Emerging Strategies in Mesenchymal Stem Cell-Based Cardiovascular Therapeutics
Abstract
1. Introduction
2. Past and Present Stem Cell-Based Therapeutic Approaches
2.1. Induced Pluripotent Stem Cells (iPSCs)
2.2. Cardiosphere-Derived Cells
2.3. Endothelial Progenitor Cells
2.4. MSCs
2.5. MSC-Derived Extracellular Vesicles
3. Biological Properties of MSCs
3.1. Multipotency and Differentiation
3.2. Immunomodulation
3.3. Paracrine Signaling
3.4. Extracellular Vesicles and Secretome
3.5. Angiogenesis
3.6. Anti-Fibrotic Effects
4. Homing and Migration of MSCs
Stimulation of Homing and Recruitment of Stem Cells
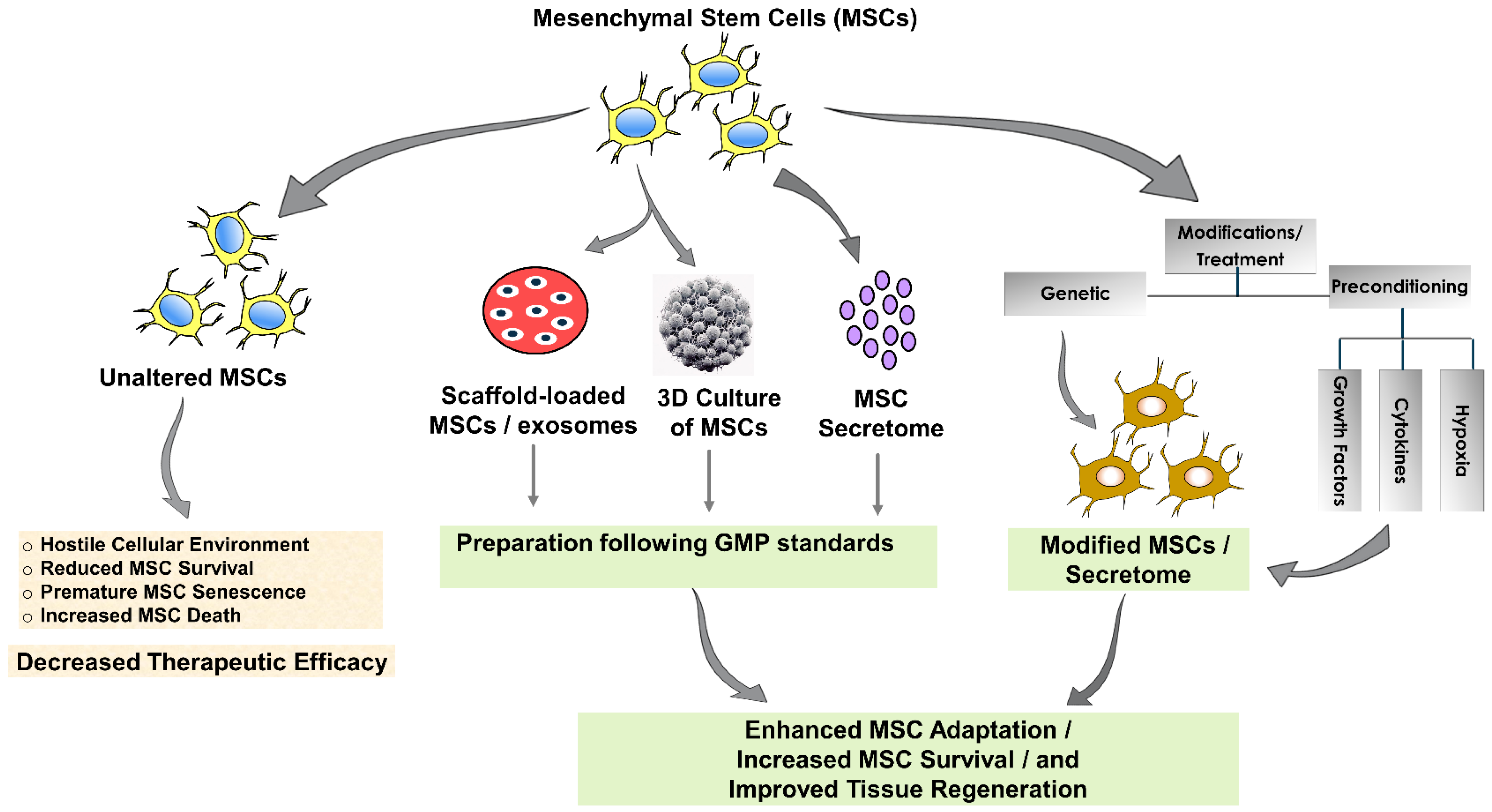
5. Challenges and Needs in MSC-Based Cardiovascular Therapeutics
5.1. Challenges
5.2. Overcoming Limitations
6. Emerging Strategies in MSC-Based Tissue Engineering and Regeneration
6.1. Cell-Free Approaches: The Power of the Secretome
6.2. Scaffold-Based Therapeutics: Enhancing Stem Cell Potential
Biomaterials
6.3. Three-Dimensional Ex Vivo Propagation and Pre-Treatment
6.3.1. Physiological Microenvironment Simulation Pre-Activation
6.3.2. Pathological Microenvironment Simulation Pre-Activation
6.4. Genetic Modifications: Tailoring MSCs for Targeted Repair
6.4.1. Viral Vector-Mediated Genetic Modification
6.4.2. Non-Viral Methods of Genetic Modification
6.5. Mechanobiologically Mediated Differentiation of Stem Cells
6.6. GMPs in Stem Cell-Based Therapeutics
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.M. Current state of stem cell-based therapies: An overview. Stem Cell Investig. 2020, 7, 8. [Google Scholar] [CrossRef]
- Thomas, E.D.; Lochte, H.L., Jr.; Lu, W.C.; Ferrebee, J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Lotz, J.; Ringes-Lichtenberg, S.; Lippolt, P.; Breidenbach, C.; Fichtner, S.; Korte, T.; Hornig, B.; Messinger, D.; et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet 2004, 364, 141–148. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Schächinger, V.; Erbs, S.; Elsässer, A.; Haberbosch, W.; Hambrecht, R.; Hölschermann, H.; Yu, J.; Corti, R.; Mathey, D.G.; Hamm, C.W.; et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the REPAIR-AMI trial. Eur. Heart J. 2006, 27, 2775–2783. [Google Scholar] [CrossRef]
- Mishra, R.; Vijayan, K.; Colletti, E.J.; Harrington, D.A.; Matthiesen, T.S.; Simpson, D.; Goh, S.K.; Walker, B.L.; Almeida-Porada, G.; Wang, D.; et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation 2011, 123, 364–373. [Google Scholar] [CrossRef]
- Sugarman, J. Ethics and stem cell therapeutics for cardiovascular disease. Prog. Cardiovasc. Dis. 2007, 50, 1–6. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Q.; Wang, Z.; Niu, B.; Jiao, Y.; An, H. APE1 promotes embryonic stem cell proliferation and teratoma formation by regulating GDNF/GFRα1 axis. Reprod. Biol. 2023, 23, 100792. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kussauer, S.; David, R.; Lemcke, H. hiPSCs Derived Cardiac Cells for Drug and Toxicity Screening and Disease Modeling: What Micro- Electrode-Array Analyses Can Tell Us. Cells 2019, 8, 1331. [Google Scholar] [CrossRef] [PubMed]
- Bonilauri, B.; Shin, H.S.; Htet, M.; Yan, C.D.; Witteles, R.M.; Sallam, K.; Wu, J.C. Generation of two induced pluripotent stem cell lines from patients with cardiac amyloidosis carrying heterozygous transthyretin (TTR) mutation. Stem Cell Res. 2023, 72, 103215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Rajasingh, R.; Isai, D.G.; Czirok, A.; Bittel, D.; Rajasingh, J. Noonan syndrome patient-specific induced cardiomyocyte model carrying SOS1 gene variant c.1654A>G. Exp. Cell Res. 2021, 400, 112508. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Choi, D.B.; Im, J.S.; Song, Y.N.; Kim, J.H.; Lee, H.; An, J.; Kim, A.; Choi, H.; Kim, J.C.; et al. Modeling acute myocardial infarction and cardiac fibrosis using human induced pluripotent stem cell-derived multi-cellular heart organoids. Cell Death Dis. 2024, 15, 308. [Google Scholar] [CrossRef]
- Liang, S.; Su, Y.; Yao, R. 3D Bioprinting of Induced Pluripotent Stem Cells and Disease Modeling. Handb. Exp. Pharmacol. 2023, 281, 29–56. [Google Scholar] [CrossRef]
- Yu, D.; Wang, X.; Ye, L. Cardiac Tissue Engineering for the Treatment of Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2021, 8, 153. [Google Scholar] [CrossRef]
- Riegler, J.; Tiburcy, M.; Ebert, A.; Tzatzalos, E.; Raaz, U.; Abilez, O.J.; Shen, Q.; Kooreman, N.G.; Neofytou, E.; Chen, V.C.; et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ. Res. 2015, 117, 720–730. [Google Scholar] [CrossRef]
- Wang, D.; Mou, H.; Li, S.; Li, Y.; Hough, S.; Tran, K.; Li, J.; Yin, H.; Anderson, D.G.; Sontheimer, E.J.; et al. Adenovirus-Mediated Somatic Genome Editing of Pten by CRISPR/Cas9 in Mouse Liver in Spite of Cas9-Specific Immune Responses. Hum. Gene Ther. 2015, 26, 432–442. [Google Scholar] [CrossRef]
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent Progress in Stem Cell Modification for Cardiac Regeneration. Stem Cells Int. 2018, 2018, 1909346. [Google Scholar] [CrossRef]
- Bian, W.; Chen, W.; Nguyen, T.; Zhou, Y.; Zhang, J. miR-199a Overexpression Enhances the Potency of Human Induced-Pluripotent Stem-Cell-Derived Cardiomyocytes for Myocardial Repair. Front. Pharmacol. 2021, 12, 673621. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marbán, E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007, 115, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, C.; Zhang, G.; Tao, J. Endothelial progenitor cells in cardiovascular diseases. Aging Med. 2018, 1, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Salybekov, A.A.; Kobayashi, S.; Asahara, T. Characterization of Endothelial Progenitor Cell: Past, Present, and Future. Int. J. Mol. Sci. 2022, 23, 7697. [Google Scholar] [CrossRef]
- Park, K.S.; Kang, S.N.; Kim, D.H.; Kim, H.B.; Im, K.S.; Park, W.; Hong, Y.J.; Han, D.K.; Joung, Y.K. Late endothelial progenitor cell-capture stents with CD146 antibody and nanostructure reduce in-stent restenosis and thrombosis. Acta Biomater. 2020, 111, 91–101. [Google Scholar] [CrossRef]
- Mitsiou, G.; Tokmakidis, S.P.; Dinas, P.C.; Smilios, I.; Nanas, S. Endothelial progenitor cell mobilization based on exercise volume in patients with cardiovascular disease and healthy individuals: A systematic review and meta-analysis. Eur. Heart J. Open 2022, 2, oeac078. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Z.; Yan, Z.; Ji, N.; Yang, X.; Gao, D.; Hu, L.; Lv, H.; Zhang, J.; Li, M. Mesenchymal Stem Cell Immunomodulation: A Novel Intervention Mechanism in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 742088. [Google Scholar] [CrossRef]
- Yan, W.; Xia, Y.; Zhao, H.; Xu, X.; Ma, X.; Tao, L. Stem cell-based therapy in cardiac repair after myocardial infarction: Promise, challenges, and future directions. J. Mol. Cell. Cardiol. 2024, 188, 1–14. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef]
- Angoulvant, D.; Ivanes, F.; Ferrera, R.; Matthews, P.G.; Nataf, S.; Ovize, M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J. Heart Lung Transplant. 2011, 30, 95–102. [Google Scholar] [CrossRef]
- Rehman, A.; Nigam, A.; Laino, L.; Russo, D.; Todisco, C.; Esposito, G.; Svolacchia, F.; Giuzio, F.; Desiderio, V.; Ferraro, G. Mesenchymal Stem Cells in Soft Tissue Regenerative Medicine: A Comprehensive Review. Medicina 2023, 59, 1449. [Google Scholar] [CrossRef] [PubMed]
- Siu, C.W.; Liao, S.Y.; Liu, Y.; Lian, Q.; Tse, H.F. Stem cells for myocardial repair. Thromb. Haemost. 2010, 104, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.H.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell Therapy for Aging Frailty. Front. Nutr. 2018, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2020, 22, 884–892. [Google Scholar] [CrossRef]
- Bartunek, J.; Behfar, A.; Dolatabadi, D.; Vanderheyden, M.; Ostojic, M.; Dens, J.; El Nakadi, B.; Banovic, M.; Beleslin, B.; Vrolix, M.; et al. Cardiopoietic stem cell therapy in heart failure: The C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 2013, 61, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Terzic, A.; Davison, B.A.; Filippatos, G.S.; Radovanovic, S.; Beleslin, B.; Merkely, B.; Musialek, P.; Wojakowski, W.; Andreka, P.; et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: Results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 2017, 38, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Borow, K.M.; Yaroshinsky, A.; Greenberg, B.; Perin, E.C. Phase 3 DREAM-HF Trial of Mesenchymal Precursor Cells in Chronic Heart Failure. Circ. Res. 2019, 125, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, L.; Zhu, X.; Li, Q.; Hu, L.; Li, H. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Acta Biochim. Biophys. Sin. 2021, 53, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, W.; Jiang, X.; Liu, Y. Mesenchymal stem cell-derived exosomes in cardiovascular and cerebrovascular diseases: From mechanisms to therapy. Biomed. Pharmacother. 2023, 163, 114817. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; You, J.; Liang, H.; Zhan, J.; Gu, X.; Zhu, Y. Engineered bone marrow mesenchymal stem cell-derived exosomes loaded with miR302 through the cardiomyocyte specific peptide can reduce myocardial ischemia and reperfusion (I/R) injury. J. Transl. Med. 2024, 22, 168. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Pan, B.; Zhou, H.; Liu, L.; Lv, T.; Zhu, J.; Huang, X.; Tian, J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J. Biomed. Sci. 2017, 24, 29. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y.J.; Qian, H.Y.; Tang, Y.D.; Wang, H.; Zhang, Q. Rosuvastatin treatment activates JAK-STAT pathway and increases efficacy of allogeneic mesenchymal stem cell transplantation in infarcted hearts. Circ. J. 2011, 75, 1476–1485. [Google Scholar] [CrossRef]
- He, X.; Li, L.; Tang, M.; Zeng, Y.; Li, H.; Yu, X. Biomimetic electrical stimulation induces rat bone marrow mesenchymal stem cells to differentiate into cardiomyocyte-like cells via TGF-beta 1 in vitro. Prog. Biophys. Mol. Biol. 2019, 148, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, H.C.; Hatzistergos, K.E.; Oskouei, B.N.; Feigenbaum, G.S.; Rodriguez, J.E.; Valdes, D.; Pattany, P.M.; Zambrano, J.P.; Hu, Q.; McNiece, I.; et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 14022–14027. [Google Scholar] [CrossRef] [PubMed]
- Khaki, M.; Salmanian, A.H.; Abtahi, H.; Ganji, A.; Mosayebi, G. Mesenchymal Stem Cells Differentiate to Endothelial Cells Using Recombinant Vascular Endothelial Growth Factor—A. Rep. Biochem. Mol. Biol. 2018, 6, 144–150. [Google Scholar] [PubMed]
- Huang, Y.; Qian, J.Y.; Cheng, H.; Li, X.M. Effects of shear stress on differentiation of stem cells into endothelial cells. World J. Stem Cells 2021, 13, 894–913. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.J.; Abdollahi, H.; Zhang, P.; McIlhenny, S.; Tulenko, T.N.; DiMuzio, P.J. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J. Surg. Res. 2011, 168, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Yamawaki, A.; Kagami, H.; Ueda, M.; Ueda, Y. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 2008, 333, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Safina, I.; Embree, M.C. Biomaterials for recruiting and activating endogenous stem cells in situ tissue regeneration. Acta Biomater. 2022, 143, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Alrumaihi, F.; Al Abdulmonem, W.; Al-Megrin, W.A.I.; Aljamaan, A.N.; Rahmani, A.H.; Khan, A.A. Recent Advances in Genome-Editing Technology with CRISPR/Cas9 Variants and Stimuli-Responsive Targeting Approaches within Tumor Cells: A Future Perspective of Cancer Management. Int. J. Mol. Sci. 2023, 24, 7052. [Google Scholar] [CrossRef]
- Shahabipour, F.; Oskuee, R.K.; Shokrgozar, M.A.; Naderi-Meshkin, H.; Goshayeshi, L.; Bonakdar, S. CRISPR/Cas9 mediated GFP-human dentin matrix protein 1 (DMP1) promoter knock-in at the ROSA26 locus in mesenchymal stem cell for monitoring osteoblast differentiation. J. Gene Med. 2020, 22, e3288. [Google Scholar] [CrossRef]
- Yuan, X.; Li, D.; Chen, X.; Han, C.; Xu, L.; Huang, T.; Dong, Z.; Zhang, M. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017, 8, 3200. [Google Scholar] [CrossRef] [PubMed]
- Meshitsuka, S.; Ninomiya, R.; Nagamura-Inoue, T.; Okada, T.; Futami, M.; Tojo, A. CRISPR/Cas9 and AAV mediated insertion of β2 microglobulin-HLA-G fusion gene protects mesenchymal stromal cells from allogeneic rejection and potentiates the use for off-the-shelf cell therapy. Regen. Ther. 2022, 21, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Shin, H.R.; Kwon, J.; Lee, S.B.; Lee, S.E.; Kim, E.Y.; Kweon, J.; Chang, E.J.; Kim, Y.; Kim, S.W. Highly efficient genome editing via CRISPR-Cas9 ribonucleoprotein (RNP) delivery in mesenchymal stem cells. BMB Rep. 2024, 57, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Oh, J.Y. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.J.; Kerr, L.D.; Sriram, S. TGF-beta inhibits IL-2-induced tyrosine phosphorylation and activation of Jak-1 and Stat 5 in T lymphocytes. J. Immunol. 1997, 159, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Herr, F.; Vernochet, A.; Mennesson, B.; Oberlin, E.; Durrbach, A. Human Fetal Liver Mesenchymal Stem Cell-Derived Exosomes Impair Natural Killer Cell Function. Stem Cells Dev. 2019, 28, 44–55. [Google Scholar] [CrossRef]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 136–143. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011, 109, 724–728. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liang, X.; Liu, B.; Huang, X.; Shen, Y.; Lin, F.; Chen, J.; Gao, X.; He, H.; Li, W.; et al. Exosomal miR-9-5p derived from iPSC-MSCs ameliorates doxorubicin-induced cardiomyopathy by inhibiting cardiomyocyte senescence. J. Nanobiotechnol. 2024, 22, 195. [Google Scholar] [CrossRef]
- Liu, B.; Wei, Y.; He, J.; Feng, B.; Chen, Y.; Guo, R.; Griffin, M.D.; Hynes, S.O.; Shen, S.; Liu, Y.; et al. Human umbilical cord-derived mesenchymal stromal cells improve myocardial fibrosis and restore miRNA-133a expression in diabetic cardiomyopathy. Stem Cell Res. Ther. 2024, 15, 120. [Google Scholar] [CrossRef]
- Zheng, R.; Wu, X.; Li, S.; Chen, X.; Yan, D.; He, J. Mechanism Exploration on the Immunoregulation of Allogeneic Heart Transplantation Rejection in Rats with Exosome miRNA and Proteins from Overexpressed IDO1 BMSCs. Cell Transplant. 2024, 33, 9636897241245796. [Google Scholar] [CrossRef]
- Zhu, W.; Du, W.; Duan, R.; Liu, Y.; Zong, B.; Jin, X.; Dong, Z.; Wang, H.; Shahab, S.; Wang, H.; et al. miR-873-5p Suppression Reinvigorates Aging Mesenchymal Stem Cells and Improves Cardiac Repair after Myocardial Infarction. ACS Pharmacol. Transl. Sci. 2024, 7, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Vignais, M.L.; Levoux, J.; Sicard, P.; Khattar, K.; Lozza, C.; Gervais, M.; Mezhoud, S.; Nakhle, J.; Relaix, F.; Agbulut, O.; et al. Transfer of Cardiac Mitochondria Improves the Therapeutic Efficacy of Mesenchymal Stem Cells in a Preclinical Model of Ischemic Heart Disease. Cells 2023, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Hatzistergos, K.E.; Quevedo, H.; Oskouei, B.N.; Hu, Q.; Feigenbaum, G.S.; Margitich, I.S.; Mazhari, R.; Boyle, A.J.; Zambrano, J.P.; Rodriguez, J.E.; et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 2010, 107, 913–922. [Google Scholar] [CrossRef]
- Tao, H.; Han, Z.; Han, Z.C.; Li, Z. Proangiogenic Features of Mesenchymal Stem Cells and Their Therapeutic Applications. Stem Cells Int. 2016, 2016, 1314709. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Mias, C.; Lairez, O.; Trouche, E.; Roncalli, J.; Calise, D.; Seguelas, M.H.; Ordener, C.; Piercecchi-Marti, M.D.; Auge, N.; Salvayre, A.N.; et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 2009, 27, 2734–2743. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Chen, T.S.; Liou, S.Y.; Lin, H.H.; Hung, M.Y.; Lin, C.C.; Lin, Y.M.; Lin, K.H.; Padma, V.V.; Yao, C.H.; Kuo, W.W.; et al. Oral administration of green tea Epigallocatechin-3-gallate reduces oxidative stress and enhances restoration of cardiac function in diabetic rats receiving autologous transplantation of adipose-derived stem cells. Arch. Physiol. Biochem. 2021, 127, 82–89. [Google Scholar] [CrossRef]
- Meng, K.; Cai, H.; Cai, S.; Hong, Y.; Zhang, X. Adiponectin Modified BMSCs Alleviate Heart Fibrosis via Inhibition TGF-beta1/Smad in Diabetic Rats. Front. Cell Dev. Biol. 2021, 9, 644160. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Sun, H.H.; Sun, P.F. MicroRNA-378 attenuates myocardial fibrosis by inhibiting MAPK/ERK pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Gubert, F.; da Silva, J.S.; Vasques, J.F.; de Jesus Gonçalves, R.G.; Martins, R.S.; de Sá, M.P.L.; Mendez-Otero, R.; Zapata-Sudo, G. Mesenchymal Stem Cells Therapies on Fibrotic Heart Diseases. Int. J. Mol. Sci. 2021, 22, 7447. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.M. The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds. Adv. Wound Care 2015, 4, 623–630. [Google Scholar] [CrossRef]
- Rüster, B.; Göttig, S.; Ludwig, R.J.; Bistrian, R.; Müller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944. [Google Scholar] [CrossRef]
- Yau, T.M.; Pagani, F.D.; Mancini, D.M.; Chang, H.L.; Lala, A.; Woo, Y.J.; Acker, M.A.; Selzman, C.H.; Soltesz, E.G.; Kern, J.A.; et al. Intramyocardial Injection of Mesenchymal Precursor Cells and Successful Temporary Weaning from Left Ventricular Assist Device Support in Patients with Advanced Heart Failure: A Randomized Clinical Trial. JAMA 2019, 321, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Haendeler, J.; Reinhold, J.; Rochwalsky, U.; Seeger, F.; Honold, J.; Hoffmann, J.; Urbich, C.; Lehmann, R.; Arenzana-Seisdesdos, F.; et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ. Res. 2005, 97, 1142–1151. [Google Scholar] [CrossRef]
- Chen, L.; Wu, F.; Xia, W.H.; Zhang, Y.Y.; Xu, S.Y.; Cheng, F.; Liu, X.; Zhang, X.Y.; Wang, S.M.; Tao, J. CXCR4 gene transfer contributes to in vivo reendothelialization capacity of endothelial progenitor cells. Cardiovasc. Res. 2010, 88, 462–470. [Google Scholar] [CrossRef][Green Version]
- He, F.; Luo, P.F.; Tang, T.; Zhang, F.; Fang, H.; Ji, S.Z.; Sun, Y.; Wu, G.S.; Pan, B.H.; Huo, Z.B.; et al. Targeted release of stromal cell-derived factor-1α by reactive oxygen species-sensitive nanoparticles results in bone marrow stromal cell chemotaxis and homing, and repair of vascular injury caused by electrical burns. PLoS ONE 2018, 13, e0194298. [Google Scholar] [CrossRef]
- Adeshina, Y.O.; Deeds, E.J.; Karanicolas, J. Machine learning classification can reduce false positives in structure-based virtual screening. Proc. Natl. Acad. Sci. USA 2020, 117, 18477–18488. [Google Scholar] [CrossRef] [PubMed]
- Terashvili, M.; Bosnjak, Z.J. Stem Cell Therapies in Cardiovascular Disease. J. Cardiothorac. Vasc. Anesth. 2019, 33, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Qiu, Y.; Tao, J. The challenges and optimization of cell-based therapy for cardiovascular disease. J. Transl. Int. Med. 2021, 9, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Jain, K.G.; Bhaskar, N.; Kaur, A.; Rawat, S.; Giri, S.; Basu, B.; Mohanty, S. Tissue-specific mesenchymal stem cell-dependent osteogenesis in highly porous chitosan-based bone analogs. Stem Cells Transl. Med. 2021, 10, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrasco, R.; Sánchez-Abarca, L.I.; Nieto-Gómez, C.; Martín García, E.; Sánchez-Guijo, F.; Argüeso, P.; Aijón, J.; Hernández-Galilea, E.; Velasco, A. Subconjunctival injection of mesenchymal stromal cells protects the cornea in an experimental model of GVHD. Ocul. Surf. 2019, 17, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O.; et al. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients with Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J. Hematol. Oncol. 2016, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Niess, H.; Huss, R.; Huber, S.; von Luettichau, I.; Nelson, P.J.; Ott, H.C.; Jauch, K.W.; Bruns, C.J. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation 2009, 119, 281–289. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Takahashi, K.; Wood, J.; Zubair, A.; Patel, T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J. Extracell. Vesicles 2015, 4, 24900. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Liao, J.; Zhang, F.; Zhou, G. Donor genetic backgrounds contribute to the functional heterogeneity of stem cells and clinical outcomes. Stem Cells Transl. Med. 2020, 9, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.; van Ginkel, J.; Groen, N.; Hulsman, M.; Mentink, A.; Reinders, M.; van Blitterswijk, C.; de Boer, J. A mesenchymal stromal cell gene signature for donor age. PLoS ONE 2012, 7, e42908. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J.; Fernández-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells from Adipose Tissue. Stem Cells Transl. Med. 2016, 5, 464–475. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Awe, O.; Rao, R.C.; Kirby, P.A.; Hitchon, P.W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J. Neurosurg. Spine 2014, 21, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Ducret, M.; Fabre, H.; Farges, J.C.; Degoul, O.; Atzeni, G.; McGuckin, C.; Forraz, N.; Mallein-Gerin, F.; Perrier-Groult, E. Production of Human Dental Pulp Cells with a Medicinal Manufacturing Approach. J. Endod. 2015, 41, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.L.; Lucarelli, E.; Guidi, S.; Ferrari, M.; Alessandri, G.; De Girolamo, L.; Pessina, A.; Ferrero, I. Ex vivo expanded mesenchymal stromal cell minimal quality requirements for clinical application. Stem Cells Dev. 2015, 24, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Codinach, M.; Blanco, M.; Ortega, I.; Lloret, M.; Reales, L.; Coca, M.I.; Torrents, S.; Doral, M.; Oliver-Vila, I.; Requena-Montero, M.; et al. Design and validation of a consistent and reproducible manufacture process for the production of clinical-grade bone marrow-derived multipotent mesenchymal stromal cells. Cytotherapy 2016, 18, 1197–1208. [Google Scholar] [CrossRef]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.T.; Handorf, A.M.; Jeon, W.B.; Li, W.J. Stem cell-based tissue engineering approaches for musculoskeletal regeneration. Curr. Pharm. Des. 2013, 19, 3429–3445. [Google Scholar] [CrossRef]
- Narayanan, G.; Bhattacharjee, M.; Nair, L.S.; Laurencin, C.T. Musculoskeletal Tissue Regeneration: The Role of the Stem Cells. Regen. Eng. Transl. Med. 2017, 3, 133–165. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.B.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef]
- Caccia, D.; Dugo, M.; Callari, M.; Bongarzone, I. Bioinformatics tools for secretome analysis. Biochim. Biophys. Acta 2013, 1834, 2442–2453. [Google Scholar] [CrossRef] [PubMed]
- Cases-Perera, O.; Blanco-Elices, C.; Chato-Astrain, J.; Miranda-Fernández, C.; Campos, F.; Crespo, P.V.; Sánchez-Montesinos, I.; Alaminos, M.; Martín-Piedra, M.A.; Garzón, I. Development of secretome-based strategies to improve cell culture protocols in tissue engineering. Sci. Rep. 2022, 12, 10003. [Google Scholar] [CrossRef]
- Tran, C.; Damaser, M.S. Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 1–11. [Google Scholar] [CrossRef]
- García-García, Ó.D.; El Soury, M.; González-Quevedo, D.; Sánchez-Porras, D.; Chato-Astrain, J.; Campos, F.; Carriel, V. Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves. Int. J. Mol. Sci. 2021, 22, 674. [Google Scholar] [CrossRef]
- Irastorza-Lorenzo, A.; Sánchez-Porras, D.; Ortiz-Arrabal, O.; de Frutos, M.J.; Esteban, E.; Fernández, J.; Janer, A.; Campos, A.; Campos, F.; Alaminos, M. Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 1923. [Google Scholar] [CrossRef]
- Martin-Piedra, M.A.; Garzon, I.; Oliveira, A.C.; Alfonso-Rodriguez, C.A.; Carriel, V.; Scionti, G.; Alaminos, M. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy 2014, 16, 266–277. [Google Scholar] [CrossRef]
- Sánchez-Porras, D.; Durand-Herrera, D.; Paes, A.B.; Chato-Astrain, J.; Verplancke, R.; Vanfleteren, J.; Sánchez-López, J.D.; García-García, Ó.D.; Campos, F.; Carriel, V. Ex Vivo Generation and Characterization of Human Hyaline and Elastic Cartilaginous Microtissues for Tissue Engineering Applications. Biomedicines 2021, 9, 292. [Google Scholar] [CrossRef]
- Constantin, A.; Comarița, I.K.; Alexandru, N.; Filippi, A.; Bojin, F.; Gherghiceanu, M.; Vîlcu, A.; Nemecz, M.; Niculescu, L.S.; Păunescu, V.; et al. Stem cell-derived extracellular vesicles reduce the expression of molecules involved in cardiac hypertrophy-In a model of human-induced pluripotent stem cell-derived cardiomyocytes. Front. Pharmacol. 2022, 13, 1003684. [Google Scholar] [CrossRef] [PubMed]
- Silveira, B.M.; Ribeiro, T.O.; Freitas, R.S.; Carreira, A.C.O.; Gonçalves, M.S.; Sogayar, M.; Meyer, R.; Birbrair, A.; Fortuna, V. Secretome from human adipose-derived mesenchymal stem cells promotes blood vessel formation and pericyte coverage in experimental skin repair. PLoS ONE 2022, 17, e0277863. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Mohd Abdul Kader Jailani, M.S.; Badrul Hisham, M.A.I.; Sunthar Raj, N.; Shamsuddin, S.A.; Ng, M.H.; Fauzi, M.B.; Maarof, M. Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products. J. Tissue Eng. 2022, 13, 20417314221114273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Qu, X.; Liu, Y.; Sun, L.; Harada, A.; Hua, Y.; Sougawa, N.; Tabata, A.; Liu, L.; et al. Development of composite functional tissue sheets using hiPSC-CMs and hADSCs to improve the cardiac function after myocardial infarction. Bioact. Mater. 2024, 37, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Qu, X.; Liu, Y.; Harada, A.; Hua, Y.; Yoshida, N.; Ishida, M.; Tabata, A.; Sun, L.; et al. Development of a thick and functional human adipose-derived stem cell tissue sheet for myocardial infarction repair in rat hearts. Stem Cell Res. Ther. 2023, 14, 380. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, S.R.; Im, J.B.; Park, C.H.; Lee, H.Y.; Hong, I.S. 3D stem cell-laden artificial endometrium: Successful endometrial regeneration and pregnancy. Biofabrication 2021, 13, 045012. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 613787. [Google Scholar] [CrossRef]
- Rice, J.J.; Martino, M.M.; De Laporte, L.; Tortelli, F.; Briquez, P.S.; Hubbell, J.A. Engineering the regenerative microenvironment with biomaterials. Adv. Healthc. Mater. 2013, 2, 57–71. [Google Scholar] [CrossRef]
- Wang, C.Y.; Hong, P.D.; Wang, D.H.; Cherng, J.H.; Chang, S.J.; Liu, C.C.; Fang, T.J.; Wang, Y.W. Polymeric Gelatin Scaffolds Affect Mesenchymal Stem Cell Differentiation and Its Diverse Applications in Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 8632. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Caramella, C.; Catenacci, L.; Conti, B.; Dorati, R.; Ferrari, F.; Genta, I.; Modena, T.; Perteghella, S.; Rossi, S.; et al. Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field. Pharmaceutics 2021, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Netti, P.A. Review on Computer-Aided Design and Manufacturing of Drug Delivery Scaffolds for Cell Guidance and Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 682133. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue engineering: Current strategies and future directions. Chonnam Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alkaya, D.; Gurcan, C.; Kilic, P.; Yilmazer, A.; Gurman, G. Where is human-based cellular pharmaceutical R&D taking us in cartilage regeneration? 3 Biotech 2020, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Brunel, L.G.; Hollinshead, M.S.; Fernando, R.C.; Gardner, L.; Duncan, I.; Moffett, A.; Best, S.; Turco, M.Y.; Burton, G.J.; et al. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus 2020, 10, 20190079. [Google Scholar] [CrossRef] [PubMed]
- Peressotti, S.; Koehl, G.E.; Goding, J.A.; Green, R.A. Self-Assembling Hydrogel Structures for Neural Tissue Repair. ACS Biomater. Sci. Eng. 2021, 7, 4136–4163. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef]
- Pearce, A.K.; O’Reilly, R.K. Polymers for Biomedical Applications: The Importance of Hydrophobicity in Directing Biological Interactions and Application Efficacy. Biomacromolecules 2021, 22, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Barthes, J.; Özçelik, H.; Hindié, M.; Ndreu-Halili, A.; Hasan, A.; Vrana, N.E. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: The recent advances. BioMed Res. Int. 2014, 2014, 921905. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef] [PubMed]
- Antmen, E.; Vrana, N.E.; Hasirci, V. The role of biomaterials and scaffolds in immune responses in regenerative medicine: Macrophage phenotype modulation by biomaterial properties and scaffold architectures. Biomater. Sci. 2021, 9, 8090–8110. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, S.; Dou, W.; Jansen, J.A.; Walboomers, X.F. Magnetic Resonance Imaging of Hard Tissues and Hard Tissue Engineered Bio-substitutes. Mol. Imaging Biol. 2019, 21, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef]
- Campostrini, G.; Windt, L.M.; van Meer, B.J.; Bellin, M.; Mummery, C.L. Cardiac Tissues from Stem Cells: New Routes to Maturation and Cardiac Regeneration. Circ. Res. 2021, 128, 775–801. [Google Scholar] [CrossRef] [PubMed]
- Abbasgholizadeh, R.; Islas, J.F.; Navran, S.; Potaman, V.N.; Schwartz, R.J.; Birla, R.K. A Highly Conductive 3D Cardiac Patch Fabricated Using Cardiac Myocytes Reprogrammed from Human Adipogenic Mesenchymal Stem Cells. Cardiovasc. Eng. Technol. 2020, 11, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, E.G.; Mulligan, J.D.; Ertel, R.L.; Kouris, N.A.; Ogle, B.M.; Raval, A.N.; Saupe, K.W. Cardiac fibroblast-derived 3D extracellular matrix seeded with mesenchymal stem cells as a novel device to transfer cells to the ischemic myocardium. Cardiovasc. Eng. Technol. 2014, 5, 119–131. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Williams, D.F. There is no such thing as a biocompatible material. Biomaterials 2014, 35, 10009–10014. [Google Scholar] [CrossRef]
- Cosgrove, B.D.; Mui, K.L.; Driscoll, T.P.; Caliari, S.R.; Mehta, K.D.; Assoian, R.K.; Burdick, J.A.; Mauck, R.L. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 2016, 15, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Ezquerra, S.; Zuleta, A.; Arancibia, R.; Estay, J.; Aulestia, F.; Carrion, F. Functional Properties of Human-Derived Mesenchymal Stem Cell Spheroids: A Meta-Analysis and Systematic Review. Stem Cells Int. 2021, 2021, 8825332. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Li, A.; Zhu, D.; McDonald, H.; Inocencio, I.M.; Chambers, D.C.; Sinclair, K.; Fang, H.; Greening, D.W.; Frith, J.E.; et al. Effect of 2D and 3D Culture Microenvironments on Mesenchymal Stem Cell-Derived Extracellular Vesicles Potencies. Front. Cell Dev. Biol. 2022, 10, 819726. [Google Scholar] [CrossRef]
- Asahara, T.; Kalka, C.; Isner, J.M. Stem cell therapy and gene transfer for regeneration. Gene Ther. 2000, 7, 451–457. [Google Scholar] [CrossRef]
- Mitrecic, D.; Nicaise, C.; Klimaschewski, L.; Gajovic, S.; Bohl, D.; Pochet, R. Genetically modified stem cells for the treatment of neurological diseases. Front. Biosci. (Elite Ed.) 2012, 4, 1170–1181. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Wan Safwani, W.K. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 448, 218–224. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujita, M.; Tanaka, Y.; Kojima, I.; Kanatani, Y.; Ishihara, M.; Tachibana, S. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. Biores Open Access 2013, 2, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int. J. Mol. Sci. 2017, 18, 1320. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.H.; Kim, H.S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef]
- Kurogoushi, R.; Hasegawa, T.; Akazawa, Y.; Iwata, K.; Sugimoto, A.; Yamaguchi-Ueda, K.; Miyazaki, A.; Narwidina, A.; Kawarabayashi, K.; Kitamura, T.; et al. Fibroblast growth factor 2 suppresses the expression of C-C motif chemokine 11 through the c-Jun N-terminal kinase pathway in human dental pulp-derived mesenchymal stem cells. Exp. Ther. Med. 2021, 22, 1356. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Zuba-Surma, E.; Kucia, M.; Reca, R.; Wojakowski, W.; Ratajczak, J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 2006, 20, 1915–1924. [Google Scholar] [CrossRef]
- Wisel, S.; Khan, M.; Kuppusamy, M.L.; Mohan, I.K.; Chacko, S.M.; Rivera, B.K.; Sun, B.C.; Hideg, K.; Kuppusamy, P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J. Pharmacol. Exp. Ther. 2009, 329, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.; Lee, Y.J.; Jiang, S.; Ahmed, R.P.; Ryon, M.; Ashraf, M. Phosphodiesterase inhibition with tadalafil provides longer and sustained protection of stem cells. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1395–H1404. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, Y.J.; Dong, Q.T.; Qian, H.Y.; Gao, R.L.; Qiao, S.B.; Shen, R.; He, Z.X.; Lu, M.J.; Zhao, S.H.; et al. Atorvastatin enhance efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase. PLoS ONE 2013, 8, e65702. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, Y.; Hou, Q.; Zhao, Y.; Zhong, L.; Fu, X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: Current status and future prospects. Stem Cell Res. Ther. 2022, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Xu, Q.; Luo, R.; Gao, J.; Chen, H.; Deng, Y.; Li, Y.; Wang, Y.; Yuan, W.; Wu, X. Atorvastatin treatment improves effects of implanted mesenchymal stem cells: Meta-analysis of animal models with acute myocardial infarction. BMC Cardiovasc. Disord. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef]
- DelaRosa, O.; Lombardo, E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: Implications on therapeutic potential. Mediat. Inflamm. 2010, 2010, 865601. [Google Scholar] [CrossRef]
- Tran, T.; Cruz, C.; Chan, A.; Awad, S.; Rajasingh, J.; Deth, R.; Gurusamy, N. Mesenchymal Stem Cell-Derived Long Noncoding RNAs in Cardiac Injury and Repair. Cells 2023, 12, 2268. [Google Scholar] [CrossRef]
- Nisole, S.; Saïb, A. Early steps of retrovirus replicative cycle. Retrovirology 2004, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, J.E. An overview of retrovirus replication and classification. Adv. Vet. Sci. Comp. Med. 1988, 32, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Nitta, S.; Kusakari, Y.; Yamada, Y.; Kubo, T.; Neo, S.; Igarashi, H.; Hisasue, M. Conversion of mesenchymal stem cells into a canine hepatocyte-like cells by Foxa1 and Hnf4a. Regen. Ther. 2020, 14, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Subbramanian, R.A.; Cohen, E.A. Molecular biology of the human immunodeficiency virus accessory proteins. J. Virol. 1994, 68, 6831–6835. [Google Scholar] [CrossRef]
- Naldini, L.; Blömer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Santoni de Sio, F.R.; Gritti, A.; Cascio, P.; Neri, M.; Sampaolesi, M.; Galli, C.; Luban, J.; Naldini, L. Lentiviral vector gene transfer is limited by the proteasome at postentry steps in various types of stem cells. Stem Cells 2008, 26, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Guo, J.; Lin, G.; Hu, M.; Hu, Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand. Cardiovasc. J. 2008, 42, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Abdul Halim, N.S.; Fakiruddin, K.S.; Ali, S.A.; Yahaya, B.H. A comparative study of non-viral gene delivery techniques to human adipose-derived mesenchymal stem cell. Int. J. Mol. Sci. 2014, 15, 15044–15060. [Google Scholar] [CrossRef] [PubMed]
- Feril, L.B., Jr. Ultrasound-mediated gene transfection. Methods Mol. Biol. 2009, 542, 179–194. [Google Scholar] [CrossRef]
- Zhou, H.; He, Y.; Xiong, W.; Jing, S.; Duan, X.; Huang, Z.; Nahal, G.S.; Peng, Y.; Li, M.; Zhu, Y.; et al. MSC based gene delivery methods and strategies improve the therapeutic efficacy of neurological diseases. Bioact. Mater. 2023, 23, 409–437. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Yang, J.; Kong, X.; Zheng, F.; Guo, L.; Zhang, L.; Huang, Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur. J. Cardiothorac. Surg. 2009, 36, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Hnatiuk, A.P.; Ong, S.G.; Olea, F.D.; Locatelli, P.; Riegler, J.; Lee, W.H.; Jen, C.H.; De Lorenzi, A.; Giménez, C.S.; Laguens, R.; et al. Allogeneic Mesenchymal Stromal Cells Overexpressing Mutant Human Hypoxia-Inducible Factor 1-α (HIF1-α) in an Ovine Model of Acute Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003714. [Google Scholar] [CrossRef]
- Gómez-Mauricio, G.; Moscoso, I.; Martín-Cancho, M.F.; Crisóstomo, V.; Prat-Vidal, C.; Báez-Díaz, C.; Sánchez-Margallo, F.M.; Bernad, A. Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Res. Ther. 2016, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.N.; Souza, B.S.F.; Vasconcelos, J.F.; Azevedo, C.M.; Valim, C.X.R.; Paredes, B.D.; Rocha, V.P.C.; Carvalho, G.B.; Daltro, P.S.; Macambira, S.G.; et al. Granulocyte-Colony Stimulating Factor-Overexpressing Mesenchymal Stem Cells Exhibit Enhanced Immunomodulatory Actions through the Recruitment of Suppressor Cells in Experimental Chagas Disease Cardiomyopathy. Front. Immunol. 2018, 9, 1449. [Google Scholar] [CrossRef]
- Huang, F.; Zhu, X.; Hu, X.Q.; Fang, Z.F.; Tang, L.; Lu, X.L.; Zhou, S.H. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int. J. Mol. Med. 2013, 31, 484–492. [Google Scholar] [CrossRef]
- Raman, N.; Imran, S.A.M.; Ahmad Amin Noordin, K.B.; Wan Kamarul Zaman, W.S.; Nordin, F. Mechanotransduction of mesenchymal stem cells (MSCs) during cardiomyocytes differentiation. Heliyon 2022, 8, e11624. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chao, Y.C.; Chen, C.N.; Chien, S.; Chen, Y.C.; Chien, C.C.; Chiu, J.J.; Linju Yen, B. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J. Biomech. 2008, 41, 813–821. [Google Scholar] [CrossRef]
- Ravishankar, P.; Tandon, I.; Balachandran, K. Effect of Cyclic Uniaxial Mechanical Strain on Endothelial Progenitor Cell Differentiation. Cardiovasc. Eng. Technol. 2022, 13, 872–885. [Google Scholar] [CrossRef]
- Sivaraman, S.; Ravishankar, P.; Rao, R.R. Differentiation and Engineering of Human Stem Cells for Smooth Muscle Generation. Tissue Eng. Part. B Rev. 2023, 29, 1–9. [Google Scholar] [CrossRef]
- Bravo-Olín, J.; Martínez-Carreón, S.A.; Francisco-Solano, E.; Lara, A.R.; Beltran-Vargas, N.E. Analysis of the role of perfusion, mechanical, and electrical stimulation in bioreactors for cardiac tissue engineering. Bioprocess Biosyst. Eng. 2024. [Google Scholar] [CrossRef]
- Chouaib, B.; Haack-Sørensen, M.; Chaubron, F.; Cuisinier, F.; Collart-Dutilleul, P.Y. Towards the Standardization of Mesenchymal Stem Cell Secretome-Derived Product Manufacturing for Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 12594. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, B.G.; Rijo, P.; Gonçalo, T.S.; Reis, C.P. Good manufacturing practices for medicinal products for human use. J. Pharm. Bioallied Sci. 2015, 7, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.R.; Ng, K.S.; Lock, L.T.; Ahsan, T.; Rowley, J.A. Peak MSC-Are We There Yet? Front. Med. 2018, 5, 178. [Google Scholar] [CrossRef]
| Method | Feasibility | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Cell-Free Approach (Secretome) | The MSC secretome encompasses a range of bioactive factors, including growth factors, cytokines, and chemokines, which contribute to cell proliferation, migration, and tissue repair. | 1. Risk of immunological rejection is minimized; 2. Secretome offers a cell-free treatment option; 3. High compatibility with host tissues; 4. Ease of delivery; 5. Lower tumorigenic potential compared to cellular therapies. | Limited by the absence of standardized protocols for secretome preparation | [57,121,122,123,124,125,131] |
| Scaffold-based Therapeutics | Scaffold-based treatments have emerged as a notable breakthrough in tissue engineering, providing promising approaches for healing injured tissues and reinstating their structure and function. | Essential for regenerating a variety of tissues, providing structural support and a conducive environment for cell attachment and growth. | Potential for host versus graft rejection and suboptimal mechanical properties that may not withstand long-term stress | [135,136,137,138,139,140,141] |
| Three-Dimensional Ex Vivo Propagation | The aggregation of MSCs in a three-dimensional (3D) structure enhanced several biological characteristics, such as the ability to differentiate into multiple cell lineages, the production of therapeutic factors, and the ability to withstand ischemic conditions. | Enhances differentiation toward skeleton-related tissues and the production of therapeutic factors. | Challenges include replicating the ECM, maintaining uniform cell distribution, and ensuring high cell viability | [157,158,159,160,161] |
| Physiological and Pathological Microenvironment Activation | Exposing MSCs to varying oxygen tensions and inflammatory cytokines simulates physiological and pathological conditions, respectively. | Physiological activation maintains stemness under hypoxia; pathological activation enhances immunomodulation and tissue regeneration responses via cytokines and growth factors. | Precise environmental control is needed to simulate conditions effectively, posing operational challenges | [167,168,169,170,171,172,173,174,175,176,177] |
| Genetic Modification | Genetic modifications of MSCs for targeted repair are performed using both viral vectors and non-viral methods to enhance specificity and efficacy. | 1. High efficiency and stability in viral gene delivery; 2. Reduced inflammatory responses and fibrosis in disease models; 3. Boosted therapeutic effectiveness for infarcted hearts with miR-126. | Side effects from viral-mediated delivery and high costs | [184,185,186,187,191,192,193] |
| Mechanobiology-Mediated Differentiation | Shear stress and mechanical strains direct MSCs and EPCs toward an endothelial phenotype, enhancing endothelial markers and functions. | 1. Promotes endothelial-like functions, enhancing tubule formation and LDL uptake; 2. Guides stem cells toward endothelial lineages, enhancing migration and tubulogenesis, which are crucial for vascular tissue engineering. | Requires precise control over mechanical conditions to ensure effective differentiation and functional outcomes in ECs | [54,196,197,198,199,200] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Mishra, N.; Tran, T.; Kumar, M.; Vijayaraghavalu, S.; Gurusamy, N. Emerging Strategies in Mesenchymal Stem Cell-Based Cardiovascular Therapeutics. Cells 2024, 13, 855. https://doi.org/10.3390/cells13100855
Kumar R, Mishra N, Tran T, Kumar M, Vijayaraghavalu S, Gurusamy N. Emerging Strategies in Mesenchymal Stem Cell-Based Cardiovascular Therapeutics. Cells. 2024; 13(10):855. https://doi.org/10.3390/cells13100855
Chicago/Turabian StyleKumar, Rishabh, Nitin Mishra, Talan Tran, Munish Kumar, Sivakumar Vijayaraghavalu, and Narasimman Gurusamy. 2024. "Emerging Strategies in Mesenchymal Stem Cell-Based Cardiovascular Therapeutics" Cells 13, no. 10: 855. https://doi.org/10.3390/cells13100855
APA StyleKumar, R., Mishra, N., Tran, T., Kumar, M., Vijayaraghavalu, S., & Gurusamy, N. (2024). Emerging Strategies in Mesenchymal Stem Cell-Based Cardiovascular Therapeutics. Cells, 13(10), 855. https://doi.org/10.3390/cells13100855







