CPEB3 Maintains Developmental Competence of the Oocyte
Abstract
1. Introduction
2. Material and Methods
2.1. Oocyte and Embryo Isolation and Cultivation
2.2. Library Preparation and RNA-Sequencing
2.3. RNA Isolation and cDNA Synthesis
2.4. PCR and qPCR
2.5. PolyA Tail Length Assay
2.6. Transcription Assay
2.7. Western Blotting
2.8. Measurement of Overall Protein Synthesis
2.9. Motive Analysis
2.10. Statistical Analysis
3. Results
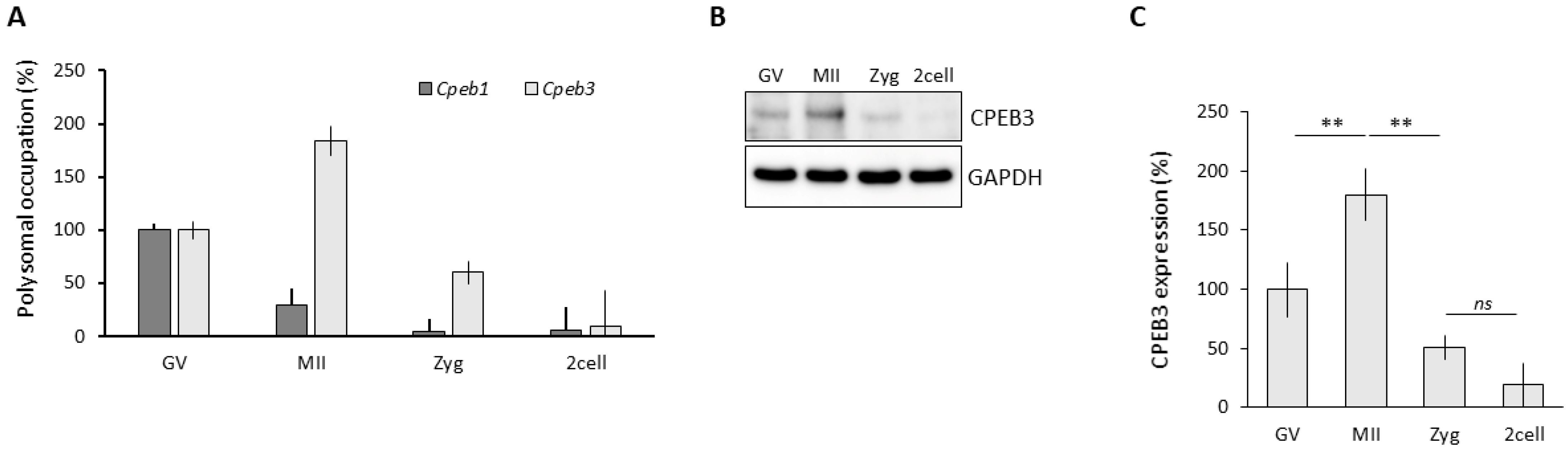
3.1. CPEB3 Is Actively Translated during Oocyte Maturation
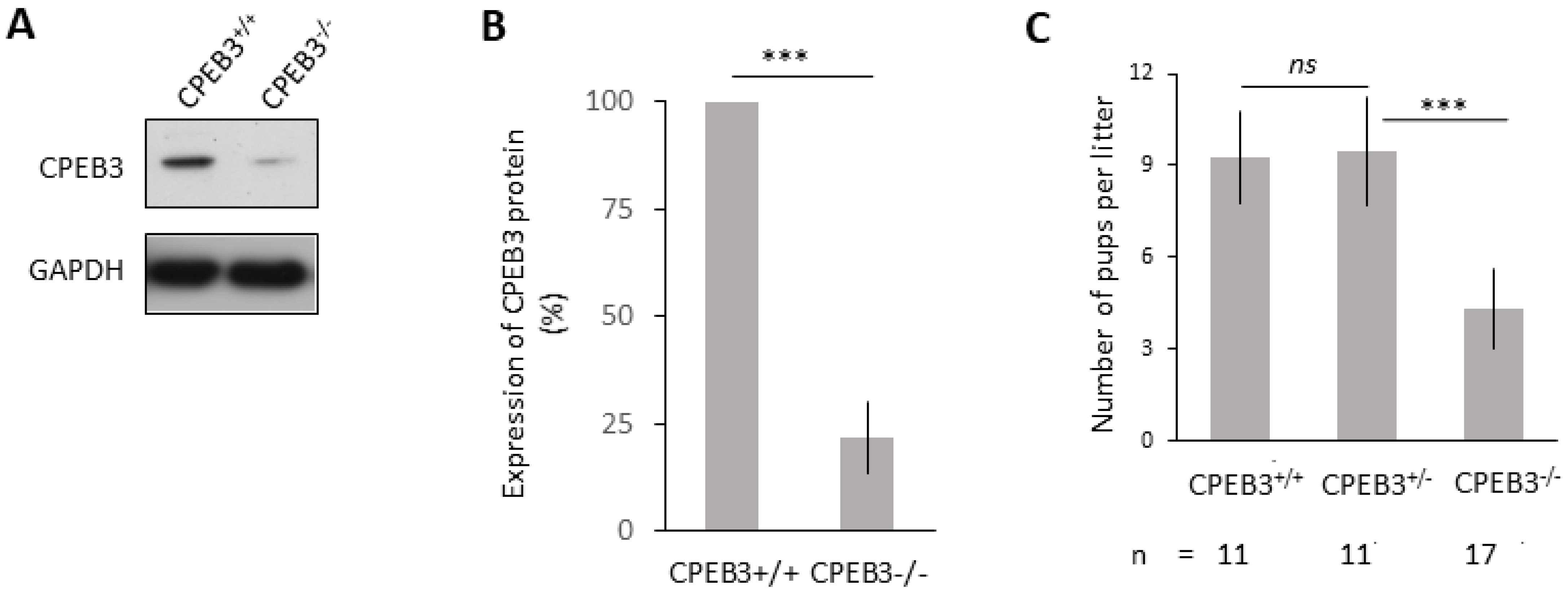
3.2. Significantly Decreased CPEB3 in the Oocytes, Leading to Subfertility
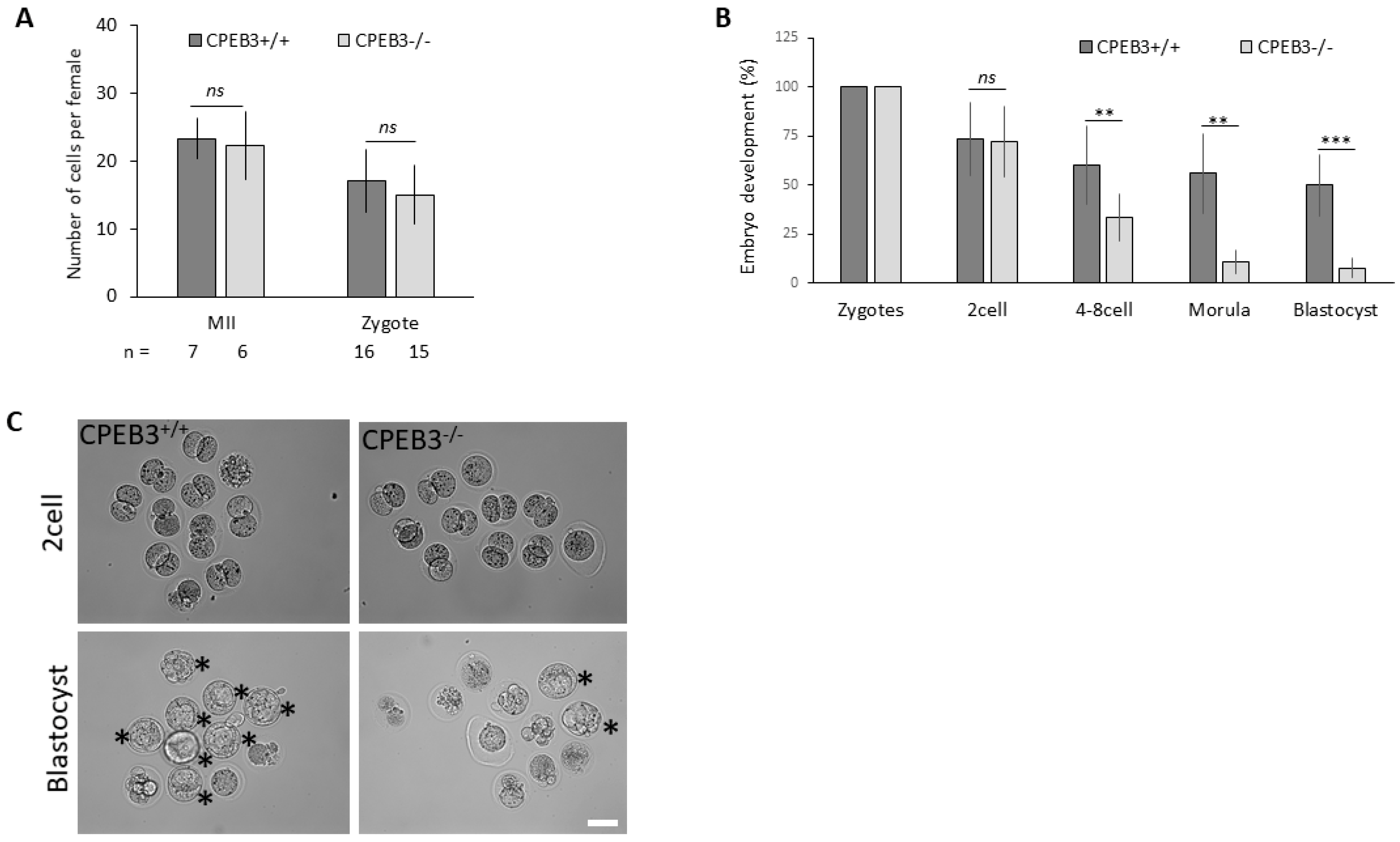
3.3. Oocytes with Downregulated CPEB3 Are Not Able to Sustain Preimplantation Embryo Development
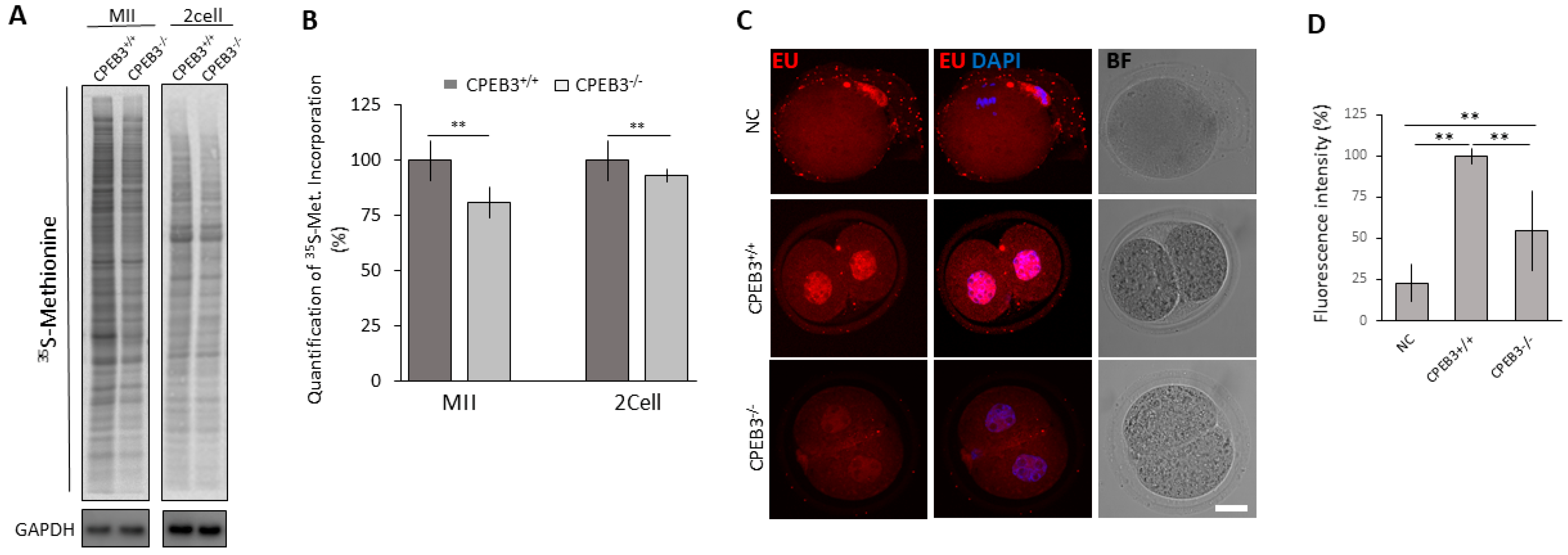
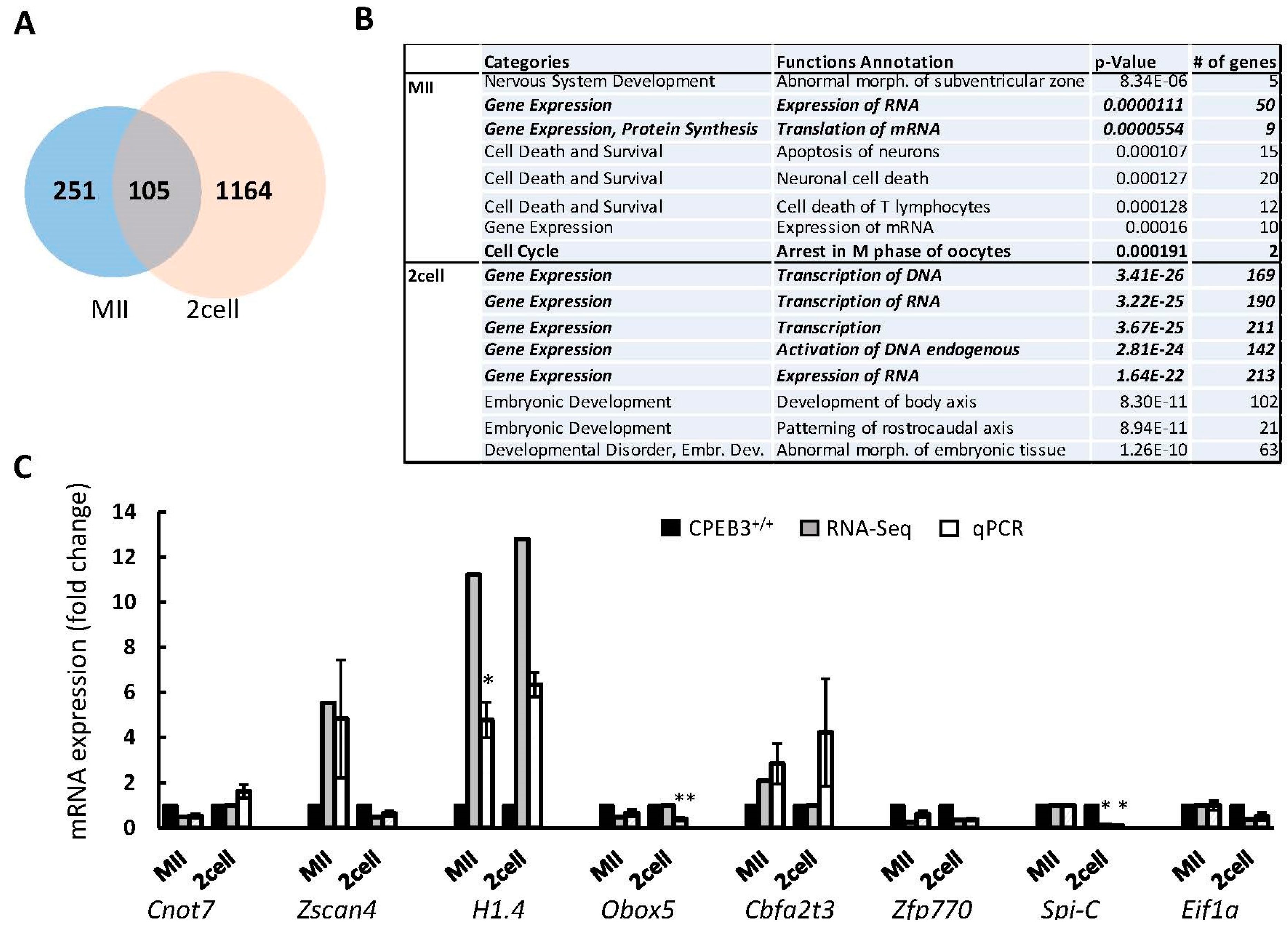
3.4. CPEB3 Depletion Results in Decreased Global Protein Synthesis and Transcriptional Activity of the 2cell Embryo
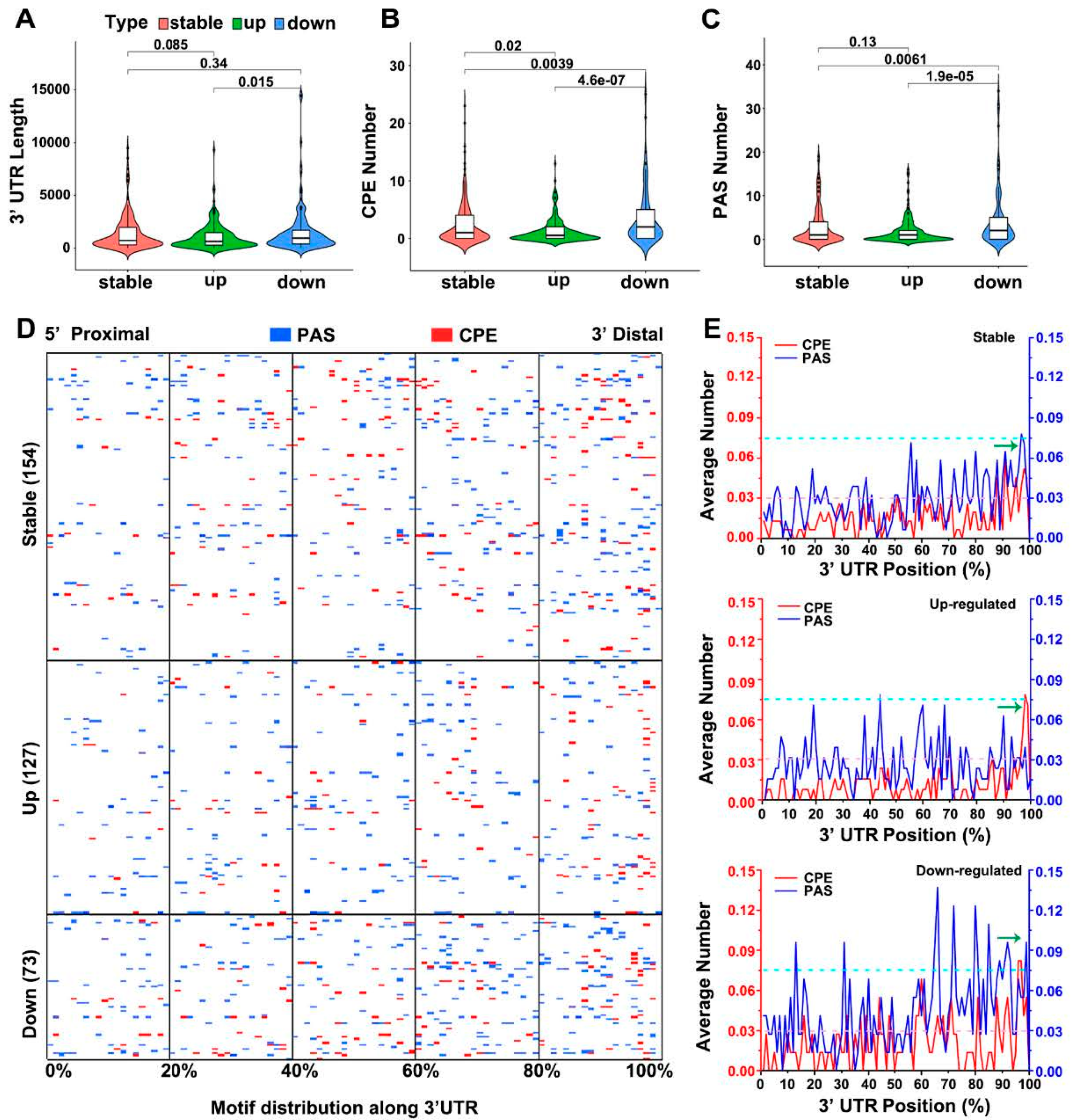
3.5. CPEB3 Absence Influences Translation of Specific mRNAs via Polyadenylation
3.6. Injection of CPEB3 Reduced the Expression of Candidate Proteins in cKO Oocytes to Wild-Type Levels
3.7. CPEB3 Regulates Translation of Specific mRNAs via 3′UTR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, M.H.; Tobler, K.J. Female Infertility. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK556033/ (accessed on 19 December 2022).
- Mtango, N.R.; Potireddy, S.; Latham, K.E. Oocyte Quality and Maternal Control of Development. Int. Rev. Cell Mol. Biol. 2008, 268, 223–290. [Google Scholar] [CrossRef] [PubMed]
- Pellestor, F.; Andréo, B.; Arnal, F.; Humeau, C.; Demaille, J. Maternal Aging and Chromosomal Abnormalities: New Data Drawn from in Vitro Unfertilized Human Oocytes. Hum. Genet. 2003, 112, 195–203. [Google Scholar] [CrossRef] [PubMed]
- De Vantéry, C.; Stutz, A.; Vassalli, J.D.; Schorderet-Slatkine, S. Acquisition of Meiotic Competence in Growing Mouse Oocytes Is Controlled at Both Translational and Posttranslational Levels. Dev. Biol. 1997, 187, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Navot, D.; Bergh, R.A.; Williams, M.A.; Garrisi, G.J.; Guzman, I.; Sandler, B.; Grunfeld, L. Poor Oocyte Quality Rather than Implantation Failure as a Cause of Age-Related Decline in Female Fertility. Lancet 1991, 337, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, R.; Viveiros, M.M.; Burns, K.H.; Adashi, E.Y.; Matzuk, M.M.; Eppig, J.J. Major Chromatin Remodeling in the Germinal Vesicle (GV) of Mammalian Oocytes Is Dispensable for Global Transcriptional Silencing but Required for Centromeric Heterochromatin Function. Dev. Biol. 2004, 275, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.J. Post-Transcriptional Control of Gene Expression During Mouse Oogenesis. Results Probl. Cell Differ. 2012, 55, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hake, L.E.; Richter, J.D. Translational Regulation of Maternal MRNA. Biochim. Biophys. Acta-Rev. Cancer 1997, 1332, M31–M38. [Google Scholar] [CrossRef]
- De Moor, C.H.; Meijer, H.; Lissenden, S. Mechanisms of Translational Control by the 3′ UTR in Development and Differentiation. Semin. Cell Dev. Biol. 2005, 16, 49–58. [Google Scholar] [CrossRef]
- Susor, A.; Jansova, D.; Cerna, R.; Danylevska, A.; Anger, M.; Toralova, T.; Malik, R.; Supolikova, J.; Cook, M.S.; Oh, J.S.; et al. Temporal and Spatial Regulation of Translation in the Mammalian Oocyte via the MTOR-EIF4F Pathway. Nat. Commun. 2015, 6, 6078. [Google Scholar] [CrossRef]
- Richter, J.D. Cytoplasmic Polyadenylation in Development and Beyond. Microbiol. Mol. Biol. Rev. 1999, 63, 446–456. [Google Scholar] [CrossRef]
- Sha, Q.Q.; Zhang, J.; Fan, H.Y. A Story of Birth and Death: MRNA Translation and Clearance at the Onset of Maternal-to-Zygotic Transition in Mammals. Biol. Reprod. 2019, 101, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, D.; Freese, P.; Alexis, M.S.; Su, A.; Hochman, M.; Palden, T.; Bazile, C.; Lambert, N.J.; Van Nostrand, E.L.; Pratt, G.A.; et al. Sequence, Structure, and Context Preferences of Human RNA Binding Proteins. Mol. Cell 2018, 70, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Castello, A.; Schwarzl, T.; Preiss, T. A Brave New World of RNA-Binding Proteins. Nat. Rev. Mol. Cell Biol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Susor, A.; Jansova, D.; Anger, M.; Kubelka, M. Translation in the Mammalian Oocyte in Space and Time. Cell Tissue Res. 2016, 363, 69–84. [Google Scholar] [CrossRef]
- Morgan, M.; Much, C.; Digiacomo, M.; Azzi, C.; Ivanova, I.; Vitsios, D.M.; Pistolic, J.; Collier, P.; Moreira, P.; Benes, V.; et al. mRNA 3′ Uridylation and Poly(A) Tail Length Sculpt the Mammalian Maternal Transcriptome. Nature 2017, 548, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, J.D.; Huarte, J.; Belin, D.; Gubler, P.; Vassalli, A.; O’Connell, M.L.; Parton, L.A.; Rickles, R.J.; Strickland, S. Regulated Polyadenylation Controls MRNA Translation during Meiotic Maturation of Mouse Oocytes. Genes Dev. 1989, 3, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Stebbins-Boaz, B.; Hake, L.E.; Richterl, J.D. CPEB Controls the Cytoplasmic Polyadenylation of Cyclin, Cdk2 and c-Mos MRNAs and Is Necessary for Oocyte Maturation in Xenopus. EMBO J. 1996, 15, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.M.; Ross, P.J. Cytoplasmic Polyadenylation in Mammalian Oocyte Maturation. Wiley Interdiscip. Rev. RNA 2016, 7, 71–89. [Google Scholar] [CrossRef]
- Wickens, M. In the Beginning Is the End: Regulation of Poly(A) Addition and Removal during Early Development. Trends Biochem. Sci. 1990, 15, 320–324. [Google Scholar] [CrossRef]
- Burns, D.M.; Richter, J.D. CPEB Regulation of Human Cellular Senescence, Energy Metabolism, and P53 MRNA Translation. Genes Dev. 2008, 22, 3449–3460. [Google Scholar] [CrossRef]
- Mcgrew, L.L.; Dworkin-Rastl, E.; Dworkin, M.B.; Richter, J.D. Poly(A) Elongation during Xenopus Oocyte Maturation Is Required for Translational Recruitment and Is Mediated by a Short Sequence Element. Genes Dev. 1989, 3, 803–815. [Google Scholar] [CrossRef]
- Yang, F.; Wang, W.; Cetinbas, M.; Sadreyev, R.I.; Blower, M.D. Genome-Wide Analysis Identifies Cis-Acting Elements Regulating MRNA Polyadenylation and Translation during Vertebrate Oocyte Maturation. RNA 2020, 26, 324–344. [Google Scholar] [CrossRef]
- Kim, J.H.; Richter, J.D. Opposing Polymerase-Deadenylase Activities Regulate Cytoplasmic Polyadenylation. Mol. Cell 2006, 24, 173–183. [Google Scholar] [CrossRef]
- Mendez, R.; Richter, J.D. Translational Control by CPEB: A Means to the End. Nat. Rev. Mol. Cell Biol. 2001, 2, 521–529. [Google Scholar] [CrossRef]
- Tay, J.; Hodgman, R.; Richter, J.D. The Control of Cyclin B1 MRNA Translation during Mouse Oocyte Maturation. Dev. Biol. 2000, 221, 1–9. [Google Scholar] [CrossRef]
- Komrskova, P.; Susor, A.; Malik, R.; Prochazkova, B.; Liskova, L.; Supolikova, J.; Hladky, S.; Kubelka, M. Aurora Kinase A Is Not Involved in CPEB1 Phosphorylation and Cyclin B1 MRNA Polyadenylation during Meiotic Maturation of Porcine Oocytes. PLoS ONE 2014, 9, 101222. [Google Scholar] [CrossRef] [PubMed]
- Theis, M.; Si, K.; Kandel, E.R. Two Previously Undescribed Members of the Mouse CPEB Family of Genes and Their Inducible Expression in the Principal Cell Layers of the Hippocampus. Proc. Natl. Acad. Sci. USA 2003, 100, 9602. [Google Scholar] [CrossRef] [PubMed]
- Jansova, D.; Tetkova, A.; Koncicka, M.; Kubelka, M.; Susor, A. Localization of RNA and Translation in the Mammalian Oocyte and Embryo. PLoS ONE 2018, 13, e0192544. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.R.; Jiang, J.C.; Fan, H.Y. Positive Feedback Stimulation of Ccnb1 and Mos MRNA Translation by MAPK Cascade during Mouse Oocyte Maturation. Front. Cell Dev. Biol. 2020, 8, 609430. [Google Scholar] [CrossRef]
- Chen, J.; Melton, C.; Suh, N.; Oh, J.S.; Horner, K.; Xie, F.; Sette, C.; Blelloch, R.; Conti, M. Genome-Wide Analysis of Translation Reveals a Critical Role for Deleted in Azoospermia-like (Dazl) at the Oocyte-to-Zygote Transition. Genes Dev. 2011, 25, 755–766. [Google Scholar] [CrossRef]
- Setoyama, D.; Yamashita, M.; Sagata, N. Mechanism of Degradation of CPEB during Xenopus Oocyte Maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 18001–18006. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, B.; Komrskova, P.; Kubelka, M. Molecular Sciences CPEB2 Is Necessary for Proper Porcine Meiotic Maturation and Embryonic Development. Int. J. Mol. Sci. 2018, 19, 3138. [Google Scholar] [CrossRef]
- Belloc, E.; Piqué, M.; Méndez, R. Sequential Waves of Polyadenylation and Deadenylation Define a Translation Circuit That Drives Meiotic Progression. Biochem. Soc. Trans. 2008, 36, 665–670. [Google Scholar] [CrossRef]
- Belloc, E.; Méndez, R. A Deadenylation Negative Feedback Mechanism Governs Meiotic Metaphase Arrest. Nature 2008, 452, 1017–1021. [Google Scholar] [CrossRef]
- Igea, A.; Méndez, R. Meiosis Requires a Translational Positive Loop Where CPEB1 Ensues Its Replacement by CPEB4. EMBO J. 2010, 29, 2182–2193. [Google Scholar] [CrossRef]
- Tsai, L.Y.; Chang, Y.W.; Lin, P.Y.; Chou, H.J.; Liu, T.J.; Lee, P.T.; Huang, W.H.; Tsou, Y.L.; Huang, Y.S. CPEB4 Knockout Mice Exhibit Normal Hippocampus-Related Synaptic Plasticity and Memory. PLoS ONE 2013, 8, 84978. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, H.; Wang, X.; Zhao, X.; Sun, L.; Cheng, Y.; Jiang, X.; Li, J.; Zhang, G. CPEB3, an RNA-Binding Protein, Modulates the Behavior of Endometriosis-Derived Stromal Cells via Regulating CXCL12. DNA Cell Biol. 2022, 41, 606–616. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.Z.; Fu, X.H.; Liu, J.B.; Peng, Y.X.; Wang, Y.J.; Han, D.X.; Zhang, Z.; Yuan, B.; Gao, Y.; et al. CPEB3 Regulates the Proliferation and Apoptosis of Bovine Cumulus Cells. Anim. Sci. J. 2020, 91, e13416. [Google Scholar] [CrossRef]
- E, F.; Zhang, H.; Yin, W.; Wang, C.; Liu, Y.; Li, Y.; Wang, L.; Wu, Y.; Zhang, R.; Zou, C.; et al. CPEB3 Deficiency in Mice Affect Ovarian Follicle Development and Causes Premature Ovarian Insufficiency. Cell Death Dis. 2021, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Boroviak, T.; Stirparo, G.G.; Dietmann, S.; Hernando-Herraez, I.; Mohammed, H.; Reik, W.; Smith, A.; Sasaki, E.; Nichols, J.; Bertone, P. Single Cell Transcriptome Analysis of Human, Marmoset and Mouse Embryos Reveals Common and Divergent Features of Preimplantation Development. Development 2018, 145, dev167833. [Google Scholar] [CrossRef]
- Potireddy, S.; Vassena, R.; Patel, B.G.; Latham, K.E. Analysis of Polysomal MRNA Populations of Mouse Oocytes and Zygotes: Dynamic Changes in Maternal MRNA Utilization and Function. Dev. Biol. 2006, 298, 155–166. [Google Scholar] [CrossRef]
- Tetkova, A.; Hancova, M. Mouse Oocyte Isolation, Cultivation and RNA Microinjection. Bio-Protocol 2016, 6, e1729. [Google Scholar] [CrossRef]
- Masek, T.; del Llano, E.; Gahurova, L.; Kubelka, M.; Susor, A.; Roucova, K.; Lin, C.-J.; Bruce, A.W.; Pospisek, M. Identifying the Translatome of Mouse NEBD-Stage Oocytes via SSP-Profiling; A Novel Polysome Fractionation Method. Int. J. Mol. Sci. 2020, 21, 1254. [Google Scholar] [CrossRef] [PubMed]
- Iyyappan, R.; Aleshkina, D.; Ming, H.; Dvoran, M.; Kakavand, K.; Anso Va, D.J.; Ar Del Llano, E.; Gahuro, V.A.L.; Br Uce, A.W.; Masek, T.; et al. The Translational Oscillation in Oocyte and Early Embryo Development. Nucleic Acids Res. 2023, 51, 12076–12091. [Google Scholar] [CrossRef] [PubMed]
- Sallés, F.J.; Strickland, S. Analysis of Poly(A) Tail Lengths by PCR: The PAT Assay. Methods Mol. Biol. 1999, 118, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Šušor, A.; Jelínková, L.; Karabínová, P.; Torner, H.; Tomek, W.; Kovářová, H.; Kubelka, M. Regulation of Cap-Dependent Translation Initiation in the Early Stage Porcine Parthenotes. Mol. Reprod. Dev. 2008, 75, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.Q.; Dai, X.X.; Dang, Y.; Tang, F.; Liu, J.; Zhang, Y.L.; Fan, H.Y. A MAPK Cascade Couples Maternal MRNA Translation and Degradation to Meiotic Cell Cycle Progression in Mouse Oocytes. Development 2017, 144, 452–463. [Google Scholar] [CrossRef]
- Lu, W.H.; Chao, H.W.; Lin, P.Y.; Lin, S.H.; Liu, T.H.; Chen, H.W.; Huang, Y.S. CPEB3-Dowregulated Nr3c1 MRNA Translation Confers Resilience to Developing Posttraumatic Stress Disorder-like Behavior in Fear-Conditioned Mice. Neuropsychopharmacology 2021, 46, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Kan, M.C.; Lin, C.L.; Richter, J.D. CPEB3 and CPEB4 in Neurons: Analysis of RNA-Binding Specificity and Translational Control of AMPA Receptor GluR2 MRNA. EMBO J. 2006, 25, 4865–4876. [Google Scholar] [CrossRef] [PubMed]
- Hervás, R.; del Carmen Fernández-Ramírez, M.; Galera-Prat, A.; Suzuki, M.; Nagai, Y.; Bruix, M.; Menéndez, M.; Laurents, D.V.; Carrión-Vázquez, M. Divergent CPEB Prion-like Domains Reveal Different Assembly Mechanisms for a Generic Amyloid-like Fold. BMC Biol. 2021, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Fioriti, L.; Myers, C.; Huang, Y.Y.; Li, X.; Stephan, J.S.; Trifilieff, P.; Colnaghi, L.; Kosmidis, S.; Drisaldi, B.; Pavlopoulos, E.; et al. The Persistence of Hippocampal-Based Memory Requires Protein Synthesis Mediated by the Prion-like Protein CPEB3. Neuron 2015, 86, 1433–1448. [Google Scholar] [CrossRef]
- Stephan, J.S.; Fioriti, L.; Lamba, N.; Colnaghi, L.; Karl, K.; Derkatch, I.L.; Kandel, E.R. The CPEB3 Protein Is a Functional Prion That Interacts with the Actin Cytoskeleton. Cell Rep. 2015, 11, 1772–1785. [Google Scholar] [CrossRef]
- Kageyama, S.I.; Liu, H.; Nagata, M.; Aoki, F. The Role of ETS Transcription Factors in Transcription and Development of Mouse Preimplantation Embryos. Biochem. Biophys. Res. Commun. 2006, 344, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Oikawa, M.; Kamimura, S.; Ogonuki, N.; Nakamura, T.; Nakano, T.; Abe, K.; Ogura, A. Trichostatin A Specifically Improves the Aberrant Expression of Transcription Factor Genes in Embryos Produced by Somatic Cell Nuclear Transfer. Sci. Rep. 2015, 5, 10127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Meng, T.G.; Li, A.; Schatten, H.; Wang, Z.B.; Sun, Q.Y. RNA-Seq Transcriptome Reveals Different Molecular Responses during Human and Mouse Oocyte Maturation and Fertilization. BMC Genom. 2020, 21, 475. [Google Scholar] [CrossRef]
- Luong, X.G.; Daldello, E.M.; Rajkovic, G.; Yang, C.R.; Conti, M. Genome-Wide Analysis Reveals a Switch in the Translational Program upon Oocyte Meiotic Resumption. Nucleic Acids Res. 2020, 48, 3257–3276. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulos, E.; Trifilieff, P.; Chevaleyre, V.; Fioriti, L.; Zairis, S.; Pagano, A.; Malleret, G.; Kandel, E.R. Neuralized1 Activates CPEB3: A Novel Function of Ubiquitination in Synaptic Plasticity and Memory Storage. Cell 2011, 147, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Jin, H.; Chen, B.P.; Liu, J.; Li, R.; Guo, W.; Tian, H. CPEB3 Regulates Neuron-Specific Alternative Splicing and Involves Neurogenesis Gene Expression. Aging (Albany N. Y.) 2020, 13, 2330–2347. [Google Scholar] [CrossRef] [PubMed]
- Falco, G.; Lee, S.L.; Stanghellini, I.; Bassey, U.C.; Hamatani, T.; Ko, M.S.H. Zscan4: A Novel Gene Expressed Exclusively in Late 2-Cell Embryos and Embryonic Stem Cells. Dev. Biol. 2007, 307, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.I.; Monti, M.; Akiyama, T.; Kimura, H.; Chikazawa-Nohtomi, N.; Sakota, M.; Sato, S.; Redi, C.A.; Ko, S.B.H.; Ko, M.S.H. Zscan4 Is Expressed Specifically during Late Meiotic Prophase in Both Spermatogenesis and Oogenesis. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 167–178. [Google Scholar] [CrossRef]
- Smith, R.; Susor, A.; Ming, H.; Tait, J.; Conti, M.; Jiang, Z.; Lin, C.-J. The H3.3 Chaperone Hira Complex Orchestrates Oocyte Developmental Competence. Development 2022, 149, dev200044. [Google Scholar] [CrossRef]
- Davis, J.N.; McGhee, L.; Meyers, S. The ETO (MTG8) Gene Family. Gene 2003, 303, 1–10. [Google Scholar] [CrossRef]
- Willcockson, M.A.; Healton, S.E.; Weiss, C.N.; Bartholdy, B.A.; Botbol, Y.; Mishra, L.N.; Sidhwani, D.S.; Wilson, T.J.; Pinto, H.B.; Maron, M.I.; et al. H1 Histones Control the Epigenetic Landscape by Local Chromatin Compaction. Nature 2021, 589, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, B.; Chen, H.; Yin, Q.; Liu, Y.; Xiang, Y.; Zhang, B.; Liu, B.; Wang, Q.; Xia, W.; et al. The Landscape of Accessible Chromatin in Mammalian Preimplantation Embryos. Nature 2016, 534, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.; Wilky, H.; Govindan, J.; Amodeo, A.A. Histone Concentration Regulates the Cell Cycle and Transcription in Early Development. Development 2019, 146, dev177402. [Google Scholar] [CrossRef]
- Pirngruber, J.; Johnsen, S.A. Induced G1 Cell-Cycle Arrest Controls Replication-Dependent Histone MRNA 3′ End Processing through P21, NPAT and CDK9. Oncogene 2010, 29, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.M.; Cunningham, C.H.; Welch, J.D.; Groh, B.; Guo, A.Y.; Wei, B.; Whitfield, M.L.; Xiong, Y.; Marzluff, W.F. A Subset of Replication-Dependent Histone MRNAs Are Expressed as Polyadenylated RNAs in Terminally Differentiated Tissues. Nucleic Acids Res. 2016, 44, 9190–9205. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Z.; Kageyama, S.I.; Aoki, F. Degradation of Maternal MRNA in Mouse Embryos: Selective Degradation of Specific MRNAs after Fertilization. Mol. Reprod. Dev. 2005, 72, 281–290. [Google Scholar] [CrossRef]
- Yu, C.; Ji, S.Y.; Sha, Q.Q.; Dang, Y.; Zhou, J.J.; Zhang, Y.L.; Liu, Y.; Wang, Z.W.; Hu, B.; Sun, Q.Y.; et al. BTG4 Is a Meiotic Cell Cycle–Coupled Maternal-Zygotic-Transition Licensing Factor in Oocytes. Nat. Struct. Mol. Biol. 2016, 23, 387–394. [Google Scholar] [CrossRef]
- Hosoda, N.; Funakoshi, Y.; Hirasawa, M.; Yamagishi, R.; Asano, Y.; Miyagawa, R.; Ogami, K.; Tsujimoto, M.; Hoshino, S.I. Anti-Proliferative Protein Tob Negatively Regulates CPEB3 Target by Recruiting Caf1 Deadenylase. EMBO J. 2011, 30, 13111323. [Google Scholar] [CrossRef]
- Jiang, J.C.; Zhang, H.; Cao, L.R.; Dai, X.X.; Zhao, L.W.; Liu, H.B.; Fan, H.Y. Oocyte Meiosis-Coupled Poly(A) Polymerase α Phosphorylation and Activation Trigger Maternal MRNA Translation in Mice. Nucleic Acids Res. 2021, 49, 5867–5880. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, J.Y.; Tian, B. Biased Alternative Polyadenylation in Human Tissues. Genome Biol. 2005, 6, R100. [Google Scholar] [CrossRef]
- Liu, D.; Brockman, J.M.; Dass, B.; Hutchins, L.N.; Singh, P.; McCarrey, J.R.; MacDonald, C.C.; Graber, J.H. Systematic Variation in MRNA 3’-Processing Signals during Mouse Spermatogenesis. Nucleic Acids Res. 2007, 35, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, L.; Shi, Q.; Zhao, G.; Wang, F. Comprehensive Analysis of APA Events and Their Association with Tumor Microenvironment in Lung Adenocarcinoma. Front. Genet. 2021, 12, 645360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, H.; Shao, F.; Zhang, Y.; Nie, H.; Zhang, J.; Li, C.; Hou, Z.; Chen, Z.J.; Wang, J.; et al. Remodeling of Maternal MRNA through Poly(A) Tail Orchestrates Human Oocyte-to-Embryo Transition. Nat. Struct. Mol. Biol. 2023, 30, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F.; Hara, K.T.; Schultz, R.M. Acquisition of Transcriptional Competence in the 1-Cell Mouse Embryo: Requirement for Recruitment of Maternal MRNAs. Mol. Reprod. Dev. 2003, 64, 270–274. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamacova, L.; Jansova, D.; Jiang, Z.; Dvoran, M.; Aleshkina, D.; Iyyappan, R.; Jindrova, A.; Fan, H.-Y.; Jiao, Y.; Susor, A. CPEB3 Maintains Developmental Competence of the Oocyte. Cells 2024, 13, 850. https://doi.org/10.3390/cells13100850
Lamacova L, Jansova D, Jiang Z, Dvoran M, Aleshkina D, Iyyappan R, Jindrova A, Fan H-Y, Jiao Y, Susor A. CPEB3 Maintains Developmental Competence of the Oocyte. Cells. 2024; 13(10):850. https://doi.org/10.3390/cells13100850
Chicago/Turabian StyleLamacova, Lucie, Denisa Jansova, Zongliang Jiang, Michal Dvoran, Daria Aleshkina, Rajan Iyyappan, Anna Jindrova, Heng-Yu Fan, Yuxuan Jiao, and Andrej Susor. 2024. "CPEB3 Maintains Developmental Competence of the Oocyte" Cells 13, no. 10: 850. https://doi.org/10.3390/cells13100850
APA StyleLamacova, L., Jansova, D., Jiang, Z., Dvoran, M., Aleshkina, D., Iyyappan, R., Jindrova, A., Fan, H.-Y., Jiao, Y., & Susor, A. (2024). CPEB3 Maintains Developmental Competence of the Oocyte. Cells, 13(10), 850. https://doi.org/10.3390/cells13100850






