Tracking the Activation of Heat Shock Signaling in Cellular Protection and Damage
Abstract
1. Heat Shock Factor 1 Is a Primary Controller of the Transcription of Heat Shock Protein Genes
2. Heat Shock Reporters Unravel the Molecular Mechanisms of Heat Shock Signaling Activation
3. Heat Shock Reporters Detect the Activation of Heat Shock Signaling upon Various Types of Cellular Stress and Injury
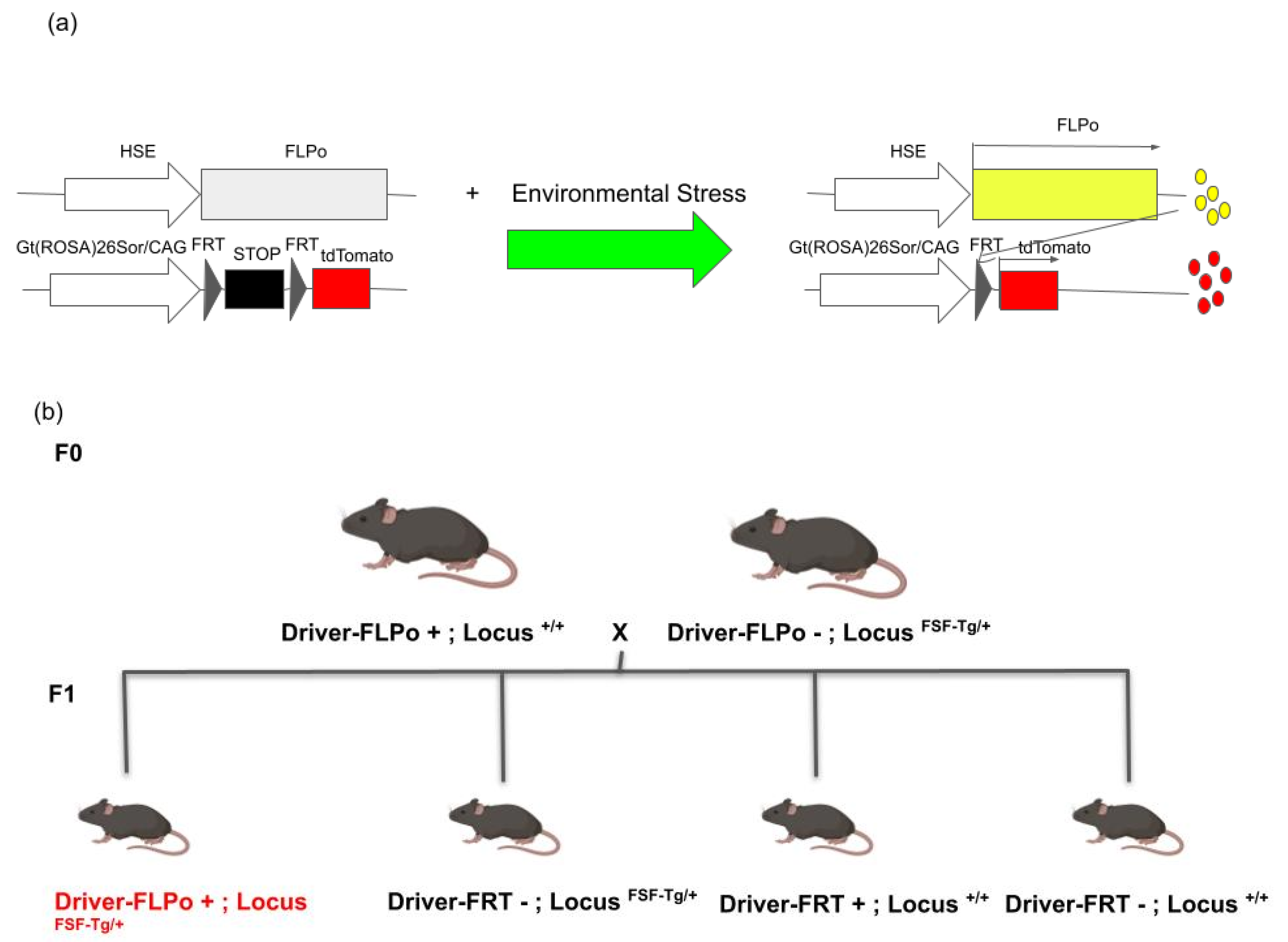
4. Generation of Permanent Tracing Systems for Cells That Activate Heat Shock Signaling
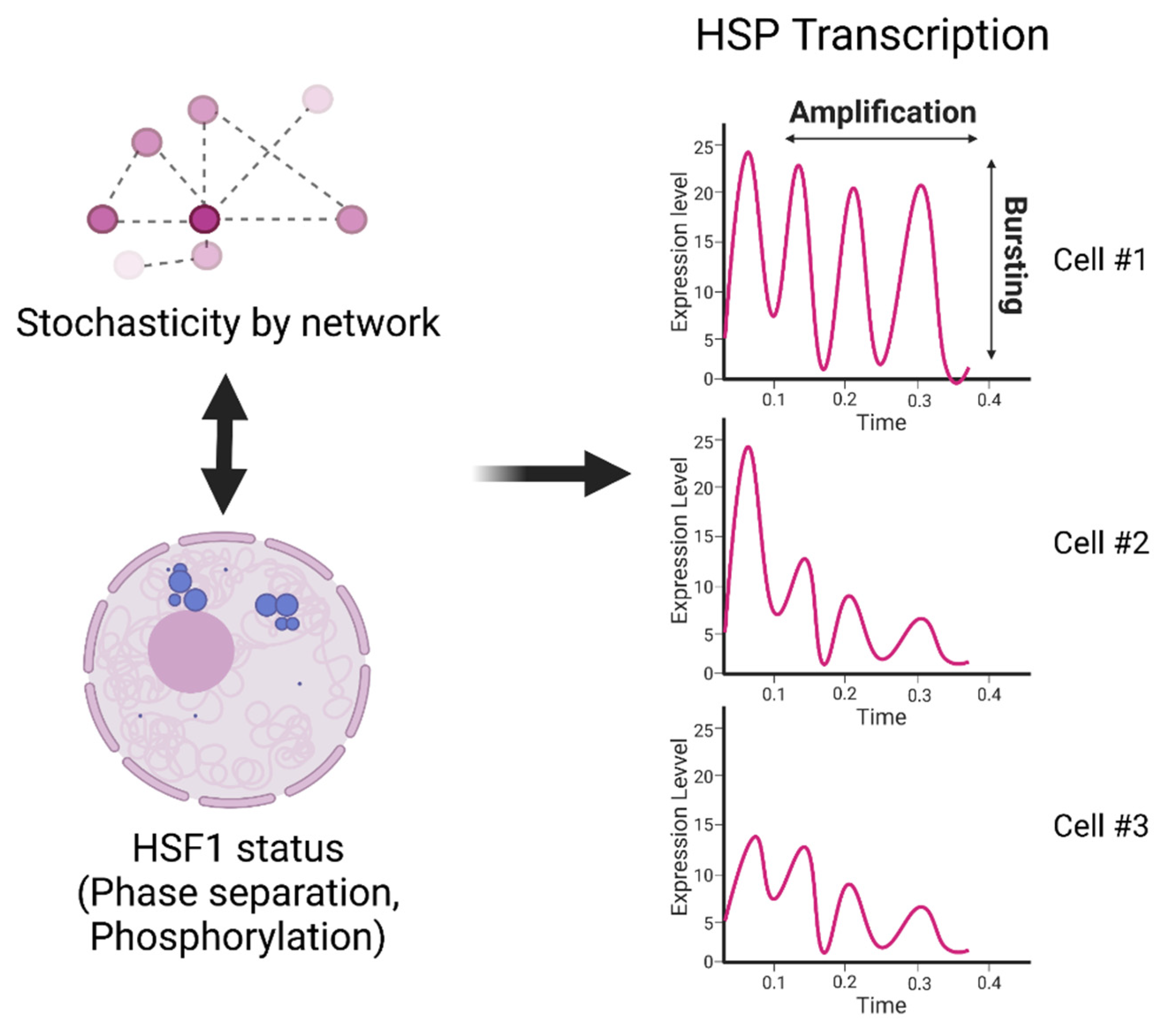
5. Mechanisms of Cell-to-Cell Heterogeneity in HS Signaling Activation
6. Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morimoto, R.I. The Heat Shock Response: Systems Biology of Proteotoxic Stress in Aging and Disease. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Joutsen, J.; Sistonen, L. Tailoring of Proteostasis Networks with Heat Shock Factors. Cold Spring Harb. Perspect. Biol. 2019, 11, a034066. [Google Scholar] [CrossRef] [PubMed]
- Anckar, J.; Sistonen, L. Regulation of HSF1 Function in the Heat Stress Response: Implications in Aging and Disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Labbadia, J.; Morimoto, R.I. Rethinking HSF1 in Stress, Development, and Organismal Health. Trends Cell Biol. 2017, 27, 895–905. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Rallu, M.; Loones, M.; Lallemand, Y.; Morimoto, R.; Morange, M.; Mezger, V. Function and regulation of heat shock factor 2 during mouse embryogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 2392–2397. [Google Scholar] [CrossRef]
- Bonner, J.J.; Carlson, T.; Fackenthal, D.L.; Paddock, D.; Storey, K.; Lea, K. Complex Regulation of the Yeast Heat Shock Transcription Factor. Mol. Biol. Cell 2000, 11, 1739–1751. [Google Scholar] [CrossRef][Green Version]
- Feder, Z.A.; Ali, A.; Singh, A.; Krakowiak, J.; Zheng, X.; Bindokas, V.P.; Wolfgeher, D.; Kron, S.J.; Pincus, D. Subcellular localization of the J-protein Sis1 regulates the heat shock response. J. Cell Biol. 2021, 220, 220. [Google Scholar] [CrossRef]
- O’Connell-Rodwell, C.E.; Mackanos, M.A.; Simanovskii, D.; Cao, Y.A.; Bachmann, M.H.; Schwettman, H.A.; Contag, C.H. In vivo analysis of heat-shock-protein-70 induction following pulsed laser irradiation in a transgenic reporter mouse. J. Biomed. Opt. 2008, 13, 030501. [Google Scholar] [CrossRef]
- Wilmink, G.J.; Opalenik, S.R.; Beckham, J.T.; Mackanos, M.A.; Nanney, L.B.; Contag, C.H.; Davidson, J.M.; Jansen, E.D. In-vivo optical imaging of hsp70 expression to assess collateral tissue damage associated with infrared laser ablation of skin. J. Biomed. Opt. 2008, 13, 054066. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, X.; Santalucia, T.; Fortin, P.Y.; Purroy, J.; Calvo, M.; Salas-Perdomo, A.; Justicia, C.; Couillaud, F.; Planas, A.M. In vivo imaging of induction of heat-shock protein-70 gene expression with fluorescence reflectance imaging and intravital confocal microscopy following brain ischaemia in reporter mice. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Ortner, V.; Ludwig, A.; Riegel, E.; Dunzinger, S.; Czerny, T. An artificial HSE promoter for efficient and selective detection of heat shock pathway activity. Cell Stress Chaperones 2015, 20, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Fortin, P.-Y.; Genevois, C.; Chapolard, M.; Santalucía, T.; Planas, A.M.; Couillaud, F. Dual-reporter in vivo imaging of transient and inducible heat-shock promoter activation. Biomed. Opt. Express 2014, 5, 457–467. [Google Scholar] [CrossRef]
- Link, C.D.; Cypser, J.R.; Johnson, C.J.; Johnson, T.E. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones 1999, 4, 235–242. [Google Scholar] [CrossRef]
- Hashimoto-Torii, K.; Sasaki, M.; Chang, Y.-W.; Hwang, H.; Waxman, S.G.; Kocsis, J.D.; Rakic, P.; Torii, M. Detection of local and remote cellular damage caused by spinal cord and peripheral nerve injury using a heat shock signaling reporter system. IBRO Rep. 2018, 5, 91–98. [Google Scholar] [CrossRef]
- Torii, M.; Sasaki, M.; Chang, Y.-W.; Ishii, S.; Waxman, S.G.; Kocsis, J.D.; Rakic, P.; Hashimoto-Torii, K. Detection of vulnerable neurons damaged by environmental insults in utero. Proc. Natl. Acad. Sci. USA 2017, 114, 2367–2372. [Google Scholar] [CrossRef]
- Ishii, S.; Torii, M.; Son, A.I.; Rajendraprasad, M.; Morozov, Y.M.; Kawasawa, Y.I.; Salzberg, A.C.; Fujimoto, M.; Brennand, K.; Nakai, A.; et al. Variations in brain defects result from cellular mosaicism in the activation of heat shock signalling. Nat. Commun. 2017, 8, 15157. [Google Scholar] [CrossRef]
- Hashimoto-Torii, K.; Torii, M.; Fujimoto, M.; Nakai, A.; El Fatimy, R.; Mezger, V.; Ju, M.J.; Ishii, S.; Chao, S.-H.; Brennand, K.J.; et al. Roles of Heat Shock Factor 1 in Neuronal Response to Fetal Environmental Risks and Its Relevance to Brain Disorders. Neuron 2014, 82, 560–572. [Google Scholar] [CrossRef]
- Mohammad, S.; Page, S.J.; Wang, L.; Ishii, S.; Li, P.; Sasaki, T.; Basha, A.; Salzberg, A.; Quezado, Z.; Imamura, F.; et al. Kcnn2 blockade reverses learning deficits in a mouse model of fetal alcohol spectrum disorders. Nat. Neurosci. 2020, 23, 533–543. [Google Scholar] [CrossRef]
- Mohammad, S.; Page, S.J.; Sasaki, T.; Ayvazian, N.; Rakic, P.; Kawasawa, Y.I.; Hashimoto-Torii, K.; Torii, M. Long-term spatial tracking of cells affected by environmental insults. J. Neurodev. Disord. 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Min, S.; Prahlad, V. Gene bookmarking by the heat shock transcription factor programs the insulin-like signaling pathway. Mol. Cell 2021, 81, 4843–4860.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, P.; Jiang, T.; Dai, D.; Wang, H.; Sun, J.; Zhu, L.; Xu, W.; Feng, L.; Shin, V.Y.; et al. Heat Shock Factor 1 Epigenetically Stimulates Glutaminase-1-Dependent mTOR Activation to Promote Colorectal Carcinogenesis. Mol. Ther. 2018, 26, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Gaglia, G.; Rashid, R.; Yapp, C.; Joshi, G.N.; Li, C.G.; Lindquist, S.L.; Sarosiek, K.A.; Whitesell, L.; Sorger, P.K.; Santagata, S. HSF1 phase transition mediates stress adaptation and cell fate decisions. Nat. Cell Biol. 2020, 22, 151–158. [Google Scholar] [CrossRef]
- Zheng, X.; Beyzavi, A.; Krakowiak, J.; Patel, N.; Khalil, A.S.; Pincus, D. Hsf1 Phosphorylation Generates Cell-to-Cell Variation in Hsp90 Levels and Promotes Phenotypic Plasticity. Cell Rep. 2018, 22, 3099–3106. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, S.; Zeng, Y.; Wang, X.; Qin, Y.; Ren, Q.; Xiang, S.; Wang, Y.; Xiao, J.; Sun, Y. Reversible phase separation of HSF1 is required for an acute transcriptional response during heat shock. Nature 2022, 24, 340–352. [Google Scholar] [CrossRef]
- Guilbert, M.; Anquez, F.; Pruvost, A.; Thommen, Q.; Courtade, E. Protein level variability determines phenotypic heterogeneity in proteotoxic stress response. FEBS J. 2020, 287, 5345–5361. [Google Scholar] [CrossRef]
- Ochiai, H.; Hayashi, T.; Umeda, M.; Yoshimura, M.; Harada, A.; Shimizu, Y.; Nakano, K.; Saitoh, N.; Liu, Z.; Yamamoto, T.; et al. Genome-wide kinetic properties of transcriptional bursting in mouse embryonic stem cells. Sci. Adv. 2020, 6, eaaz6699. [Google Scholar] [CrossRef]
- Kim, J.; Venkata, N.C.; Gonzalez, G.A.H.; Khanna, N.; Belmont, A.S. Gene expression amplification by nuclear speckle association. J. Cell Biol. 2019, 219, e201904046. [Google Scholar] [CrossRef]
- Tunnacliffe, E.; Chubb, J.R. What Is a Transcriptional Burst? Trends Genet. 2020, 36, 288–297. [Google Scholar] [CrossRef]
- Luo, L.; Ambrozkiewicz, M.C.; Benseler, F.; Chen, C.; Dumontier, E.; Falkner, S.; Furlanis, E.; Gomez, A.M.; Hoshina, N.; Huang, W.-H.; et al. Optimizing Nervous System-Specific Gene Targeting with Cre Driver Lines: Prevalence of Germline Recombination and Influencing Factors. Neuron 2020, 106, 37–65.e5. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, N.; Tavernarakis, N. Cellular stress response pathways and ageing: Intricate molecular relationships. EMBO J. 2011, 30, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Lamech, L.T.; Haynes, C.M. The unpredictability of prolonged activation of stress response pathways. J. Cell Biol. 2015, 209, 781–787. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torii, S.; Rakic, P. Tracking the Activation of Heat Shock Signaling in Cellular Protection and Damage. Cells 2022, 11, 1561. https://doi.org/10.3390/cells11091561
Torii S, Rakic P. Tracking the Activation of Heat Shock Signaling in Cellular Protection and Damage. Cells. 2022; 11(9):1561. https://doi.org/10.3390/cells11091561
Chicago/Turabian StyleTorii, Shisui, and Pasko Rakic. 2022. "Tracking the Activation of Heat Shock Signaling in Cellular Protection and Damage" Cells 11, no. 9: 1561. https://doi.org/10.3390/cells11091561
APA StyleTorii, S., & Rakic, P. (2022). Tracking the Activation of Heat Shock Signaling in Cellular Protection and Damage. Cells, 11(9), 1561. https://doi.org/10.3390/cells11091561




