Nutrient-Response Pathways in Healthspan and Lifespan Regulation
Abstract
1. Introduction
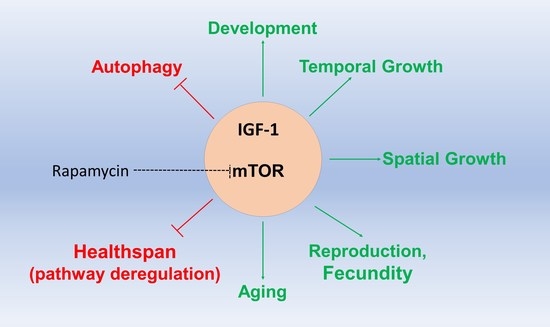
2. The Growth Hormone-Insulin Growth Factor Signaling in Lifespan and Healthspan
2.1. Inhibiting IGF-1 for Increased Health- and Lifespan
2.2. Dietary Modulation of IGF-1 Signaling
2.3. Boosting IGF-1 towards Increasing Healthspan
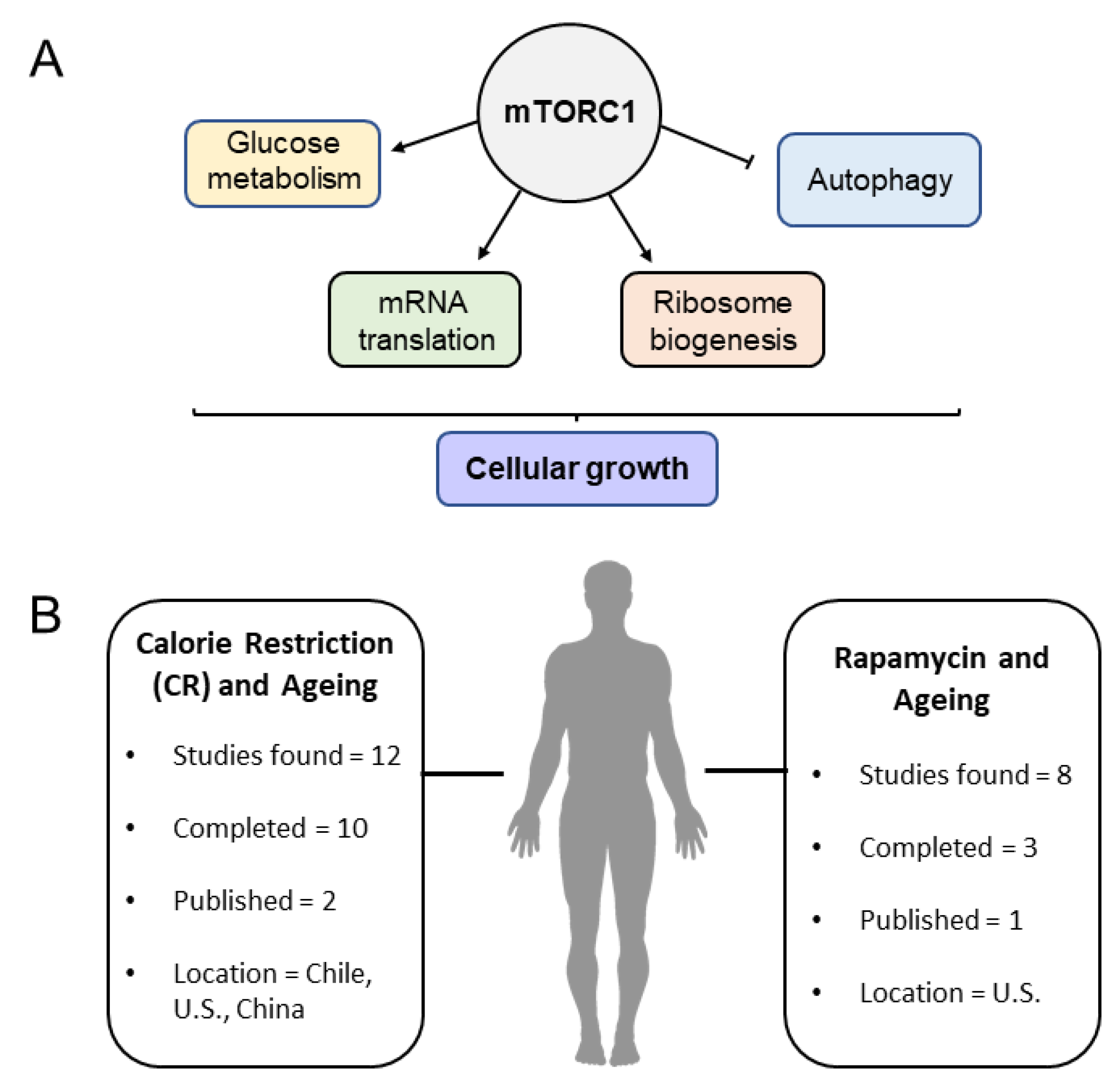
3. The Mechanistic Target of Rapamycin (mTOR) Signaling Pathway in Lifespan and Healthspan Regulation
3.1. Amino Acid Sensing and mTOR Activity
3.2. Rapamycin: Lifespan and Healthspan Regulation
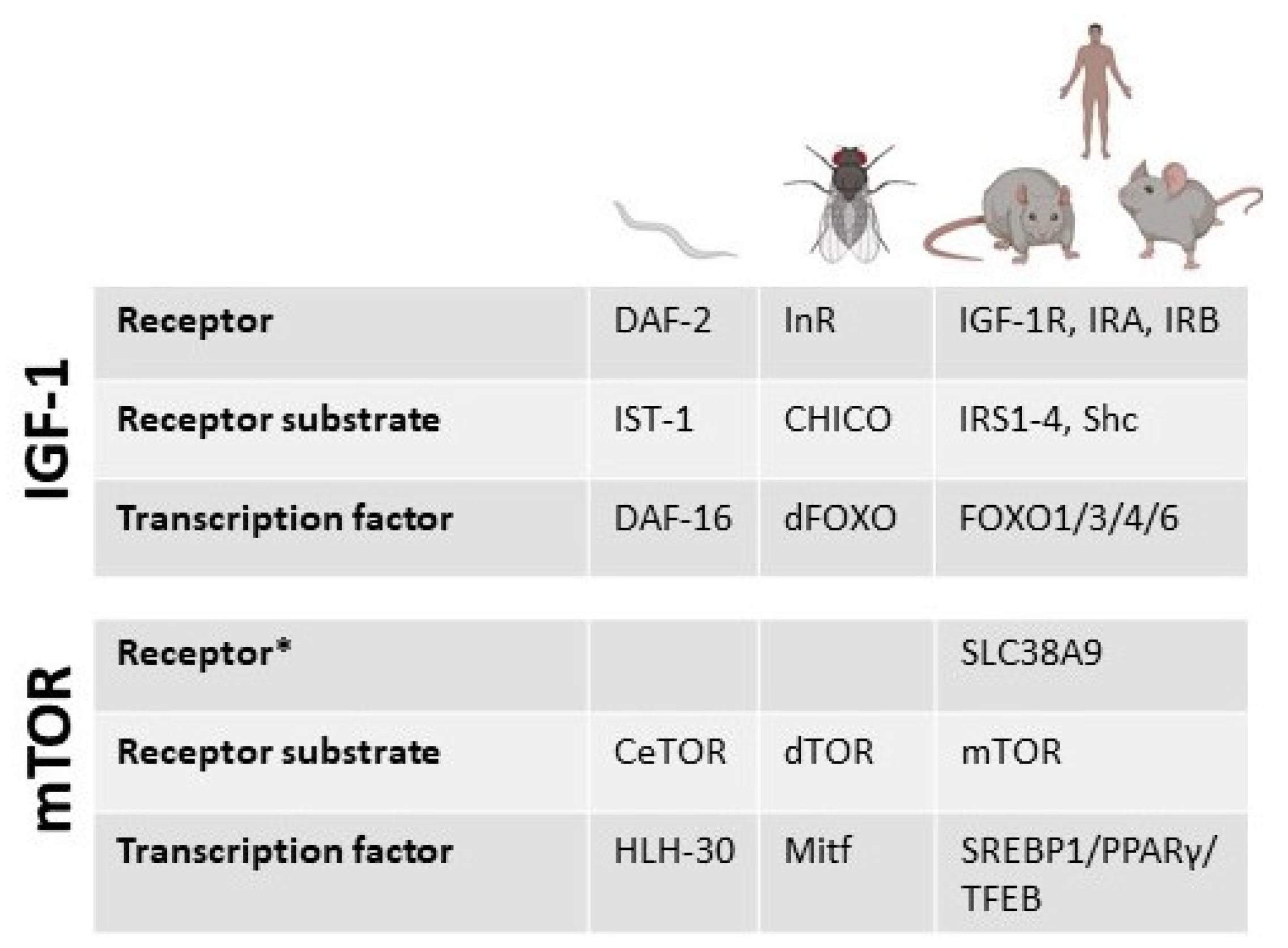
3.3. Models for mTOR Studies and Investigation in Humans
4. Human Aging, Lifespan, and Healthspan Analysis Studies
4.1. GWAS Studies on Lifespan and Healthspan
4.2. EWAS Studies in Human Lifespan and Healthspan: Links to Diet?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the Global Challenges of Ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, I.; Rallis, C. The Target of Rapamycin Signalling Pathway in Ageing and Lifespan Regulation. Genes 2020, 11, 1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ye, J.; Shi, R.; Zhao, B.; Liu, Z.; Lin, W.; Liu, X. Dietary Protein and Amino Acid Restriction: Roles in Metabolic Health and Aging-Related Diseases. Free Radic. Biol. Med. 2022, 178, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Alì, S.; Davinelli, S.; Accardi, G.; Aiello, A.; Caruso, C.; Duro, G.; Ligotti, M.E.; Pojero, F.; Scapagnini, G.; Candore, G. Healthy Ageing and Mediterranean Diet: A Focus on Hormetic Phytochemicals. Mech. Ageing Dev. 2021, 200, 111592. [Google Scholar] [CrossRef] [PubMed]
- Catterson, J.H.; Khericha, M.; Dyson, M.C.; Vincent, A.J.; Callard, R.; Haveron, S.M.; Rajasingam, A.; Ahmad, M.; Partridge, L. Short-Term, Intermittent Fasting Induces Long-Lasting Gut Health and TOR-Independent Lifespan Extension. Curr. Biol. 2018, 28, 1714–1724. [Google Scholar] [CrossRef]
- Gems, D.; Partridge, L. Stress-Response Hormesis and Aging: “That Which Does Not Kill Us Makes Us Stronger”. Cell Metab. 2008, 7, 200–203. [Google Scholar] [CrossRef]
- Gonzalez, S.; Rallis, C. The TOR Signaling Pathway in Spatial and Temporal Control of Cell Size and Growth. Front. Cell Dev. Biol. 2017, 5, 61. [Google Scholar] [CrossRef]
- Castillo-Quan, J.I.; Tain, L.S.; Kinghorn, K.J.; Li, L.; Grönke, S.; Hinze, Y.; Blackwell, T.K.; Bjedov, I.; Partridge, L. A Triple Drug Combination Targeting Components of the Nutrient-Sensing Network Maximizes Longevity. Proc. Natl. Acad. Sci. USA 2019, 116, 20817–20819. [Google Scholar] [CrossRef]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The Genetics of Human Ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- Wilfond, B.S.; Porter, K.M.; Creevy, K.E.; Kaeberlein, M.; Promislow, D. Research to Promote Longevity and Health Span in Companion Dogs: A Pediatric Perspective. Am. J. Bioeth. 2018, 18, 64–65. [Google Scholar] [CrossRef]
- Lee, M.B.; Kaeberlein, M. Translational Geroscience: From Invertebrate Models to Companion Animal and Human Interventions. Transl. Med. Aging 2018, 2, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Rayon, T.; Stamataki, D.; Perez-Carrasco, R.; Garcia-Perez, L.; Barrington, C.; Melchionda, M.; Exelby, K.; Lazaro, J.; Tybulewicz, V.L.J.; Fisher, E.M.C.; et al. Species-Specific Pace of Development Is Associated with Differences in Protein Stability. Science 2020, 369, eaba7667. [Google Scholar] [CrossRef] [PubMed]
- Rudman, D.; Feller, A.G.; Nagraj, H.S.; Gergans, G.A.; Lalitha, P.Y.; Goldberg, A.F.; Schlenker, R.A.; Cohn, L.; Rudman, I.W.; Mattson, D.E. Effects of Human Growth Hormone in Men over 60 Years Old. N. Engl. J. Med. 1990, 323, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brown-Borg, H.M.; Borg, K.E.; Meliska, C.J.; Bartke, A. Dwarf Mice and the Ageing Process. Nature 1996, 384, 33. [Google Scholar] [CrossRef]
- Bartke, A. Growth Hormone and Aging: Updated Review. World J. Mens Health 2019, 37, 19. [Google Scholar] [CrossRef]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 Axis in Ageing and Longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef]
- Bartke, A.; Brown-Borg, H. Mutations Affecting Mammalian Aging: GH and GHR vs. IGF-1 and Insulin. Front. Genet. 2021, 12, 667355. [Google Scholar] [CrossRef]
- Friedman, D.B.; Johnson, T.E. A Mutation in the Age-1 Gene in Caenorhabditis Elegans Lengthens Life and Reduces Hermaphrodite Fertility. Genetics 1988, 118, 75–86. [Google Scholar] [CrossRef]
- Morris, J.Z.; Tissenbaum, H.A.; Ruvkun, G. A Phosphatidylinositol-3-OH Kinase Family Member Regulating Longevity and Diapause in Caenorhabditis Elegans. Nature 1996, 382, 536–539. [Google Scholar] [CrossRef]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. Daf-2, an Insulin Receptor-like Gene That Regulates Longevity and Diapause in Caenorhabditis Elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A Mutant Drosophila Insulin Receptor Homolog That Extends Life-Span and Impairs Neuroendocrine Function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of Life-Span by Loss of CHICO, a Drosophila Insulin Receptor Substrate Protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Aging and Immortality: Quasi-Programmed Senescence and Its Pharmacologic Inhibition. Cell Cycle 2006, 5, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Dillin, A.; Crawford, D.K.; Kenyon, C. Timing Requirements for Insulin/IGF-1 Signaling in C. Elegans. Science 2002, 298, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.I.; Ravindran, S.; Sekajova, Z.; Carlsson, H.; Hinas, A.; Maklakov, A.A. Experimentally Reduced Insulin/IGF-1 Signaling in Adulthood Extends Lifespan of Parents and Improves Darwinian Fitness of Their Offspring. Evol. Lett. 2019, 3, 207–216. [Google Scholar] [CrossRef]
- Venz, R.; Pekec, T.; Katic, I.; Ciosk, R.; Ewald, C.Y. End-of-Life Targeted Degradation of DAF-2 Insulin/IGF-1 Receptor Promotes Longevity Free from Growth-Related Pathologies. eLife 2021, 10, e71335. [Google Scholar] [CrossRef]
- Moll, L.; Roitenberg, N.; Bejerano-Sagie, M.; Boocholez, H.; Carvalhal Marques, F.; Volovik, Y.; Elami, T.; Siddiqui, A.A.; Grushko, D.; Biram, A.; et al. The Insulin/IGF Signaling Cascade Modulates SUMOylation to Regulate Aging and Proteostasis in Caenorhabditis Elegans. eLife 2018, 7, e38635. [Google Scholar] [CrossRef]
- Roitenberg, N.; Bejerano-Sagie, M.; Boocholez, H.; Moll, L.; Marques, F.C.; Golodetzki, L.; Nevo, Y.; Elami, T.; Cohen, E. Modulation of Caveolae by Insulin/ IGF -1 Signaling Regulates Aging of Caenorhabditis Elegans. EMBO Rep. 2018, 19, e45673. [Google Scholar] [CrossRef]
- Son, H.G.; Seo, M.; Ham, S.; Hwang, W.; Lee, D.; An, S.W.A.; Artan, M.; Seo, K.; Kaletsky, R.; Arey, R.N.; et al. RNA Surveillance via Nonsense-Mediated MRNA Decay Is Crucial for Longevity in Daf-2/Insulin/IGF-1 Mutant C. Elegans. Nat. Commun. 2017, 8, 14749. [Google Scholar] [CrossRef]
- Mao, K.; Quipildor, G.F.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K.; et al. Late-Life Targeting of the IGF-1 Receptor Improves Healthspan and Lifespan in Female Mice. Nat. Commun. 2018, 9, 2394. [Google Scholar] [CrossRef]
- François, J.-C.; Aïd, S.; Chaker, Z.; Lacube, P.; Xu, J.; Fayad, R.; Côté, F.; Even, P.; Holzenberger, M. Disrupting IGF Signaling in Adult Mice Conditions Leanness, Resilient Energy Metabolism, and High Growth Hormone Pulses. Endocrinology 2017, 158, 2269–2283. [Google Scholar] [CrossRef] [PubMed]
- Milman, S.; Atzmon, G.; Huffman, D.M.; Wan, J.; Crandall, J.P.; Cohen, P.; Barzilai, N. Low Insulin-like Growth Factor-1 Level Predicts Survival in Humans with Exceptional Longevity. Aging Cell 2014, 13, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Brugts, M.P.; Ogliari, G.; Castaldi, D.; Fatti, L.M.; Varewijck, A.J.; Lamberts, S.W.; Monti, D.; Bucci, L.; Cevenini, E.; et al. Low Circulating IGF-I Bioactivity Is Associated with Human Longevity: Findings in Centenarians’ Offspring. Aging 2012, 4, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Sablin, M.-P.; Bergh, J.; Im, S.-A.; Lu, Y.-S.; Martínez, N.; Neven, P.; Lee, K.S.; Morales, S.; Pérez-Fidalgo, J.A.; et al. A Phase Ib/II Study of Xentuzumab, an IGF-Neutralising Antibody, Combined with Exemestane and Everolimus in Hormone Receptor-Positive, HER2-Negative Locally Advanced/Metastatic Breast Cancer. Breast Cancer Res. 2021, 23, 8. [Google Scholar] [CrossRef]
- de Bono, J.; Lin, C.-C.; Chen, L.-T.; Corral, J.; Michalarea, V.; Rihawi, K.; Ong, M.; Lee, J.-H.; Hsu, C.-H.; Yang, J.C.-H.; et al. Two First-in-Human Studies of Xentuzumab, a Humanised Insulin-like Growth Factor (IGF)-Neutralising Antibody, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1324–1332. [Google Scholar] [CrossRef]
- Dunn, S.E.; Kari, F.W.; French, J.; Leininger, J.R.; Travlos, G.; Wilson, R.; Barrett, J.C. Dietary Restriction Reduces Insulin-like Growth Factor I Levels, Which Modulates Apoptosis, Cell Proliferation, and Tumor Progression in P53-Deficient Mice. Cancer Res. 1997, 57, 4667–4672. [Google Scholar] [PubMed]
- Jia, K.; Levine, B. Autophagy Is Required for Dietary Restriction-Mediated Life Span Extension in C. Elegans. Autophagy 2007, 3, 597–599. [Google Scholar] [CrossRef]
- Campion, R.; Bloxam, L.; Burrow, K.; Brownridge, P.J.; Pentland, D.R.; Thomas, P.; Gourlay, C.W.; Eyers, C.E.; Barclay, J.W.; Morgan, A. Proteomic Analysis of Dietary Restriction in Yeast Reveals a Role for Hsp26 in Replicative Lifespan Extension. Biochem. J. 2021, 478, 4153–4167. [Google Scholar] [CrossRef]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of Caloric Restriction on Health and Survival in Rhesus Monkeys from the NIA Study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef]
- Pifferi, F.; Terrien, J.; Marchal, J.; Dal-Pan, A.; Djelti, F.; Hardy, I.; Chahory, S.; Cordonnier, N.; Desquilbet, L.; Hurion, M.; et al. Caloric Restriction Increases Lifespan but Affects Brain Integrity in Grey Mouse Lemur Primates. Commun. Biol. 2018, 1, 30. [Google Scholar] [CrossRef]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-Term Effects of Calorie or Protein Restriction on Serum IGF-1 and IGFBP-3 Concentration in Humans. Aging Cell 2008, 7, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Villareal, D.T.; Das, S.K.; Smith, S.R.; Meydani, S.N.; Pittas, A.G.; Klein, S.; Bhapkar, M.; Rochon, J.; Ravussin, E.; et al. Effects of 2-year Calorie Restriction on Circulating Levels of IGF-1, IGF-binding Proteins and Cortisol in Nonobese Men and Women: A Randomized Clinical Trial. Aging Cell 2016, 15, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Plummer, J.D.; Postnikoff, S.D.; Tyler, J.K.; Johnson, J.E. Selenium Supplementation Inhibits IGF-1 Signaling and Confers Methionine Restriction-like Healthspan Benefits to Mice. eLife 2021, 10, e62483. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, B.-K.; Park, S.-K. Supplementation with Phosphatidylethanolamine Confers Anti-Oxidant and Anti-Aging Effects via Hormesis and Reduced Insulin/IGF-1-like Signaling in C. Elegans. Mech. Ageing Dev. 2021, 197, 111498. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, H.-Y.; Yu, M.; Yeom, E.; Lee, J.-H.; Yoon, A.; Lee, K.-S.; Min, K.-J. Extension of Drosophila Lifespan by Korean Red Ginseng through a Mechanism Dependent on DSir2 and Insulin/IGF-1 Signaling. Aging 2019, 11, 9369–9387. [Google Scholar] [CrossRef]
- Lo, W.-C.; Chiou, C.-S.; Tsai, F.-C.; Chan, C.-H.; Mao, S.; Deng, Y.-H.; Wu, C.-Y.; Peng, B.-Y.; Deng, W.-P. Platelet-Derived Biomaterials Inhibit Nicotine-Induced Intervertebral Disc Degeneration Through Regulating IGF-1/AKT/IRS-1 Signaling Axis. Cell Transplant. 2021, 30, 096368972110453. [Google Scholar] [CrossRef]
- Trejo, J.; Piriz, J.; Llorens-Martin, M.V.; Fernandez, A.M.; Bolós, M.; LeRoith, D.; Nuñez, A.; Torres-Aleman, I. Central Actions of Liver-Derived Insulin-like Growth Factor I Underlying Its pro-Cognitive Effects. Mol. Psychiatry 2007, 12, 1118–1128. [Google Scholar] [CrossRef]
- Pardo, J.; Uriarte, M.; Cónsole, G.M.; Reggiani, P.C.; Outeiro, T.F.; Morel, G.R.; Goya, R.G. Insulin-like Growth Factor-I Gene Therapy Increases Hippocampal Neurogenesis, Astrocyte Branching and Improves Spatial Memory in Female Aging Rats. Eur. J. Neurosci. 2016, 44, 2120–2128. [Google Scholar] [CrossRef]
- Farias Quipildor, G.E.; Mao, K.; Hu, Z.; Novaj, A.; Cui, M.-H.; Gulinello, M.; Branch, C.A.; Gubbi, S.; Patel, K.; Moellering, D.R.; et al. Central IGF-1 Protects against Features of Cognitive and Sensorimotor Decline with Aging in Male Mice. GeroScience 2019, 41, 185–208. [Google Scholar] [CrossRef]
- Tang, J.J.; Podratz, J.L.; Lange, M.; Scrable, H.J.; Jang, M.-H.; Windebank, A.J. Mechano Growth Factor, a Splice Variant of IGF-1, Promotes Neurogenesis in the Aging Mouse Brain. Mol. Brain 2017, 10, 23. [Google Scholar] [CrossRef]
- Atilano, M.L.; Grönke, S.; Niccoli, T.; Kempthorne, L.; Hahn, O.; Morón-Oset, J.; Hendrich, O.; Dyson, M.; Adams, M.L.; Hull, A.; et al. Enhanced Insulin Signalling Ameliorates C9orf72 Hexanucleotide Repeat Expansion Toxicity in Drosophila. eLife 2021, 10, e58565. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Nuñez, A.; Busiguina, S.; Torres-Aleman, I. Circulating Insulin-Like Growth Factor I Mediates Effects of Exercise on the Brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Bressel, E.; Kim, D. Effects of Aquatic Exercise on Insulin-like Growth Factor-1, Brain-Derived Neurotrophic Factor, Vascular Endothelial Growth Factor, and Cognitive Function in Elderly Women. Exp. Gerontol. 2020, 132, 110842. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Wang, C.-H.; Pan, C.-Y.; Chen, F.-C. The Effects of Long-Term Resistance Exercise on the Relationship between Neurocognitive Performance and GH, IGF-1, and Homocysteine Levels in the Elderly. Front. Behav. Neurosci. 2015, 9, 10-3389. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Cholerton, B.A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; et al. Aerobic Exercise Improves Cognition for Older Adults with Glucose Intolerance, A Risk Factor for Alzheimer’s Disease. JAD 2010, 22, 569–579. [Google Scholar] [CrossRef]
- Maass, A.; Düzel, S.; Brigadski, T.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L.; et al. Relationships of Peripheral IGF-1, VEGF and BDNF Levels to Exercise-Related Changes in Memory, Hippocampal Perfusion and Volumes in Older Adults. NeuroImage 2016, 131, 142–154. [Google Scholar] [CrossRef]
- Behrendt, T.; Kirschnick, F.; Kröger, L.; Beileke, P.; Rezepin, M.; Brigadski, T.; Leßmann, V.; Schega, L. Comparison of the Effects of Open vs. Closed Skill Exercise on the Acute and Chronic BDNF, IGF-1 and IL-6 Response in Older Healthy Adults. BMC Neurosci. 2021, 22, 71. [Google Scholar] [CrossRef]
- Arazi, H.; Babaei, P.; Moghimi, M.; Asadi, A. Acute Effects of Strength and Endurance Exercise on Serum BDNF and IGF-1 Levels in Older Men. BMC Geriatr. 2021, 21, 50. [Google Scholar] [CrossRef]
- Żebrowska, A.; Hall, B.; Maszczyk, A.; Banaś, R.; Urban, J. Brain-Derived Neurotrophic Factor, Insulin like Growth Factor-1 and Inflammatory Cytokine Responses to Continuous and Intermittent Exercise in Patients with Type 1 Diabetes. Diabetes Res. Clin. Pract. 2018, 144, 126–136. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S. Mitochondria Impact Brain Function and Cognition. Proc. Natl. Acad. Sci. USA 2014, 111, 7–8. [Google Scholar] [CrossRef]
- Pharaoh, G.; Owen, D.; Yeganeh, A.; Premkumar, P.; Farley, J.; Bhaskaran, S.; Ashpole, N.; Kinter, M.; Van Remmen, H.; Logan, S. Disparate Central and Peripheral Effects of Circulating IGF-1 Deficiency on Tissue Mitochondrial Function. Mol. Neurobiol. 2020, 57, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira, C.R.; Rego, A.C. Insulin and IGF-1 Improve Mitochondrial Function in a PI-3K/Akt-Dependent Manner and Reduce Mitochondrial Generation of Reactive Oxygen Species in Huntington’s Disease Knock-in Striatal Cells. Free. Radic. Biol. Med. 2014, 74, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Riis, S.; Murray, J.B.; O’Connor, R. IGF-1 Signalling Regulates Mitochondria Dynamics and Turnover through a Conserved GSK-3β–Nrf2–BNIP3 Pathway. Cells 2020, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.; Coleman, M.; Riis, S.; Favre, C.; O’Flanagan, C.H.; Zhdanov, A.V.; Papkovsky, D.B.; Hursting, S.D.; O’Connor, R. Insulin-like Growth Factor 1 Signaling Is Essential for Mitochondrial Biogenesis and Mitophagy in Cancer Cells. J. Biol. Chem. 2017, 292, 16983–16998. [Google Scholar] [CrossRef]
- Ding, Y.; Li, J.; Liu, Z.; Liu, H.; Li, H.; Li, Z. IGF-1 Potentiates Sensory Innervation Signalling by Modulating the Mitochondrial Fission/Fusion Balance. Sci. Rep. 2017, 7, 43949. [Google Scholar] [CrossRef]
- Tarantini, S.; Tucsek, Z.; Valcarcel-Ares, M.N.; Toth, P.; Gautam, T.; Giles, C.B.; Ballabh, P.; Wei, J.Y.; Wren, J.D.; Ashpole, N.M.; et al. Circulating IGF-1 Deficiency Exacerbates Hypertension-Induced Microvascular Rarefaction in the Mouse Hippocampus and Retrosplenial Cortex: Implications for Cerebromicrovascular and Brain Aging. AGE 2016, 38, 273–289. [Google Scholar] [CrossRef]
- Fulop, G.A.; Ramirez-Perez, F.I.; Kiss, T.; Tarantini, S.; Valcarcel Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Conley, S.M.; Ballabh, P.; Martinez-Lemus, L.A.; et al. IGF-1 Deficiency Promotes Pathological Remodeling of Cerebral Arteries: A Potential Mechanism Contributing to the Pathogenesis of Intracerebral Hemorrhages in Aging. J. Gerontol. Ser. A 2019, 74, 446–454. [Google Scholar] [CrossRef]
- Bake, S.; Okoreeh, A.K.; Alaniz, R.C.; Sohrabji, F. Insulin-Like Growth Factor (IGF)-I Modulates Endothelial Blood-Brain Barrier Function in Ischemic Middle-Aged Female Rats. Endocrinology 2016, 157, 61–69. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. MTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Zhao, Y.; Cholewa, J.; Shang, H.; Yang, Y.; Ding, X.; Wang, Q.; Su, Q.; Zanchi, N.E.; Xia, Z. Advances in the Role of Leucine-Sensing in the Regulation of Protein Synthesis in Aging Skeletal Muscle. Front. Cell Dev. Biol 2021, 9, 646482. [Google Scholar] [CrossRef] [PubMed]
- Deleyto-Seldas, N.; Efeyan, A. The MTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol 2021, 9, 655731. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, S.; Khoutorsky, A.; Mathews, M.B.; Sonenberg, N. Translation Deregulation in Human Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Hsu, S.-C.; Ann, D.K.; Yen, Y.; Kung, H.-J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541. [Google Scholar] [CrossRef] [PubMed]
- Castejón-Vega, B.; Rubio, A.; Pérez-Pulido, A.J.; Quiles, J.L.; Lane, J.D.; Fernández-Domínguez, B.; Cachón-González, M.B.; Martín-Ruiz, C.; Sanz, A.; Cox, T.M.; et al. L-Arginine Ameliorates Defective Autophagy in GM2 Gangliosidoses by MTOR Modulation. Cells 2021, 10, 3122. [Google Scholar] [CrossRef]
- Dyachok, J.; Earnest, S.; Iturraran, E.N.; Cobb, M.H.; Ross, E.M. Amino Acids Regulate MTORC1 by an Obligate Two-Step Mechanism. J. Biol. Chem. 2016, 291, 22414–22426. [Google Scholar] [CrossRef]
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Vander Heiden, M.G.; Sabatini, D.M. MTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell 2017, 171, 642–654. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 Is a Leucine Sensor for the MTORC1 Pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Weichhart, T. MTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Chen, J.; Ou, Y.; Luo, R.; Wang, J.; Wang, D.; Guan, J.; Li, Y.; Xia, P.; Chen, P.R.; Liu, Y. SAR1B Senses Leucine Levels to Regulate MTORC1 Signalling. Nature 2021, 596, 281–284. [Google Scholar] [CrossRef]
- Bjedov, I.; Toivonen, J.M.; Kerr, F.; Slack, C.; Jacobson, J.; Foley, A.; Partridge, L. Mechanisms of Life Span Extension by Rapamycin in the Fruit Fly Drosophila Melanogaster. Cell Metab. 2010, 11, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R. Meta-Analysis of 29 Experiments Evaluating the Effects of Rapamycin on Life Span in the Laboratory Mouse. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1024–1032. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stead, E.R.; Castillo-Quan, J.I.; Miguel, V.E.M.; Lujan, C.; Ketteler, R.; Kinghorn, K.J.; Bjedov, I. Agephagy—Adapting Autophagy for Health During Aging. Front. Cell Dev. Biol. 2019, 7, 308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Wang, S. The Role of Rapamycin in Healthspan Extension via the Delay of Organ Aging. Ageing Res. Rev. 2021, 70, 101376. [Google Scholar] [CrossRef]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of Rapamycin on Aging and Age-Related Diseases-Past and Future. Geroscience 2021, 43, 1135–1158. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Fernandez, E.; Flurkey, K.; Han, M.; Javors, M.A.; Li, X.; Nadon, N.L.; Nelson, J.F.; et al. Rapamycin-Mediated Lifespan Increase in Mice Is Dose and Sex Dependent and Metabolically Distinct from Dietary Restriction. Aging Cell 2014, 13, 468–477. [Google Scholar] [CrossRef]
- Kraig, E.; Linehan, L.A.; Liang, H.; Romo, T.Q.; Liu, Q.; Wu, Y.; Benavides, A.D.; Curiel, T.J.; Javors, M.A.; Musi, N.; et al. A Randomized Control Trial to Establish the Feasibility and Safety of Rapamycin Treatment in an Older Human Cohort: Immunological, Physical Performance, and Cognitive Effects. Exp. Gerontol. 2018, 105, 53–69. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, J.; Chen, E.B.; Wang, J.J.; Cao, L.; Narayan, N.; Fergusson, M.M.; Rovira, I.I.; Allen, M.; Springer, D.A.; et al. Increased Mammalian Lifespan and a Segmental and Tissue-Specific Slowing of Aging after Genetic Reduction of MTOR Expression. Cell Rep. 2013, 4, 913–920. [Google Scholar] [CrossRef]
- Walters, H.E.; Deneka-Hannemann, S.; Cox, L.S. Reversal of Phenotypes of Cellular Senescence by Pan-MTOR Inhibition. Aging 2016, 8, 231–244. [Google Scholar] [CrossRef]
- Harries, L.W.; Fellows, A.D.; Pilling, L.C.; Hernandez, D.; Singleton, A.; Bandinelli, S.; Guralnik, J.; Powell, J.; Ferrucci, L.; Melzer, D. Advancing Age Is Associated with Gene Expression Changes Resembling MTOR Inhibition: Evidence from Two Human Populations. Mech. Ageing Dev. 2012, 133, 556–562. [Google Scholar] [CrossRef]
- Passtoors, W.M.; Beekman, M.; Deelen, J.; van der Breggen, R.; Maier, A.B.; Guigas, B.; Derhovanessian, E.; van Heemst, D.; de Craen, A.J.M.; Gunn, D.A.; et al. Gene Expression Analysis of MTOR Pathway: Association with Human Longevity. Aging Cell 2013, 12, 24–31. [Google Scholar] [CrossRef]
- Hwangbo, D.-S.; Lee, H.-Y.; Abozaid, L.S.; Min, K.-J. Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients 2020, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.E.; Konon, E.N.; Schuster, H.S.; Mitchell, A.T.; Boyle, C.; Rodgers, A.C.; Finke, M.; Haider, L.R.; Yu, D.; Flores, V.; et al. Lifelong Restriction of Dietary Branched-Chain Amino Acids Has Sex-Specific Benefits for Frailty and Lifespan in Mice. Nat. Aging 2021, 1, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Schächter, F.; Faure-Delanef, L.; Guénot, F.; Rouger, H.; Froguel, P.; Lesueur-Ginot, L.; Cohen, D. Genetic Associations with Human Longevity at the APOE and ACE Loci. Nat. Genet. 1994, 6, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Murray, M.E.; Li, X.; Zhao, N.; Wang, N.; Heckman, M.G.; Shue, F.; Martens, Y.; Li, Y.; Raulin, A.-C.; et al. APOE3-Jacksonville (V236E) Variant Reduces Self-Aggregation and Risk of Dementia. Sci. Transl. Med. 2021, 13, eabc9375. [Google Scholar] [CrossRef] [PubMed]
- Nacmias, B.; Bagnoli, S.; Tedde, A.; Cellini, E.; Bessi, V.; Guarnieri, B.; Ortensi, L.; Piacentini, S.; Bracco, L.; Sorbi, S. Angiotensin Converting Enzyme Insertion/Deletion Polymorphism in Sporadic and Familial Alzheimer’s Disease and Longevity. Arch. Gerontol. Geriatr. 2007, 45, 201–206. [Google Scholar] [CrossRef]
- Huang, Y.-W.A.; Zhou, B.; Wernig, M.; Südhof, T.C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell 2017, 168, 427–441. [Google Scholar] [CrossRef]
- Botchway, B.O.; Okoye, F.C.; Chen, Y.; Arthur, W.E.; Fang, M. Alzheimer Disease: Recent Updates on Apolipoprotein E and Gut Microbiome Mediation of Oxidative Stress, and Prospective Interventional Agents. Aging Dis. 2022, 13, 87–102. [Google Scholar] [CrossRef]
- Aleshkov, S.; Abraham, C.R.; Zannis, V.I. Interaction of Nascent ApoE2, ApoE3, and ApoE4 Isoforms Expressed in Mammalian Cells with Amyloid Peptide Beta (1-40). Relevance to Alzheimer’s Disease. Biochemistry 1997, 36, 10571–10580. [Google Scholar] [CrossRef]
- Li, Z.; Shue, F.; Zhao, N.; Shinohara, M.; Bu, G. APOE2: Protective Mechanism and Therapeutic Implications for Alzheimer’s Disease. Mol. Neurodegener. 2020, 15, 63. [Google Scholar] [CrossRef]
- Keeney, J.T.-R.; Ibrahimi, S.; Zhao, L. Human ApoE Isoforms Differentially Modulate Glucose and Amyloid Metabolic Pathways in Female Brain: Evidence of the Mechanism of Neuroprotection by ApoE2 and Implications for Alzheimer’s Disease Prevention and Early Intervention. J. Alzheimers Dis. 2015, 48, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.A.; Zhou, B.; Nabet, A.M.; Wernig, M.; Südhof, T.C. Differential Signaling Mediated by ApoE2, ApoE3, and ApoE4 in Human Neurons Parallels Alzheimer’s Disease Risk. J. Neurosci. 2019, 39, 7408–7427. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank Resource with Deep Phenotyping and Genomic Data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Timmers, P.R.; Mounier, N.; Lall, K.; Fischer, K.; Ning, Z.; Feng, X.; Bretherick, A.D.; Clark, D.W.; eQTLGen Consortium; Agbessi, M.; et al. Genomics of 1 Million Parent Lifespans Implicates Novel Pathways and Common Diseases and Distinguishes Survival Chances. eLife 2019, 8, e39856. [Google Scholar] [CrossRef]
- Wright, K.M.; Rand, K.A.; Kermany, A.; Noto, K.; Curtis, D.; Garrigan, D.; Slinkov, D.; Dorfman, I.; Granka, J.M.; Byrnes, J.; et al. A Prospective Analysis of Genetic Variants Associated with Human Lifespan. G3 2019, 9, 2863–2878. [Google Scholar] [CrossRef]
- Pilling, L.C.; Kuo, C.-L.; Sicinski, K.; Tamosauskaite, J.; Kuchel, G.A.; Harries, L.W.; Herd, P.; Wallace, R.; Ferrucci, L.; Melzer, D. Human Longevity: 25 Genetic Loci Associated in 389,166 UK Biobank Participants. Aging 2017, 9, 2504–2520. [Google Scholar] [CrossRef]
- Welter, D.; MacArthur, J.; Morales, J.; Burdett, T.; Hall, P.; Junkins, H.; Klemm, A.; Flicek, P.; Manolio, T.; Hindorff, L.; et al. The NHGRI GWAS Catalog, a Curated Resource of SNP-Trait Associations. Nucl. Acids Res. 2014, 42, D1001–D1006. [Google Scholar] [CrossRef]
- Joshi, P.K.; Pirastu, N.; Kentistou, K.A.; Fischer, K.; Hofer, E.; Schraut, K.E.; Clark, D.W.; Nutile, T.; Barnes, C.L.K.; Timmers, P.R.H.J.; et al. Genome-Wide Meta-Analysis Associates HLA-DQA1/DRB1 and LPA and Lifestyle Factors with Human Longevity. Nat. Commun. 2017, 8, 910. [Google Scholar] [CrossRef]
- Deelen, J.; Evans, D.S.; Arking, D.E.; Tesi, N.; Nygaard, M.; Liu, X.; Wojczynski, M.K.; Biggs, M.L.; van der Spek, A.; Atzmon, G.; et al. A Meta-Analysis of Genome-Wide Association Studies Identifies Multiple Longevity Genes. Nat. Commun. 2019, 10, 3669. [Google Scholar] [CrossRef]
- Eline Slagboom, P.; van den Berg, N.; Deelen, J. Phenome and Genome Based Studies into Human Ageing and Longevity: An Overview. Biochim. Biophys. Acta 2018, 1864, 2742–2751. [Google Scholar] [CrossRef]
- Lin, K.; Dorman, J.B.; Rodan, A.; Kenyon, C. Daf-16: An HNF-3/Forkhead Family Member That Can Function to Double the Life-Span of Caenorhabditis Elegans. Science 1997, 278, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The Fork Head Transcription Factor DAF-16 Transduces Insulin-like Metabolic and Longevity Signals in C. Elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Burgering, B.M.T. FOXOs: Masters of the Equilibrium. FEBS J. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Butler, L.; Perkins, A.; Kershaw, N.J.; Babon, J.J. The Role of LNK (SH2B3) in the Regulation of JAK-STAT Signalling in Haematopoiesis. Pharmaceuticals 2021, 15, 24. [Google Scholar] [CrossRef]
- Fortney, K.; Dobriban, E.; Garagnani, P.; Pirazzini, C.; Monti, D.; Mari, D.; Atzmon, G.; Barzilai, N.; Franceschi, C.; Owen, A.B.; et al. Genome-Wide Scan Informed by Age-Related Disease Identifies Loci for Exceptional Human Longevity. PLoS Genet. 2015, 11, e1005728. [Google Scholar] [CrossRef]
- Allenspach, E.J.; Shubin, N.J.; Cerosaletti, K.; Mikacenic, C.; Gorman, J.A.; MacQuivey, M.A.; Rosen, A.B.I.; Timms, A.E.; Wray-Dutra, M.N.; Niino, K.; et al. The Autoimmune Risk R262W Variant of the Adaptor SH2B3 Improves Survival in Sepsis. J. Immunol. 2021, 207, 2710–2719. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Joaquim, M.; Kuchel, G.A.; Ferrucci, L.; Harries, L.W.; Pilling, L.C.; Melzer, D. The Longevity-Associated SH2B3 (LNK) Genetic Variant: Selected Aging Phenotypes in 379,758 Subjects. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1656–1662. [Google Scholar] [CrossRef]
- Song, W.; Ren, D.; Li, W.; Jiang, L.; Cho, K.W.; Huang, P.; Fan, C.; Song, Y.; Liu, Y.; Rui, L. SH2B Regulation of Growth, Metabolism, and Longevity in Both Insects and Mammals. Cell Metab. 2010, 11, 427–437. [Google Scholar] [CrossRef]
- Slack, C.; Werz, C.; Wieser, D.; Alic, N.; Foley, A.; Stocker, H.; Withers, D.J.; Thornton, J.M.; Hafen, E.; Partridge, L. Regulation of Lifespan, Metabolism, and Stress Responses by the Drosophila SH2B Protein, Lnk. PLoS Genet. 2010, 6, e1000881. [Google Scholar] [CrossRef]
- Shen, Y.; Xia, Y.; Meng, S.; Lim, N.K.H.; Wang, W.; Huang, F. SH2B1 Is Involved in the Accumulation of Amyloid-Β42 in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 835–847. [Google Scholar] [CrossRef]
- Morris, D.L.; Cho, K.W.; Rui, L. Critical Role of the Src Homology 2 (SH2) Domain of Neuronal SH2B1 in the Regulation of Body Weight and Glucose Homeostasis in Mice. Endocrinology 2010, 151, 3643–3651. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Argetsinger, L.S.; Stadler, L.K.J.; Malaga, A.E.; Vander, P.B.; DeSantis, L.C.; Joe, R.M.; Cline, J.M.; Keogh, J.M.; Henning, E.; et al. Crucial Role of the SH2B1 PH Domain for the Control of Energy Balance. Diabetes 2019, 68, 2049–2062. [Google Scholar] [CrossRef] [PubMed]
- Desbuquois, B.; Carré, N.; Burnol, A.-F. Regulation of Insulin and Type 1 Insulin-like Growth Factor Signaling and Action by the Grb10/14 and SH2B1/B2 Adaptor Proteins. FEBS J. 2013, 280, 794–816. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Suzuki-Yamazaki, N.; Takaki, S. Lnk/Sh2b3 Regulates Adipose Inflammation and Glucose Tolerance through Group 1 ILCs. Cell Rep. 2018, 24, 1830–1841. [Google Scholar] [CrossRef]
- Silva, P.N.O.; Gigek, C.O.; Leal, M.F.; Bertolucci, P.H.F.; de Labio, R.W.; Payão, S.L.M.; Smith, M.D.A.C. Promoter Methylation Analysis of SIRT3, SMARCA5, HTERT and CDH1 Genes in Aging and Alzheimer’s Disease. J. Alzheimers Dis. 2008, 13, 173–176. [Google Scholar] [CrossRef]
- Kim, K.; Friso, S.; Choi, S.-W. DNA Methylation, an Epigenetic Mechanism Connecting Folate to Healthy Embryonic Development and Aging. J. Nutr. Biochem. 2009, 20, 917–926. [Google Scholar] [CrossRef]
- Hernandez, D.G.; Nalls, M.A.; Gibbs, J.R.; Arepalli, S.; van der Brug, M.; Chong, S.; Moore, M.; Longo, D.L.; Cookson, M.R.; Traynor, B.J.; et al. Distinct DNA Methylation Changes Highly Correlated with Chronological Age in the Human Brain. Hum. Mol. Genet. 2011, 20, 1164–1172. [Google Scholar] [CrossRef]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- van Dijk, S.J.; Tellam, R.L.; Morrison, J.L.; Muhlhausler, B.S.; Molloy, P.L. Recent Developments on the Role of Epigenetics in Obesity and Metabolic Disease. Clin. Epigenetics 2015, 7, 66. [Google Scholar] [CrossRef]
- Fogel, O.; Richard-Miceli, C.; Tost, J. Epigenetic Changes in Chronic Inflammatory Diseases. Adv. Protein Chem. Struct. Biol. 2017, 106, 139–189. [Google Scholar] [CrossRef]
- Bell, J.T.; Tsai, P.-C.; Yang, T.-P.; Pidsley, R.; Nisbet, J.; Glass, D.; Mangino, M.; Zhai, G.; Zhang, F.; Valdes, A.; et al. Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population. PLoS Genet. 2012, 8, e1002629. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.; Gonzalez, S.; Hillson, O.; Tunnacliffe, E.; Codlin, S.; Tallada, V.A.; Bähler, J.; Rallis, C. The GATA Transcription Factor Gaf1 Represses TRNAs, Inhibits Growth, and Extends Chronological Lifespan Downstream of Fission Yeast TORC1. Cell Rep. 2020, 30, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Rallis, C.; Codlin, S.; Bähler, J. TORC1 Signaling Inhibition by Rapamycin and Caffeine Affect Lifespan, Global Gene Expression, and Cell Proliferation of Fission Yeast. Aging Cell 2013, 12, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Karabegović, I.; Portilla-Fernandez, E.; Li, Y.; Ma, J.; Maas, S.C.E.; Sun, D.; Hu, E.A.; Kühnel, B.; Zhang, Y.; Ambatipudi, S.; et al. Epigenome-Wide Association Meta-Analysis of DNA Methylation with Coffee and Tea Consumption. Nat. Commun. 2021, 12, 2830. [Google Scholar] [CrossRef]
- Westerman, K.; Kelly, J.M.; Ordovás, J.M.; Booth, S.L.; DeMeo, D.L. Epigenome-Wide Association Study Reveals a Molecular Signature of Response to Phylloquinone (Vitamin K1) Supplementation. Epigenetics 2020, 15, 859–870. [Google Scholar] [CrossRef]
- Mohajeri, M.S.A.; Eslahi, A.; Khazaii, Z.; Moradi, M.R.; Pazhoomand, R.; Farrokhi, S.; Feizabadi, M.H.; Alizadeh, F.; Mojarrad, M. TMEM263: A Novel Candidate Gene Implicated in Human Autosomal Recessive Severe Lethal Skeletal Dysplasia. Hum. Genom. 2021, 15, 42. [Google Scholar] [CrossRef]
- Fraszczyk, E.; Luijten, M.; Spijkerman, A.M.W.; Snieder, H.; Wackers, P.F.K.; Bloks, V.W.; Nicoletti, C.F.; Nonino, C.B.; Crujeiras, A.B.; Buurman, W.A.; et al. The Effects of Bariatric Surgery on Clinical Profile, DNA Methylation, and Ageing in Severely Obese Patients. Clin. Epigenetics 2020, 12, 14. [Google Scholar] [CrossRef]

| Study Title and Phase | Status | Interventions | Enrollment (Estimated) |
|---|---|---|---|
| Participatory Evaluation (of) Aging (With) Rapamycin (for) Longevity Study [Phase II] | Recruiting | Rapamycin and placebo | 150 participants |
| The Role of Sirolimus in Preventing Functional Decline in Older Adults [Phase II] | Not yet recruiting | Sirolimus | 14 participants |
| VIAging Deceleration Trial Using Metformin, Dasatinib, Rapamycin and Nutritional Supplements [Phase I] | Not yet recruiting | Study drugs (including Rapamycin) and nutritional supplements | 50 participants |
| Effect of mTOR inhibition and Other Metabolism Modulating Interventions on the Elderly [Phase II] | Completed—has results | Rapamycin and placebo | 34 participants |
| Effect of mTOR Inhibition and Other Metabolism Modulating Interventions on the Elderly (Substudy Rapa and cMRI to Evaluate Cardiac Function) [Phase II] | Recruiting | Rapamycin | 12 participants |
| Exercise and Low-Dose Rapamycin in Older Adults With CAD: Cardiac Rehabilitation and Rapamycin in Elderly (CARE) trial [Phase I] | Completed—no results available | Rapamycin | 13 participants |
| Phase I Study of the Effects of Combining Topical FDA-approved Drugs on Age-related Pathways on the Skin of Healthy Volunteers | Completed | Sirolimus, Metformin, Diclofenac | 10 participants |
| Topical-RAPA Use in Inflammation Reversal and Re-setting of the Epigenetic Clock [Early Phase I] | Active, not recruiting | Rapamycin topical ointment and placebo | 50 participants |
| Study Title and Phase | Status | Interventions | Enrollment (Estimated) |
|---|---|---|---|
| Calorie Restriction Retards the Aging Process | Unknown | Energy restricted Mediterranean-type diet; 25% calorie restriction | 48 participants |
| The Effect of Food Stimuli on the Calorie Restriction Response in Healthy Subjects | Completed | Stimuli of food smell and vision vs. no stimuli of food smell and vision | 12 participants |
| Effect of Age and Weight Loss on Inflammation and Iron Homeostasis | Completed | Calorie restriction | 44 participants |
| Effect of Resvida, a Comparison with Calorie Restriction Regimen | Completed | Resveratrol, placebo, and calorie restriction | 58 participants |
| Metformin Induces a Dietary Restriction-like State in Human [Phase IV] | Unknown status | Metformin 0.85 twice daily for six months, calorie restriction | 60 participants |
| CALERIE Phase II Ancillary: Metabolic [Phase II] | Completed | Calorie Restriction | 75 participants |
| CALERIE (Tufts)—Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy [Phase I] | Completed | Calorie Restriction | 44 participants |
| Long-term Caloric Restriction and Cellular Aging Markers | Completed—Has results | No interventions | 71 participants |
| CALERIE (Washington University): Comprehensive Assessment of Long-Term Effect of Reducing Intake of Energy [Phase I] | Completed | Calorie Restriction | 48 participants |
| The Effect of Time-restricted Feeding on Physiological Function in Middle-aged and Older Adults [Phase I/II] | Unknown status | Time-restricted feeding | 12 participants |
| CALERIE: Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy | Completed—has results | Calorie restriction and control | 238 participants |
| CALERIE (PBRC, Baton Rouge)—Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy | Completed | Calorie restriction and exercise | 48 participants |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabrowska, A.; Kumar, J.; Rallis, C. Nutrient-Response Pathways in Healthspan and Lifespan Regulation. Cells 2022, 11, 1568. https://doi.org/10.3390/cells11091568
Dabrowska A, Kumar J, Rallis C. Nutrient-Response Pathways in Healthspan and Lifespan Regulation. Cells. 2022; 11(9):1568. https://doi.org/10.3390/cells11091568
Chicago/Turabian StyleDabrowska, Aleksandra, Juhi Kumar, and Charalampos Rallis. 2022. "Nutrient-Response Pathways in Healthspan and Lifespan Regulation" Cells 11, no. 9: 1568. https://doi.org/10.3390/cells11091568
APA StyleDabrowska, A., Kumar, J., & Rallis, C. (2022). Nutrient-Response Pathways in Healthspan and Lifespan Regulation. Cells, 11(9), 1568. https://doi.org/10.3390/cells11091568