The Role of FKBPs in Complex Disorders: Neuropsychiatric Diseases, Cancer, and Type 2 Diabetes Mellitus
Abstract
1. Introduction
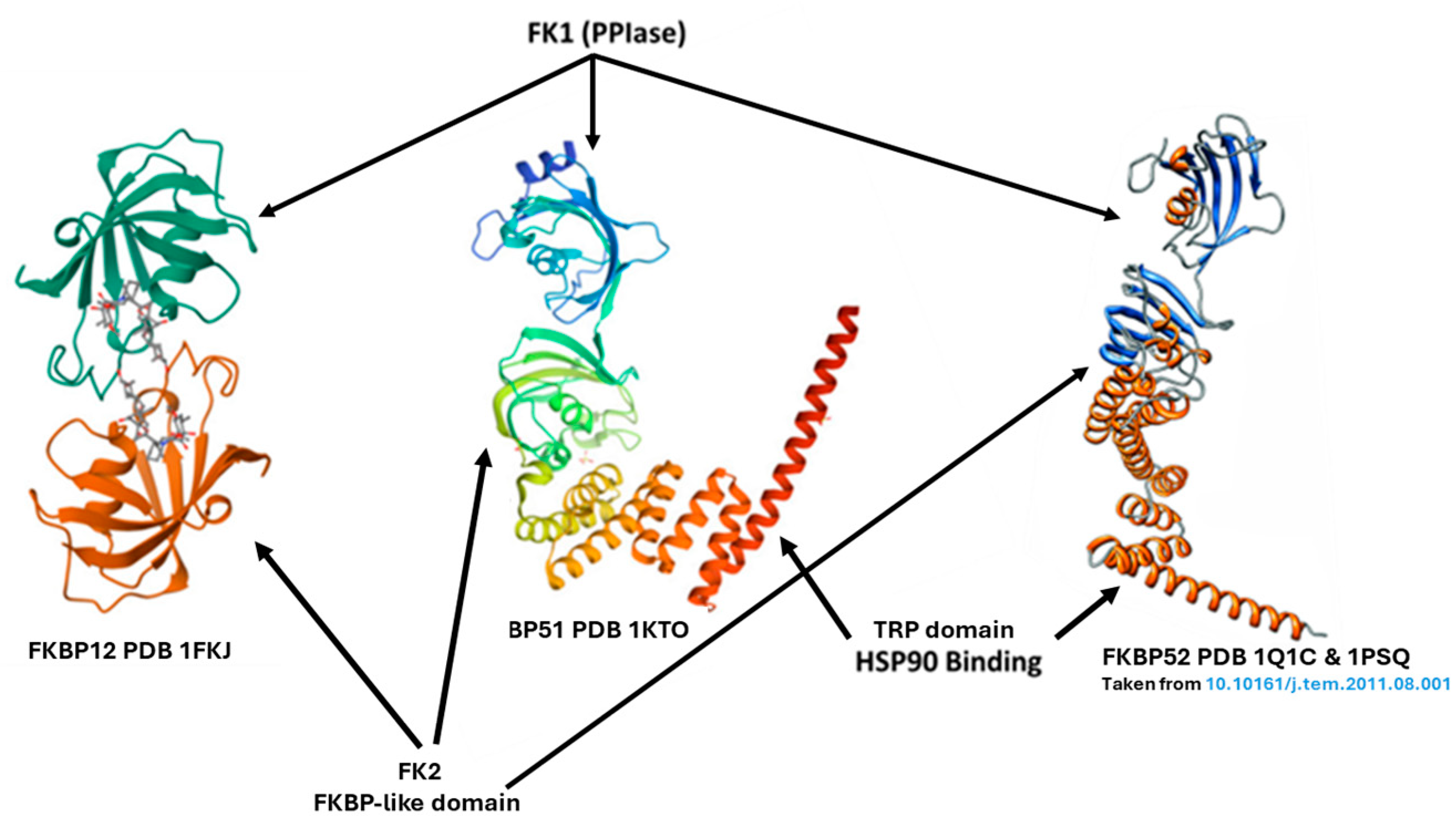
2. The FKBP5/FKBP4 Genes and Their Products FKBP51/52, Respectively
3. Complex Disorders, Stress, and FKBP5 and Its Products
3.1. Neuropsychiatric Diseases
3.2. Type 2 Diabetes Mellitus (T2DM)
3.3. Cancer
4. Methods
5. Involvement of FKBP5/FKBP51 in Neuropsychiatric Diseases
5.1. Neurological Disorders
5.1.1. Huntington’s Disease (HD)
5.1.2. Alzheimer’s Disease (AD)
5.1.3. Parkinson’s Disease (PD)
5.2. Mental Disorders
Genetics and Epigenetics of FKBP5
5.3. Involvement of FKBP5/FKBP51 in Cancer
5.3.1. The FKBP51–Hsp90–p23 Super-Chaperone Complex
5.3.2. FKBP51 Regulation of the NF-κB Pathway
5.3.3. FKBP51 as a Negative Regulator of the AKt Pathway
5.4. Involvement of FKBP5/FKBP51 in T2DM
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AKt | Serine/threonine protein kinase |
| AR | Androgen receptor |
| FKBP | FK-506 binding protein |
| GR | Glucocorticoid receptor |
| HD | Huntington’s disease |
| HPA | Hypothalamic–pituitary–adrenal |
| Hsp | Heat-shock protein |
| HTT | Huntingtin |
| KO | Knockout |
| mHTT | Mutant huntingtin |
| PD | Parkinson’s disease |
| PHLPP | PH domain and leucine-rich repeat protein phosphatase |
| PPARγ | Peroxisome proliferator-activated receptor-γ |
| PTSD | Post-traumatic stress disorder |
| SNP | Single nucleotide polymorphism |
| α-SYN | α-synuclein |
| Tau | Tubulin associated unit |
| T2DM | Type 2 diabetes mellitus |
| α-SYN | α-synuclein |
| Tau | Tubulin associated unit |
| T2DM | Type 2 diabetes mellitus |
References
- Harding, M.W.; Galat, A.; Uehling, D.E.; Schreiber, S.L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature 1989, 341, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Siekierka, J.J.; Hung, S.H.; Poe, M.; Lin, C.S.; Sigal, N.H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature 1989, 341, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Faber, L.E.; Toft, D.O. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J. Biol. Chem. 1990, 265, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Zgajnar, N.R.; De Leo, S.A.; Lotufo, C.M.; Erlejman, A.G.; Piwien-Pilipuk, G.; Galigniana, M.D. Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52. Biomolecules 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, P.; Liu, Y.; Lou, Z.; Ding, Y.; Shu, C.; Ye, S.; Bartlam, M.; Shen, B.; Rao, Z. 3D structure of human FK506-binding protein 52: Implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc. Natl. Acad. Sci. USA 2004, 101, 8348–8353. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Jiang, Y. FK506-Binding Proteins and Their Diverse Functions. Curr. Mol. Pharmacol. 2015, 9, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Storer, C.L.; Dickey, C.A.; Galigniana, M.D.; Rein, T.; Cox, M.B. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol. Metab. 2011, 22, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hahle, A.; Merz, S.; Meyners, C.; Hausch, F. The Many Faces of FKBP51. Biomolecules 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.I.; Lagadari, M.; Piwien-Pilipuk, G.; Galigniana, M.D. The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J. Biol. Chem. 2011, 286, 30152–30160. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Rein, T.; Binder, E.B.; Porter, J.T.; Koren, J., 3rd; Blair, L.J. Hsp90 and FKBP51: Complex regulators of psychiatric diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160532. [Google Scholar] [CrossRef]
- Zannas, A.S.; Jia, M.; Hafner, K.; Baumert, J.; Wiechmann, T.; Pape, J.C.; Arloth, J.; Ködel, M.; Martinelli, S.; Roitman, M.; et al. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-kappaB-driven inflammation and cardiovascular risk. Proc. Natl. Acad. Sci. USA 2019, 116, 11370–11379. [Google Scholar] [CrossRef] [PubMed]
- Kästle, M.; Kistler, B.; Lamla, T.; Bretschneider, T.; Lamb, D.; Nicklin, P.; Wyatt, D. FKBP51 modulates steroid sensitivity and NFkappaB signalling: A novel anti-inflammatory drug target. Eur. J. Immunol. 2018, 48, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Zannas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology 2016, 41, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.L.; Roberts, P.J.; Chirillo, S.C.; Cheung-Flynn, J.; Prapapanich, V.; Ratajczak, T.; Gaber, R.; Picard, D.; Smith, D.F. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003, 22, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.H.; Ning, Y.M.; Sanchez, E.R. A new first step in activation of steroid receptors: Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2002, 277, 4597–4600. [Google Scholar] [CrossRef] [PubMed]
- Wochnik, G.M.; Ruegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.I.; Ghini, A.A.; Piwien Pilipuk, G.; Galigniana, M.D. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry 2007, 46, 14044–14057. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D.; Erlejman, A.G.; Monte, M.; Gomez-Sanchez, C.; Piwien-Pilipuk, G. The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol. Cell Biol. 2010, 30, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D.; Radanyi, C.; Renoir, J.M.; Housley, P.R.; Pratt, W.B. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem. 2001, 276, 14884–14889. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J. What is complex about complex disorders? Genome Biol. 2012, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Huybrechts, I.; Michels, N. Psychosocial stress and cancer risk: A narrative review. Eur. J. Cancer Prev. 2022, 31, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.A.; Steptoe, A. Type 2 diabetes mellitus and psychological stress—A modifiable risk factor. Nat. Rev. Endocrinol. 2017, 13, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Yan, Z.; Popoli, M. The stressed synapse 2.0: Pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat. Rev. Neurosci. 2022, 23, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shelton, R.C.; Dwivedi, Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: A systematic review and meta-analysis. J. Affect. Disord. 2018, 225, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J.C. The concept of mental disorder: Diagnostic implications of the harmful dysfunction analysis. World Psychiatry 2007, 6, 149–156. [Google Scholar] [PubMed]
- Andrade, L.; Caraveo-Anduaga, J.J.; Berglund, P.; Bijl, R.; Kessler, R.C.; Demler, O.; Walters, E.; Kylyc, C.; Offord, D.; Ustun, T.B.; et al. Cross-national comparisons of the prevalences and correlates of mental disorders. WHO International Consortium in Psychiatric Epidemiology. Bull. World Health Organ. 2000, 78, 413–426. [Google Scholar]
- Baeuerle, P.A.; Baltimore, D. I kappa B: A specific inhibitor of the NF-kappa B transcription factor. Science 1988, 242, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S.; et al. Diabetes in older adults. Diabetes Care 2012, 35, 2650–2664. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Gassen, N.C.; Schmidt, U.; Rein, T. The FKBP51-Glucocorticoid Receptor Balance in Stress-Related Mental Disorders. Curr. Mol. Pharmacol. 2015, 9, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Hausl, A.S.; Balsevich, G.; Gassen, N.C.; Schmidt, M.V. Focus on FKBP51: A molecular link between stress and metabolic disorders. Mol. Metab. 2019, 29, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Balsevich, G.; Häusl, A.S.; Meyer, C.W.; Karamihalev, S.; Feng, X.; Pöhlmann, M.L.; Dournes, C.; Uribe-Marino, A.; Santarelli, S.; Labermaier, C.; et al. Stress-responsive FKBP51 regulates AKT2-AS160 signaling and metabolic function. Nat. Commun. 2017, 8, 1725. [Google Scholar] [CrossRef] [PubMed]
- Staibano, S.; Mascolo, M.; Ilardi, G.; Siano, M.; De Rosa, G. Immunohistochemical analysis of FKBP51 in human cancers. Curr. Opin. Pharmacol. 2011, 11, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bailus, B.J.; Scheeler, S.M.; Simons, J.; Sanchez, M.A.; Tshilenge, K.T.; Creus-Muncunill, J.; Naphade, S.; Lopez-Ramirez, A.; Zhang, N.; Lakshika Madushani, K.; et al. Modulating FKBP5/FKBP51 and autophagy lowers HTT (huntingtin) levels. Autophagy 2021, 17, 4119–4140. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.C.; Burgess, J.K.; Chen, J.H.; Thomas, J.H.; Schellenberg, G.D. Molecular pathways that influence human tau-induced pathology in Caenorhabditis elegans. Hum. Mol. Genet. 2006, 15, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Chambraud, B.; Sardin, E.; Giustiniani, J.; Dounane, O.; Schumacher, M.; Goedert, M.; Baulieu, E.E. A role for FKBP52 in Tau protein function. Proc. Natl. Acad. Sci. USA 2010, 107, 2658–2663. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Konsolaki, M. FKBP immunophilins and Alzheimer’s disease: A chaperoned affair. J. Biosci. 2011, 36, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Hiltunen, M.; Soininen, H. Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer’s disease. Prog. Neurobiol. 2011, 93, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Giustiniani, J.; Sineus, M.; Sardin, E.; Dounane, O.; Panchal, M.; Sazdovitch, V.; Duyckaerts, C.; Chambraud, B.; Baulieu, E.E. Decrease of the immunophilin FKBP52 accumulation in human brains of Alzheimer’s disease and FTDP-17. J. Alzheimer’s Dis. 2012, 29, 471–483. [Google Scholar] [CrossRef]
- Shelton, L.B.; Koren, J., 3rd; Blair, L.J. Imbalances in the Hsp90 Chaperone Machinery: Implications for Tauopathies. Front. Neurosci. 2017, 11, 724. [Google Scholar] [CrossRef]
- Jinwal, U.K.; Koren, J.; Borysov, S.I.; Schmid, A.B.; Abisambra, J.F.; Blair, L.J.; Johnson, A.G.; Jones, J.R.; Shults, C.L.; O’Leary, J.C.; et al. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J. Neurosci. 2010, 30, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.J.; Nordhues, B.A.; Hill, S.E.; Scaglione, K.M.; O’Leary, J.C.; Fontaine, S.N.; Breydo, L.; Zhang, B.; Li, P.; Wang, L.; et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Investig. 2013, 123, 4158–4169. [Google Scholar] [CrossRef] [PubMed]
- Giustiniani, J.; Chambraud, B.; Sardin, E.; Dounane, O.; Guillemeau, K.; Nakatani, H.; Paquet, D.; Kamah, A.; Landrieu, I.; Lippens, G.; et al. Immunophilin FKBP52 induces Tau-P301L filamentous assembly in vitro and modulates its activity in a model of tauopathy. Proc. Natl. Acad. Sci. USA 2014, 111, 4584–4589. [Google Scholar] [CrossRef] [PubMed]
- Giustiniani, J.; Guillemeau, K.; Dounane, O.; Sardin, E.; Huvent, I.; Schmitt, A.; Hamdane, M.; Buée, L.; Landrieu, I.; Lippens, G.; et al. The FK506-binding protein FKBP52 in vitro induces aggregation of truncated Tau forms with prion-like behavior. FASEB J. 2015, 29, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Gebru, N.T.; Blazier, D.M.; Gould, L.A.; Baker, J.D.; Beaulieu-Abdelahad, D.; Blair, L.J. Hsp90 co-chaperones, FKBP52 and Aha1, promote tau pathogenesis in aged wild-type mice. Acta Neuropathol. Commun. 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Kolos, J.M.; Voll, A.M.; Bauder, M.; Hausch, F. FKBP Ligands-Where We Are and Where to Go? Front. Pharmacol. 2018, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Sinars, C.R.; Cheung-Flynn, J.; Rimerman, R.A.; Scammell, J.G.; Smith, D.F.; Clardy, J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc. Natl. Acad. Sci. USA 2003, 100, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.W.E.; Samso, M. The FKBP12 subunit modifies the long-range allosterism of the ryanodine receptor. J. Struct. Biol. 2019, 205, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Shou, W.; Aghdasi, B.; Armstrong, D.L.; Guo, Q.; Bao, S.; Charng, M.J.; Mathews, L.M.; Schneider, M.D.; Hamilton, S.L.; Matzuk, M.M. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature 1998, 391, 489–492. [Google Scholar] [CrossRef]
- Deleersnijder, A.; Van Rompuy, A.S.; Desender, L.; Pottel, H.; Buée, L.; Debyser, Z.; Baekelandt, V.; Gerard, M. Comparative analysis of different peptidyl-prolyl isomerases reveals FK506-binding protein 12 as the most potent enhancer of alpha-synuclein aggregation. J. Biol. Chem. 2011, 286, 26687–26701. [Google Scholar] [CrossRef] [PubMed]
- Gerard, M.; Debyser, Z.; Desender, L.; Baert, J.; Brandt, I.; Baekelandt, V. Engelborghs YFK506 binding protein 12 differentially accelerates fibril formation of wild type alpha-synuclein its clinical mutants A30P or, A.5.3.T. J. Neurochem. 2008, 106, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Honjo, Y.; Ayaki, T.; Horibe, T.; Ito, H.; Takahashi, R.; Kawakami, K. FKBP12-immunopositive inclusions in patients with alpha-synucleinopathies. Brain Res. 2018, 1680, 39–45. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.C., 3rd; Zhang, B.; Koren, J., 3rd; Blair, L.; Dickey, C.A. The role of FKBP5 in mood disorders: Action of FKBP5 on steroid hormone receptors leads to questions about its evolutionary importance. CNS Neurol. Disord. Drug Targets. 2013, 12, 1157–1162. [Google Scholar] [PubMed]
- Mendonca, M.S.; Mangiavacchi, P.M.; Rios, A.F.L. Regulatory functions of FKBP5 intronic regions associated with psychiatric disorders. J. Psychiatr. Res. 2021, 143, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Keers, R.; Breen, G.; Coleman, J.R.; Jöhren, P.; Kepa, A.; Lester, K.J.; Margraf, J.; Scheider, S.; Teismann, T.; et al. DNA methylation of FKBP5 and response to exposure-based psychological therapy. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2019, 180, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Flasbeck, V.; Brune, M. Association between childhood maltreatment, psychopathology and DNA methylation of genes involved in stress regulation: Evidence from a study in Borderline Personality Disorder. PLoS ONE 2021, 16, e0248514. [Google Scholar] [CrossRef]
- Lai, W.; Li, W.; Du, X.; Guo, Y.; Wang, W.; Guo, L.; Lu, C. Association Between Childhood Maltreatment, FKBP5 Gene Methylation, and Anxiety Symptoms Among Chinese Adolescents: A Nested Case-Control Study. Front. Psychiatry 2022, 13, 761898. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.; Schwahn, C.; Mahler, J.; Schulz, A.; Spitzer, C.; Fenske, K.; Stender, J.; Barnow, S.; John, U.; Teumer, A.; et al. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology 2011, 36, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Diaz, Y.; González-Castro, T.B.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Perez-Hernandez, N.; Rodríguez-Pérez, J.M.; Genis-Mendoza, A.D. Association between FKBP5 polymorphisms and depressive disorders or suicidal behavior: A systematic review and meta-analysis study. Psychiatry Res. 2019, 271, 658–668. [Google Scholar] [CrossRef]
- Saito, T.; Shinozaki, G.; Koga, M.; Tanichi, M.; Takeshita, S.; Nakagawa, R.; Nagamine, M.; Cho, H.R.; Morimoto, Y.; Kobayashi, Y.; et al. Effect of interaction between a specific subtype of child abuse and the FKBP5 rs1360780 SNP on DNA methylation among patients with bipolar disorder. J. Affect. Disord. 2020, 272, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal-Narváez, P.; Sheinbaum, T.; Rosa, A.; Ballespí, S.; de Castro-Catala, M.; Peña, E.; Kwapil, T.R.; Barrantes-Vidal, N. The Interaction between Childhood Bullying and the FKBP5 Gene on Psychotic-Like Experiences and Stress Reactivity in Real Life. PLoS ONE 2016, 11, e0158809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheerin, C.M.; Lind, M.J.; Bountress, K.E.; Marraccini, M.E.; Amstadter, A.B.; Bacanu, S.A.; Nugent, N.R. Meta-Analysis of Associations Between Hypothalamic-Pituitary-Adrenal Axis Genes and Risk of Posttraumatic Stress Disorder. J. Trauma. Stress 2020, 33, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Yao, Y.; Ryan, J.; Li, T.; Wang, D.; Zheng, C.; Xu, Y.; Xu, Q. Common variants in FKBP5 gene and major depressive disorder (MDD) susceptibility: A comprehensive meta-analysis. Sci. Rep. 2016, 6, 32687. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, C.; Bierer, L.M.; Flory, J.D.; Gautam, A.; Bader, H.N.; Lehrner, A.; Makotkine, I.; Desarnaud, F.; Miller, S.A.; et al. Longitudinal genome-wide methylation study of PTSD treatment using prolonged exposure and hydrocortisone. Transl. Psychiatry. 2021, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Wang, F.; Feng, X.L.; Li, W.F.; Tao, J.H.; Pan, F.M.; Huang, F.; Su, H. Meta-analysis of FKBP5 gene polymorphisms association with treatment response in patients with mood disorders. Neurosci. Lett. 2010, 484, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Großmann, N.L.; Weihs, A.; Kühn, L.; Sauer, S.; Röh, S.; Wiechmann, T.; Rex-Haffner, M.; Völzke, H.; Völker, U.; Binder, E.B.; et al. Methylation Patterns of the FKBP5 Gene in Association with Childhood Maltreatment and Depressive Disorders. Int. J. Mol. Sci. 2024, 25, 1485. [Google Scholar] [CrossRef] [PubMed]
- Schiele, M.A.; Gottschalk, M.G.; Domschke, K. The applied implications of epigenetics in anxiety, affective and stress-related disorders—A review and synthesis on psychosocial stress, psychotherapy and prevention. Clin. Psychol. Rev. 2020, 77, 101830. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Richter, J.; Mahr, M.; Gajewska, A.; Schiele, M.A.; Gehrmann, A.; Schmidt, B.; Lesch, K.P.; Lang, T.; Helbig-Lang, S.; et al. MAOA gene hypomethylation in panic disorder-reversibility of an epigenetic risk pattern by psychotherapy. Transl. Psychiatry 2016, 6, e773. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, R. The role of epigenetics for understanding mental health difficulties and its implications for psychotherapy research. Psychol. Psychother. 2019, 92, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Niesink, R.J.; van Laar, M.W. Does Cannabidiol Protect Against Adverse Psychological Effects of THC? Front Psychiatry 2013, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xie, Z.; Chen, L.; Peng, X.; Luan, F.; Hu, J.; Xie, H.; Liu, R.; Zeng, N. Rosmarinic acid alleviate CORT-induced depressive-like behavior by promoting neurogenesis and regulating BDNF/TrkB/PI3K signaling axis. Biomed. Pharmacother. 2024, 170, 115994. [Google Scholar] [CrossRef]
- Li, L.; Lou, Z.; Wang, L. The role of FKBP5 in cancer aetiology and chemoresistance. Br. J. Cancer 2011, 104, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, H.; Kauhanen, M.; Paakinaho, V.; Jaaskelainen, T.; Palvimo, J.J. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic Acids Res. 2009, 37, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Febbo, P.G.; Lowenberg, M.; Thorner, A.R.; Brown, M.; Loda, M.; Golub, T.R. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J. Urol. 2005, 173, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Jubb, A.W.; Boyle, S.; Hume, D.A.; Bickmore, W.A. Glucocorticoid Receptor Binding Induces Rapid and Prolonged Large-Scale Chromatin Decompaction at Multiple Target Loci. Cell Rep. 2017, 21, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yang, C.S.; Gioeli, D.; Frierson, H.; Toft, D.O.; Paschal, B.M. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol. Cell Biol. 2010, 30, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Alahdab, F.; Amit, A.M.L.; Bärnighausen, T.W.; Beghi, E.; Beheshti, M.; Chavan, P.P.; Criqui, M.H.; Desai, R.; et al. Burden of Neurological Disorders Across the US From 1990–2017: A Global Burden of Disease Study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar] [PubMed]
- Avellino, R.; Romano, S.; Parasole, R.; Bisogni, R.; Lamberti, A.; Poggi, V.; Venuta, S.; Romano, M.F. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood 2005, 106, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Grădinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, L.; Fridley, B.L.; Jenkins, G.D.; Kalari, K.R.; Lingle, W.; Petersen, G.; Lou, Z.; Wang, L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 2009, 16, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Mu, J.; Kim, J.K.; Thorvaldsen, J.L.; Chu, Q.; Crenshaw, E.B., III.; Kaestner, K.H.; Bartolomei, M.S.; Shulman, G.I.; Birnbaum, M.J. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 2001, 292, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Mukhopadhyay, A.; Narasimhan, S.D.; Tesz, G.; Czech, M.P.; Tissenbaum, H.A. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell 2009, 136, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Brognard, J.; Newton, A.C. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol. Metab. 2008, 19, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Pietrocola, F.; Guilbaud, E.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostini, M.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 2023, 30, 1097–1154. [Google Scholar] [PubMed]
- Sidibeh, C.O.; Pereira, M.J.; Abalo, X.M.; JBoersma, G.; Skrtic, S.; Lundkvist, P.; Katsogiannos, P.; Hausch, F.; Castillejo-López, C.; Eriksson, J.W. FKBP5 expression in human adipose tissue: Potential role in glucose and lipid metabolism, adipogenesis and type 2 diabetes. Endocrine 2018, 62, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, C.; Lambadiari, V.; Maratou, E.; Geromeriati, C.; Artemiadis, A.; Dimitriadis, G.; Moutsatsou, P. Insufficient glucocorticoid receptor signaling and flattened salivary cortisol profile are associated with metabolic and inflammatory indices in type 2 diabetes. J. Endocrinol. Investig. 2021, 44, 37–48. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, J.; Smit, E.; Mohren, R.; Boekschoten, M.V.; de Groot, P.; van den Berg, S.A.; Bijland, S.; Voshol, P.J.; van Dijk, K.W.; de Wit, N.W.; et al. An 8-week high-fat diet induces obesity and insulin resistance with small changes in the muscle transcriptome of C57BL/6J mice. J. Nutrigenet Nutr. 2009, 2, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Willmer, T.; Goedecke, J.H.; Dias, S.; Louw, J.; Pheiffer, C. DNA methylation of FKBP5 in South African women: Associations with obesity and insulin resistance. Clin. Epigenetics 2020, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Joseph, J.J.; Lee, R.; Wand, G.S.; Golden, S.H. Type 2 diabetes and cardiometabolic risk may be associated with increase in DNA methylation of FKBP5. Clin. Epigenetics 2018, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D.; Stechschulte, L.A.; Elkhairi, F.; Sanchez, E.R. Analysis of FK506, timcodar (VX-853) and FKBP51 and FKBP52 chaperones in control of glucocorticoid receptor activity and phosphorylation. Pharmacol. Res. Perspect. 2014, 2, e00076. [Google Scholar] [CrossRef] [PubMed]
- Stechschulte, L.A.; Qiu, B.; Warrier, M.; Hinds, T.D., Jr.; Zhang, M.; Gu, H.; Xu, Y.; Khuder, S.S.; Russo, L.; Najjar, S.M.; et al. FKBP51 Null Mice Are Resistant to Diet-Induced Obesity and the PPARgamma Agonist Rosiglitazone. Endocrinology 2016, 157, 3888–3900. [Google Scholar] [CrossRef] [PubMed]
- Smedlund, K.B.; Sanchez, E.R.; Hinds, T.D., Jr. FKBP51 and the molecular chaperoning of metabolism. Trends Endocrinol. Metab. 2021, 32, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.; Lahti, J.; Kajantie, E.; Eriksson, J.G.; Raikkonen, K. Early Life Stress, FKBP5 Polymorphisms, and Quantitative Glycemic Traits. Psychosom. Med. 2017, 79, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, M.; Shekouh, D.; Safavinia, M.E.; Shiralipour, S.; Jalouli, M.; Mortezanejad, S.; Azarpira, N.; Ebrahimi, N.D. Role of FKBP5 and its genetic mutations in stress-induced psychiatric disorders: An opportunity for drug discovery. Front. Psychiatry 2023, 14, 1182345. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Wagner, K.V.; Liebl, C.; Scharf, S.H.; Wang, X.D.; Wolf, M.; Hausch, F.; Rein, T.; Schmidt, U.; Touma, C.; et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 2012, 62, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Codagnone, M.G.; Kara, N.; Ratsika, A.; Levone, B.R.; van de Wouw, M.; Tan, L.A.; Cunningham, J.I.; Sanchez, C.; Cryan, J.F.; O’Leary, O.F. Inhibition of FKBP51 induces stress resilience and alters hippocampal neurogenesis. Mol. Psychiatry 2022, 27, 4928–4938. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Tamashiro, K.L.; Yang, X.; Purcell, R.H.; Harvey, A.; Willour, V.L.; Huo, Y.; Rongione, M.; Wand, G.S.; Potash, J.B. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology 2010, 151, 4332–4343. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, N.; Roeh, S.; Sotillos Elliott, L.; Chang, S.; Loganathan, S.; Urbina-Treviño, L.; Fröhlich, A.S.; Sauer, S.; Ködel, M.; Matosin, N.; et al. DNA methylation patterns of FKBP5 regulatory regions in brain and blood of humanized mice and humans. Mol. Psychiatry 2024. [Google Scholar] [CrossRef]
- Klinger-König, J.; Hertel, J.; Van der Auwera, S.; Frenzel, S.; Pfeiffer, L.; Waldenberger, M.; Golchert, J.; Teumer, A.; Nauck, M.; Homuth, G.; et al. Methylation of the FKBP5 gene in association with FKBP5 genotypes, childhood maltreatment and depression. Neuropsychopharmacology 2019, 44, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Su, P.; Lai, T.K.; Jiang, A.; Liu, J.; Zhai, D.; Campbell, C.T.; Lee, F.H.; Yong, W. The glucocorticoid receptor-FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. J. Clin. Investig. 2020, 130, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Buffa, V.; Knaup, F.H.; Heymann, T.; Springer, M.; Schmidt, M.V.; Hausch, F. Analysis of the Selective Antagonist SAFit2 as a Chemical Probe for the FK506-Binding Protein 51. ACS Pharmacol Transl Sci. 2023, 6, 361–371. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.T.; Iwai, A.; Feau, C.; Garcia, Y.; Balsiger, H.A.; Storer, C.L.; Suro, R.M.; Garza, K.M.; Lee, S.; Sang Kim, Y.; et al. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 11878–11883. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Habara, M.; Kawaguchi, M.; Matsumoto, H.; Hanaki, S.; Masaki, T.; Sato, Y.; Matsuyama, H.; Kunieda, K.; Nakagawa, H.; et al. FKBP51 and FKBP52 regulate androgen receptor dimerization and proliferation in prostate cancer cells. Mol. Oncol. 2022, 16, 940–956. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Warrier, M.; Tillekeratne, M.P.; Shou, W.; Sanchez, E.R. The immunophilin ligands cyclosporin A and FK506 suppress prostate cancer cell growth by androgen receptor-dependent and -independent mechanisms. Endocrinology 2007, 148, 4716–4726. [Google Scholar] [CrossRef]
- Voll, A.M.; Meyners, C.; Taubert, M.C.; Bajaj, T.; Heymann, T.; Merz, S.; Charalampidou, A.; Kolos, J.; Purder, P.L.; Geiger, T.M.; et al. Macrocyclic FKBP51 Ligands Define a Transient Binding Mode with Enhanced Selectivity. Angew. Chem. Int. Ed. Engl. 2021, 60, 13257–13263. [Google Scholar] [CrossRef]
 A mouse model of PD.
A mouse model of PD.  mRNA.
mRNA.  DNA.
DNA.  Fast proliferating cells (cancer).
Fast proliferating cells (cancer). 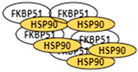 Over-expression of the chaperone complex consisting of FKBP51 and HSP90.
Over-expression of the chaperone complex consisting of FKBP51 and HSP90.  Inhibition.
Inhibition. 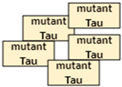 Aggregation of mutant Tau.
Aggregation of mutant Tau.
 A mouse model of PD.
A mouse model of PD.  mRNA.
mRNA.  DNA.
DNA.  Fast proliferating cells (cancer).
Fast proliferating cells (cancer).  Over-expression of the chaperone complex consisting of FKBP51 and HSP90.
Over-expression of the chaperone complex consisting of FKBP51 and HSP90.  Inhibition.
Inhibition.  Aggregation of mutant Tau.
Aggregation of mutant Tau.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agam, G.; Atawna, B.; Damri, O.; Azab, A.N. The Role of FKBPs in Complex Disorders: Neuropsychiatric Diseases, Cancer, and Type 2 Diabetes Mellitus. Cells 2024, 13, 801. https://doi.org/10.3390/cells13100801
Agam G, Atawna B, Damri O, Azab AN. The Role of FKBPs in Complex Disorders: Neuropsychiatric Diseases, Cancer, and Type 2 Diabetes Mellitus. Cells. 2024; 13(10):801. https://doi.org/10.3390/cells13100801
Chicago/Turabian StyleAgam, Galila, Bayan Atawna, Odeya Damri, and Abed N. Azab. 2024. "The Role of FKBPs in Complex Disorders: Neuropsychiatric Diseases, Cancer, and Type 2 Diabetes Mellitus" Cells 13, no. 10: 801. https://doi.org/10.3390/cells13100801
APA StyleAgam, G., Atawna, B., Damri, O., & Azab, A. N. (2024). The Role of FKBPs in Complex Disorders: Neuropsychiatric Diseases, Cancer, and Type 2 Diabetes Mellitus. Cells, 13(10), 801. https://doi.org/10.3390/cells13100801






