N. Automated method for the rapid and precise estimation of adherent cell culture characteristics from phase contrast microscopy images. Biotechnol. Bioeng. 2014, 111, 504–517. [Google Scholar] [CrossRef]
- Hallal, S.; Tűzesi, Á.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the extracellular vesicle surface for clinical molecular biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef]
- Lam, K.C.K.; Lam, M.K.N.; Chim, C.S.; Chan, G.C.F.; Li, J.C.B. The functional role of surface molecules on extracellular vesicles in cancer, autoimmune diseases, and coagulopathy. J. Leucoc. Biol. 2020, 108, 1565–1573. [Google Scholar] [CrossRef]
- Roland, C.L.; Harken, A.H.; Sarr, M.G.; Barnett, C.C., Jr. ICAM-1 expression determines malignant potential of cancer. Surgery 2007, 141, 705–707. [Google Scholar] [CrossRef]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Z.; Li, J.; Yang, X.; Wang, Y.; Yu, Y.; Ye, J.; Xu, C.; Qin, W.; Zhang, Z. MCAM is a novel metastasis marker and regulates spreading, apoptosis and invasion of ovarian cancer cells. Tumor Biol. 2012, 33, 1619–1628. [Google Scholar] [CrossRef]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The role of epidermal growth factor receptor in cancer metastasis and microenvironment. BioMed Res. Int. 2013, 2013, 546318. [Google Scholar] [CrossRef]
- Wang, M.H.; Sun, R.; Zhou, X.M.; Zhang, M.Y.; Lu, J.B.; Yang, Y.; Zeng, L.S.; Yang, X.Z.; Shi, L.; Xiao, R.W.; et al. Epithelial cell adhesion molecule overexpression regulates epithelial-mesenchymal transition, stemness and metastasis of nasopharyngeal carcinoma cells via the PTEN/AKT/mTOR pathway. Cell Death Dis. 2018, 9, 2. [Google Scholar] [CrossRef]
- Darvishi, B.; Boroumandieh, S.; Majidzadeh, A.K.; Salehi, M.; Jafari, F.; Farahmand, L. The role of activated leukocyte cell adhesion molecule (ALCAM) in cancer progression, invasion, metastasis and recurrence: A novel cancer stem cell marker and tumor-specific prognostic marker. Exp. Mol. Pathol. 2020, 115, 104443. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Q.; Wang, S.; Chen, X.; Wang, Y. MCAM is associated with metastasis and poor prognosis in osteosarcoma by modulating tumor cell migration. J. Clin. Lab. Anal. 2022, 36, e24214. [Google Scholar] [CrossRef]
- Rege, T.A.; Hagood, J.S. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006, 20, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Loghmani, H.; Conway, E.M. Exploring traditional and nontraditional roles for thrombomodulin. Blood 2018, 132, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Wu, H.L.; Chen, S.H.; Wang, Y.T.; Wu, C.C. Thrombomodulin facilitates peripheral nerve regeneration through regulating M1/M2 switching. J. Neuroinflamm. 2020, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Zhuravin, I.A.; Turner, A.J. Neprilysin expression and functions in development, ageing and disease. Mech. Ageing Dev. 2020, 192, 111363. [Google Scholar] [CrossRef] [PubMed]
- Sankhe, R.; Pai, S.R.K.; Kishore, A. Tumour suppression through modulation of neprilysin signaling: A comprehensive review. Eur. J. Pharmacol. 2021, 891, 173727. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhan, X.Z.; Malola, J.; Li, Z.Y.; Pawar, J.S.; Zhang, H.T.; Zha, Z.G. The multiple roles of Thy-1 in cell differentiation and regeneration. Differentiation 2020, 113, 38–48. [Google Scholar] [CrossRef]
- Sedov, E.; Koren, E.; Chopra, S.; Ankawa, R.; Yosefzon, Y.; Yusupova, M.; Weiss, L.E.; Mahly, A.; Soffer, A.; Feldman, A.; et al. THY1-mediated mechanisms converge to drive YAP activation in skin homeostasis and repair. Nat. Cell Biol. 2022, 24, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Mavragani, I.V.; Laskaratou, D.A.; Frey, B.; Candéias, S.M.; Gaipl, U.S.; Lumniczky, K.; Georgakilas, A.G. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol. Res. 2015, 5, 12–33. [Google Scholar] [CrossRef]
- Branscome, H.; Khatkar, P.; Al Sharif, S.; Yin, D.; Jacob, S.; Cowen, M.; Kim, Y.; Erickson, J.; Brantner, C.A.; El-Hage, N.; et al. Retroviral infection of human neurospheres and use of stem Cell EVs to repair cellular damage. Sci. Rep. 2022, 12, 2019. [Google Scholar] [CrossRef]
- Öztürk, S.; Elçin, A.E.; Koca, A.; Elçin, Y.M. Therapeutic Applications of Stem Cells and Extracellular Vesicles in Emergency Care: Futuristic Perspectives. Stem Cell Rev. Rep. 2021, 17, 390–410. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells 2020, 9, 707. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Wolbank, S.; Stadler, G.; Peterbauer, A.; Gillich, A.; Karbiener, M.; Streubel, B.; Wieser, M.; Katinger, H.; van Griensven, M.; Redl, H.; et al. Telomerase immortalized human amnion- and adipose-derived mesenchymal stem cells: Maintenance of differentiation and immunomodulatory characteristics. Tissue Eng. Part A 2009, 15, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol. Cell. Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Q.; Xia, Y.; You, B.; Shan, Y.; Bao, L.; Li, L.; You, Y.; Gu, Z. Mesenchymal stem cell-derived exosomes facilitate nasopharyngeal carcinoma progression. Am. J. Cancer Res. 2016, 6, 459–472. [Google Scholar] [PubMed]
- Qin, F.; Tang, H.; Zhang, Y.; Zhang, Z.; Huang, P.; Zhu, J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J. Cell Physiol. 2020, 235, 4734–4745. [Google Scholar] [CrossRef]
- Gómez-Ferrer, M.; Villanueva-Badenas, E.; Sánchez-Sánchez, R.; Sánchez-López, C.M.; Baquero, M.C.; Sepúlveda, P.; Dorronsoro, A. HIF-1α and Pro-Inflammatory Signaling Improves the Immunomodulatory Activity of MSC-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 3416. [Google Scholar] [CrossRef]
- Haghighitalab, A.; Matin, M.M.; Amin, A.; Minaee, S.; Bidkhori, H.R.; Doeppner, T.R.; Bahrami, A.R. Investigating the effects of IDO1, PTGS2, and TGF-β1 overexpression on immunomodulatory properties of hTERT-MSCs and their extracellular vesicles. Sci. Rep. 2021, 11, 7825. [Google Scholar] [CrossRef]
- Anand, S.; Samuel, M.; Mathivanan, S. Exomeres: A New Member of Extracellular Vesicles Family. Subcell Biochem. 2021, 97, 89–97. [Google Scholar] [PubMed]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E. Extracellular Vesicles and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037275. [Google Scholar] [CrossRef]
- Kischel, P.; Bellahcene, A.; Deux, B.; Lamour, V.; Dobson, R.; DE Pauw, E.; Clezardin, P.; Castronovo, V. Overexpression of CD9 in human breast cancer cells promotes the development of bone metastases. Anticancer. Res. 2012, 32, 5211–5220. [Google Scholar] [PubMed]
- Lorico, A.; Lorico-Rappa, M.; Karbanová, J.; Corbeil, D.; Pizzorno, G. CD9, a tetraspanin target for cancer therapy? Exp. Biol. Med. 2021, 246, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Nigri, J.; Leca, J.; Tubiana, S.S.; Finetti, P.; Guillaumond, F.; Martinez, S.; Lac, S.; Iovanna, J.L.; Audebert, S.; Camoin, L.; et al. CD9 mediates the uptake of extracellular vesicles from cancer-associated fibroblasts that promote pancreatic cancer cell aggressiveness. Sci. Signal. 2022, 15, eabg8191. [Google Scholar] [CrossRef] [PubMed]
- Lupia, A.; Peppicelli, S.; Witort, E.; Bianchini, F.; Carloni, V.; Pimpinelli, N.; Urso, C.; Borgognoni, L.; Capaccioli, S.; Calorini, L.; et al. CD63 tetraspanin is a negative driver of epithelial-to-mesenchymal transition in human melanoma cells. J. Investig. Dermatol. 2014, 134, 947–2956. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Li, X.; Zhu, X.L.; Hou, M.L.; Zhao, W. CD63 inhibits the cell migration and invasion ability of tongue squamous cell carcinoma. Oncol. Lett. 2018, 15, 9033–9042. [Google Scholar] [CrossRef]
- Yu, S.; Chen, J.; Quan, M.; Li, L.; Li, Y.; Gao, Y. CD63 negatively regulates hepatocellular carcinoma development through suppression of inflammatory cytokine-induced STAT3 activation. J. Cell. Mol. Med. 2021, 25, 1024–1034. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- An, Y.; Wei, N.; Cheng, X.; Li, Y.; Liu, H.; Wang, J.; Xu, Z.; Sun, Z.; Zhang, X. MCAM abnormal expression and clinical outcome associations are highly cancer dependent as revealed through pan-cancer analysis. Brief. Bioinform. 2020, 21, 709–718. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leucoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in physiological processes and diseases (Review). Int. J. Mol. Med. 2018, 42, 1771–1785. [Google Scholar] [CrossRef]
- Lee, M.J.; Shin, J.O.; Jung, H.S. Thy-1 knockdown retards wound repair in mouse skin. J. Dermatol. Sci. 2013, 69, 95–104. [Google Scholar] [CrossRef]
- Martin, F.A.; Murphy, R.P.; Cummins, P.M. Thrombomodulin and the vascular endothelium: Insights into functional, regulatory, and therapeutic aspects. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1585–H1597. [Google Scholar] [CrossRef]
- Saalbach, A.; Anderegg, U. Thy-1: More than a marker for mesenchymal stromal cells. FASEB J. 2019, 33, 6689–6696. [Google Scholar] [CrossRef]
- Cao, X.; Wen, P.; Fu, Y.; Gao, Y.; Qi, X.; Chen, B.; Tao, Y.; Wu, L.; Xu, A.; Lu, H.; et al. Radiation induces apoptosis primarily through the intrinsic pathway in mammalian cells. Cell. Signal. 2019, 62, 109337. [Google Scholar] [CrossRef]
- Teyssier, F.; Bay, J.O.; Dionet, C.; Verrelle, P. Régulation du cycle cellulaire des cellules exposées aux radiations ionisantes [Cell cycle regulation after exposure to ionizing radiation]. Bull. Cancer 1999, 86, 345–357. [Google Scholar] [PubMed]
- Jones, M.; Sabatini, P.J.; Lee, F.S.; Bendeck, M.P.; Langille, B.L. N-cadherin upregulation and function in response of smooth muscle cells to arterial injury. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, M. E-cadherin Is Important for the Maintenance of Intestinal Epithelial Homeostasis Under Basal and Inflammatory Conditions. Dig. Dis. Sci. 2015, 60, 4. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 10. [Google Scholar] [CrossRef]
- Ray, S.; Menezes, M.R.; Senejani, A.; Sweasy, J.B. Cellular roles of DNA polymerase beta. Yale J. Biol. Med. 2013, 86, 4. [Google Scholar]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y. The impact of VEGF on cancer metastasis and systemic disease. Semin Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Gu, H.; Ji, R.; Zhang, X.; Wang, M.; Zhu, W.; Qian, H.; Chen, Y.; Jiang, P.; Xu, W. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol. Med. Rep. 2016, 14, 3452–3458. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Y.; Lan, Q.; Wu, W.; Li, Z.; Ma, X.; Yu, L. Exosomes Derived from Mesenchymal Stem Cells Ameliorate Hypoxia/Reoxygenation-Injured ECs via Transferring MicroRNA-126. Stem Cells Int. 2019, 2019, 2831756. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xu, Y.; Chen, Q.; Chen, H.; Zhu, X.; Li, Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020, 11, 290, Erratum in Cell Death Dis. 2020, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Ernandez, T.; Mayadas, T.N. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009, 76, 262–276. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Hewett, S.J.; Jackman, N.A.; Claycomb, R.J. Interleukin-1β in Central Nervous System Injury and Repair. Eur. J. Neurodegener. Dis. 2012, 1, 195–211. [Google Scholar]
- Tachibana, S.; Zhang, X.; Ito, K.; Ota, Y.; Cameron, A.M.; Williams, G.M.; Sun, Z. Interleukin-6 is required for cell cycle arrest and activation of DNA repair enzymes after partial hepatectomy in mice. Cell Biosci. 2014, 4, 6. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.; Ding, Y.; Tan, K.; Ni, W.; Ouyang, W.; Tang, J.; Ding, X.; Zhao, J.; Hao, Y.; et al. IL-6 coaxes cellular dedifferentiation as a pro-regenerative intermediate that contributes to pericardial ADSC-induced cardiac repair. Stem Cell Res. Ther. 2022, 13, 44. [Google Scholar] [CrossRef]
- Aslan, C.; Kiaie, S.H.; Zolbanin, N.M.; Lotfinejad, P.; Ramezani, R.; Kashanchi, F.; Jafari, R. Exosomes for mRNA delivery: A novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. 2021, 21, 20. [Google Scholar] [CrossRef]
- Gorshkov, A.; Purvinsh, L.; Brodskaia, A.; Vasin, A. Exosomes as Natural Nanocarriers for RNA-Based Therapy and Prophylaxis. Nanomaterials 2022, 12, 524. [Google Scholar] [CrossRef]
- Nuzzi, R.; Trossarello, M.; Bartoncini, S.; Marolo, P.; Franco, P.; Mantovani, C.; Ricardi, U. Ocular Complications after Radiation Therapy: An Observational Study. Clin. Ophthalmol. 2020, 14, 3153–3166. [Google Scholar] [CrossRef] [PubMed]
- Fish, D.E.; Kim, A.; Ornelas, C.; Song, S.; Pangarkar, S. The risk of radiation exposure to the eyes of the interventional pain physician. Radiol. Res. Pract. 2011, 2011, 609537. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Rae, W.I.D.; Sweetlove, M.A.; Ngetu, L.; Benadjaoud, M.A.; Marais, W. Radiation induced cataracts in interventionalists occupationally exposed to ionising radiation. SA J. Radiol. 2022, 26, 2495. [Google Scholar] [CrossRef]


| Antibody | Vendor | Catalog |
|---|---|---|
| α-CD81 | SBI (Palo Alto, CA, USA) | EXOAB-CD81A-1 |
| α-CD63 | SBI | EXOAB-CD63A-1 |
| α-CD9 | SBI | EXOAB-CD9A-1 |
| α-GAPDH | Santa Cruz Biotechnology (Dallas, TX, USA) | sc-47724 |
| α-Caspase 3 | Santa Cruz Biotechnology | sc-7148 |
| α-Cyclin A | Santa Cruz Biotechnology | sc-751 |
| α-Cyclin D1 | Santa Cruz Biotechnology | sc-753 |
| α-Cyclin E | Santa Cruz Biotechnology | sc-198 |
| α-Actin | Santa Cruz Biotechnology | sc-8432 |
| α-N-cadherin | Cell Signaling Technology (Danvers, MA, USA) | 14215S |
| α-E-cadherin | Cell Signaling Technology | 3195T |
| α-CD142 | Cell Signaling Technology | 97438T |
| α-VEGF | Santa Cruz Biotechnology | sc-57496 |
| α-DNA Polymerase β | Novus Biologicals (Centennial, CO, USA) | NBP2-38600 |
| α-p-AKT | Santa Cruz Biotechnology | sc-16646-R |
| α-AKT | Cell Signaling Technology | 9272S |
| Primer | Forward Sequence | Reverse Sequence | Tm |
|---|---|---|---|
| S100A6 | 5’-GTGACAAGCACACCCTGAGCAA-3’ | 5’-GGAAGTTCACCTCCTGGTCCTT-3’ | 58.1 °C |
| RPS23 | 5’-AGGAAGTGT GTAAGGGTCCAGC-3’ | 5’-CACCAACAGCATGACCTTTGCG-3’ | 58.9 °C |
| LGALS1 | 5’-AGCAGCGGGAGGCTGTCTTTC-3’ | 5’-ATCCATCTGGCAGCTTGACGGT-3′ | 61.2 °C |
| GAPDH | 5’-GAAGGTGAAGGTCGG AGTCAAC-3’ | 5’-CAGAGTTAAAAGCAGCCCTGGT-3’ | 57.5 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hindle, J.; Williams, A.; Kim, Y.; Kim, D.; Patil, K.; Khatkar, P.; Osgood, Q.; Nelson, C.; Routenberg, D.A.; Howard, M.; et al. hTERT-Immortalized Mesenchymal Stem Cell-Derived Extracellular Vesicles: Large-Scale Manufacturing, Cargo Profiling, and Functional Effects in Retinal Epithelial Cells. Cells 2024, 13, 861. https://doi.org/10.3390/cells13100861
Hindle J, Williams A, Kim Y, Kim D, Patil K, Khatkar P, Osgood Q, Nelson C, Routenberg DA, Howard M, et al. hTERT-Immortalized Mesenchymal Stem Cell-Derived Extracellular Vesicles: Large-Scale Manufacturing, Cargo Profiling, and Functional Effects in Retinal Epithelial Cells. Cells. 2024; 13(10):861. https://doi.org/10.3390/cells13100861
Chicago/Turabian StyleHindle, Jessica, Anastasia Williams, Yuriy Kim, Dongsung Kim, Kajal Patil, Pooja Khatkar, Quinn Osgood, Collin Nelson, David A. Routenberg, Marissa Howard, and et al. 2024. "hTERT-Immortalized Mesenchymal Stem Cell-Derived Extracellular Vesicles: Large-Scale Manufacturing, Cargo Profiling, and Functional Effects in Retinal Epithelial Cells" Cells 13, no. 10: 861. https://doi.org/10.3390/cells13100861
APA StyleHindle, J., Williams, A., Kim, Y., Kim, D., Patil, K., Khatkar, P., Osgood, Q., Nelson, C., Routenberg, D. A., Howard, M., Liotta, L. A., Kashanchi, F., & Branscome, H. (2024). hTERT-Immortalized Mesenchymal Stem Cell-Derived Extracellular Vesicles: Large-Scale Manufacturing, Cargo Profiling, and Functional Effects in Retinal Epithelial Cells. Cells, 13(10), 861. https://doi.org/10.3390/cells13100861









