Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis
Abstract
1. Introduction
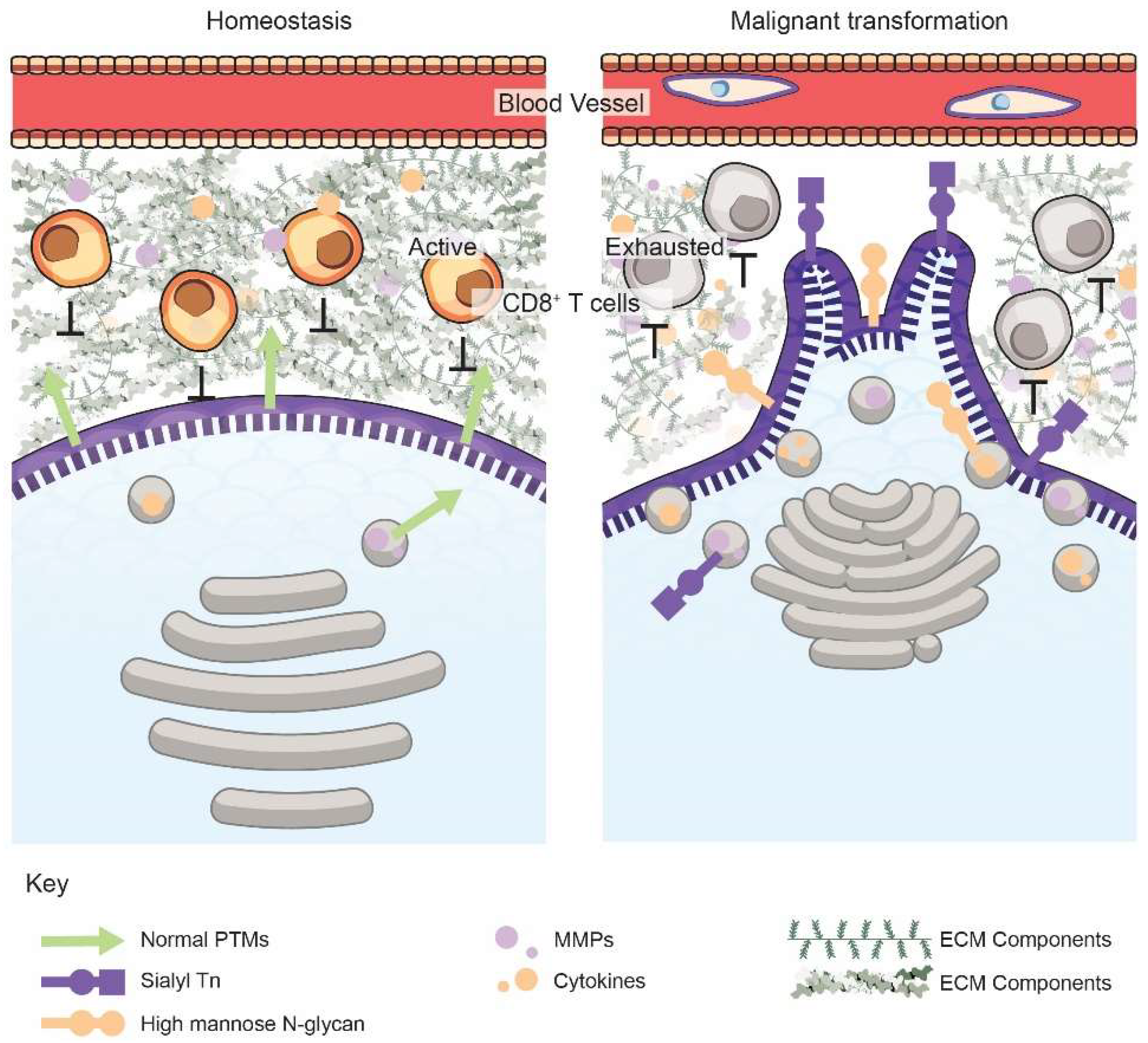
2. The Golgi Alters the Cancer Cell Secretome, the Tumor ECM, and Immune Surveillance
3. Golgi Dynamics in Cancer Metastasis
3.1. Golgi-Mediated Vesicular Trafficking and Exocytosis
3.2. Golgi Orientation Governs Cell Polarity and Directional Migration
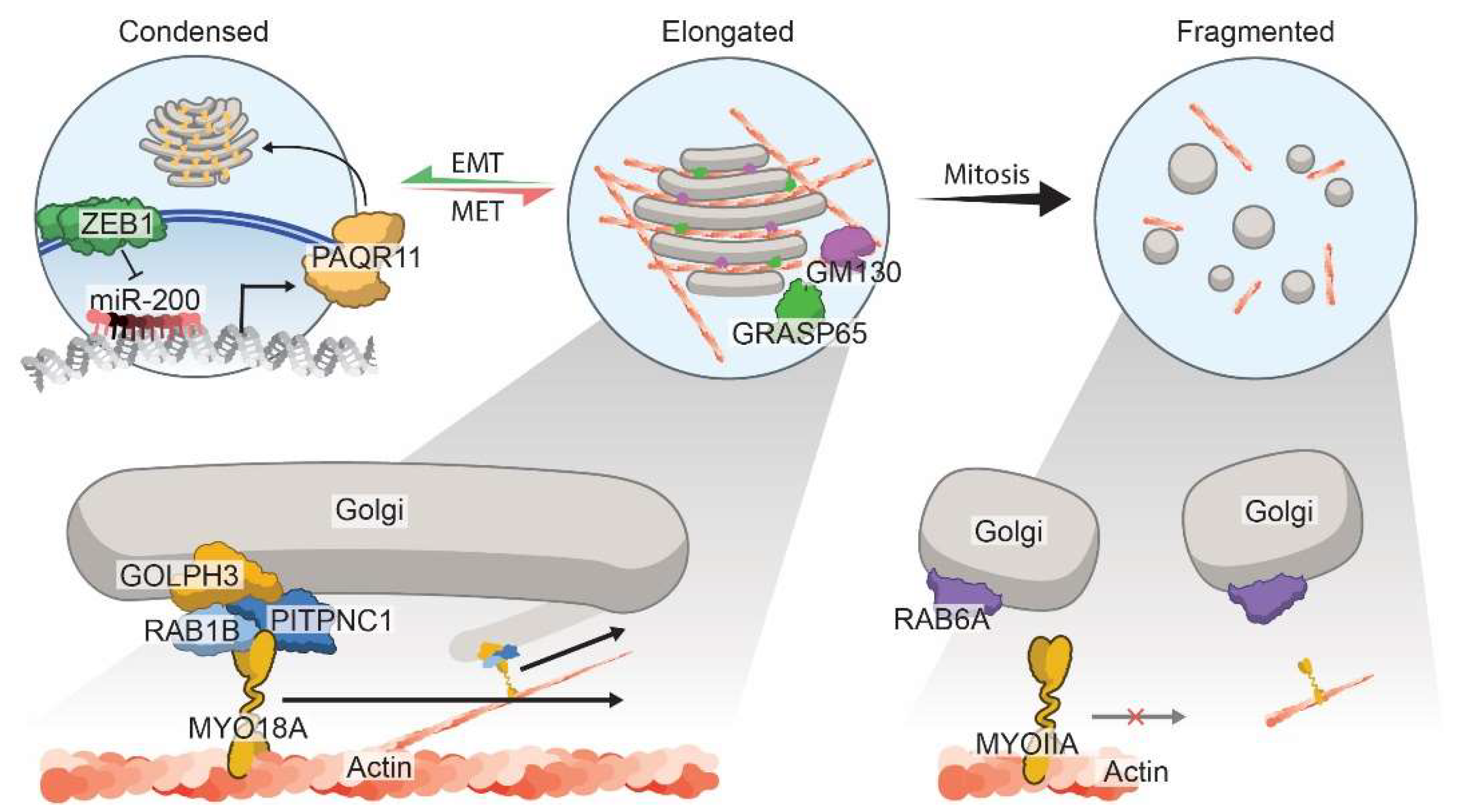
3.3. Morphology of the Golgi Apparatus
4. Golgi-Mediated Post-Translational Modifications (PTMs)
5. Therapeutic Targeting of Tumor Secretion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ER | Endoplasmic reticulum |
| PM | Plasma membrane |
| EC | Extracellular |
| CPS | Conventional protein secretion |
| UPS | Unconventional protein secretion |
| PTM | Post-translational modification |
| ECM | Extracellular matrix |
| KDELR | KDEL receptor |
| NSCLC | Non-small cell lung cancer |
| BM | Basement membrane |
| MMPs | Matrix metalloproteases |
| TME | Tumor microenvironment |
| GEFs | Guanine exchange factors |
| GAPs | GTPase activating proteins |
| ARF | ADP-ribosylation factor |
| IMPAD1 | Inositol monophosphatase domain containing 1 |
| NO | Nitric oxide |
| UPR | Unfolded protein response |
| EMT | Epithelial-to-mesenchymal transition |
| miR-200 | miRNA-200 family |
| PAQR11 | Progestin and adipoQ receptor family member 11 |
| TIME | Tumor immune microenvironment |
| GAGs | Glycosaminoglycans |
| GPI | Glycosylphosphatidylinositol |
| GSL | Glycosphingolipids |
| CS | Chondroitin sulfate |
| HS | Heparan sulfate |
| BFA | Brefeldin-A |
References
- Viotti, C. ER to Golgi-Dependent Protein Secretion: The Conventional Pathway. Methods Mol. Biol. 2016, 1459, 3–29. [Google Scholar] [PubMed]
- Palade, G. Intracellular Aspects of the Process of Protein Synthesis. Science 1975, 189, 347–358. [Google Scholar] [CrossRef]
- Paltridge, J.L.; Belle, L.; Khew-Goodall, Y. The secretome in cancer progression. Biochim. Biophys Acta 2013, 1834, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Rabouille, C. Pathways of Unconventional Protein Secretion. Trends Cell Biol. 2017, 27, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Rabouille, C.; Malhotra, V.; Nickel, W. Diversity in unconventional protein secretion. J. Cell Sci. 2012, 125 Pt 22, 5251–5255. [Google Scholar] [CrossRef] [PubMed]
- Nickel, W.; Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 2009, 10, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, M.; Jones, E.G.; Mazzarello, P.; Ribak, C.E.; Shepherd, G.M.; Swanson, L.W. Camillo Golgi and modern neuroscience. Brain Res. Rev. 2011, 66, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- GM, C. The Endoplasmic Reticulum. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Cooper, G. The Golgi Apparatus. In The Cell: A Molecular Approach; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Lodish, H.B.A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Overview of the Secretory Pathway. In Molecular Cell Biology, 4th ed.; W. H. Freeman: New York, NY, USA, 2000. [Google Scholar]
- Lodish, H.B.A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Protein Glycosylation in the ER and Golgi Complex. In Molecular Cell Biology; W. H. Freeman: New York, NY, USA, 2000. [Google Scholar]
- Wiersma, V.R.; Michalak, M.; Abdullah, T.M.; Bremer, E.; Eggleton, P. Mechanisms of Translocation of ER Chaperones to the Cell Surface and Immunomodulatory Roles in Cancer and Autoimmunity. Front. Oncol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Tan, X.; Banerjee, P.; Pham, E.A.; Rutaganira, F.U.N.; Basu, K.; Bota-Rabassedas, N.; Guo, H.F.; Grzeskowiak, C.L.; Liu, X.; Yu, J.; et al. PI4KIIIβ is a therapeutic target in chromosome 1q-amplified lung adenocarcinoma. Sci. Transl. Med. 2020, 12, eaax3772. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Halberg, N.; Sengelaub, C.A.; Navrazhina, K.; Molina, H.; Uryu, K.; Tavazoie, S.F. PITPNC1 Recruits RAB1B to the Golgi Network to Drive Malignant Secretion. Cancer Cell 2016, 29, 339–353. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Jwa, N.S.; Lebrun, M.H.; Job, D.; Rakwal, R. Plant secretome: Unlocking secrets of the secreted proteins. Proteomics 2010, 10, 799–827. [Google Scholar] [CrossRef]
- Han, H.M.; Bouchet-Marquis, C.; Huebinger, J.; Grabenbauer, M. Golgi apparatus analyzed by cryo-electron microscopy. Histochem. Cell Biol. 2013, 140, 369–381. [Google Scholar] [CrossRef]
- Kurokawa, K.; Ishii, M.; Suda, Y.; Ichihara, A.; Nakano, A. Live cell visualization of Golgi membrane dynamics by super-resolution confocal live imaging microscopy. Methods Cell Biol. 2013, 118, 235–242. [Google Scholar]
- Snapp, E.L. Photobleaching methods to study Golgi complex dynamics in living cells. Methods Cell Biol. 2013, 118, 195–216. [Google Scholar]
- Tannous, B.A. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat. Protoc. 2009, 4, 582–591. [Google Scholar] [CrossRef]
- Egea, G.; Franci, C.; Gambus, G.; Lesuffleur, T.; Zweibaum, A.; Real, F.X. cis-Golgi resident proteins and O-glycans are abnormally compartmentalized in the RER of colon cancer cells. J. Cell Sci. 1993, 105 Pt 3, 819–830. [Google Scholar] [CrossRef]
- Kellokumpu, S.; Sormunen, R.; Kellokumpu, I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: Dependence on intra-Golgi pH. Febs. Lett. 2002, 516, 217–224. [Google Scholar] [CrossRef]
- Albacete-Albacete, L.; Sanchez-Alvarez, M.; Del Pozo, M.A. Extracellular Vesicles: An Emerging Mechanism Governing the Secretion and Biological Roles of Tenascin-C. Front. Immunol. 2021, 12, 671485. [Google Scholar] [CrossRef]
- Duffy, M.J.; Maguire, T.M.; Hill, A.; McDermott, E.; O’Higgins, N. Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000, 2, 252. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.C.; Harris, D.A. Organizing ‘Elements’: Facilitating Exocytosis and Promoting Metastasis. Trends Cancer 2020, 6, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Sbai, O.; Ferhat, L.; Bernard, A.; Gueye, Y.; Ould-Yahoui, A.; Thiolloy, S.; Charrat, E.; Charton, G.; Tremblay, E.; Risso, J.; et al. vesicular trafficking and secretion of matrix metalloproteinases-2, -9 and tissue inhibitor of metalloproteinases-1 in neuronal cells. Mol. Cell. Neurosci. 2008, 39, 549–568. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, T.; Hasegawa, K.; Aoki, Y.; Watanabe, T.; Otagiri, Y.; Arasaki, K.; Wakana, Y.; Asano, K.; Tanaka, M.; Yamaguchi, H.; et al. MT1-MMP recruits the ER-Golgi SNARE Bet1 for efficient MT1-MMP transport to the plasma membrane. Cancer Cell Biol. 2019, 218, 3355–3371. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 1999, 43, S42–S51. [Google Scholar] [CrossRef]
- Jin, D.; Tao, J.; Li, D.; Wang, Y.; Li, L.; Hu, Z.; Zhou, Z.; Chang, X.; Qu, C.; Zhang, H. Golgi protein 73 activation of MMP-13 promotes hepatocellular carcinoma cell invasion. Oncotarget 2015, 6, 33523–33533. [Google Scholar] [CrossRef]
- Condamine, T.; Ramachandran, I.; Youn, J.I.; Gabrilovich, D.I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 2015, 66, 97–110. [Google Scholar] [CrossRef]
- Song, J.W.; Zhu, J.; Wu, X.X.; Tu, T.; Huang, J.Q.; Chen, G.Z.; Liang, L.Y.; Zhou, C.H.; Xu, X.; Gong, L.Y. GOLPH3/CKAP4 promotes metastasis and tumorigenicity by enhancing the secretion of exosomal WNT3A in non-small-cell lung cancer. Cell Death Dis. 2021, 12, 976. [Google Scholar] [CrossRef]
- Schinzari, V.; Timperi, E.; Pecora, G.; Palmucci, F.; Gallerano, D.; Grimaldi, A.; Covino, D.A.; Guglielmo, N.; Melandro, F.; Manzi, E.; et al. Wnt3a/β-Catenin Signaling Conditions Differentiation of Partially Exhausted T-effector Cells in Human Cancers. Cancer Immunol. Res. 2018, 6, 941–952. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Pavlou, M.P.; Diamandis, E.P. Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol. Oncol. 2010, 4, 496–510. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Marone, G.; Mantovani, A. Cancer Inflammation and Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028662. [Google Scholar] [CrossRef]
- Lieu, Z.Z.; Lock, J.G.; Hammond, L.A.; La Gruta, N.L.; Stow, J.L.; Gleeson, P.A. A trans-Golgi network golgin is required for the regulated secretion of TNF in activated macrophages in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 3351–3356. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Tessitore, A.; Alesse, E.; Capalbo, C.; Zazzeroni, F. Cancer secretome and inflammation: The bright and the dark sides of NF-κB. Semin. Cell Dev. Biol. 2018, 78, 51–61. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Sehgal, P.B. Interleukin-6 at the Host-Tumor Interface: STAT3 in Biomolecular Condensates in Cancer Cells. Cells 2022, 11, 1164. [Google Scholar] [CrossRef]
- Goldenring, J.R. A central role for vesicle trafficking in epithelial neoplasia: Intracellular highways to carcinogenesis. Nat. Reviews. Cancer 2013, 13, 813–820. [Google Scholar] [CrossRef]
- Bhuin, T.; Roy, J.K. Rab proteins: The key regulators of intracellular vesicle transport. Exp. Cell Res. 2014, 328, 1–19. [Google Scholar] [CrossRef]
- Mughees, M.; Chugh, H.; Wajid, S. Vesicular trafficking-related proteins as the potential therapeutic target for breast cancer. Protoplasma 2020, 257, 345–352. [Google Scholar] [CrossRef]
- Tzeng, H.-T.; Wang, Y.-C. Rab-mediated vesicle trafficking in cancer. J. Biomed. Sci. 2016, 23, 70. [Google Scholar] [CrossRef]
- Acob, A.; Jing, J.; Lee, J.; Schedin, P.; Gilbert, S.M.; Peden, A.A.; Junutula, J.R.; Prekeris, R. Rab40b regulates trafficking of MMP2 and MMP9 during invadopodia formation and invasion of breast cancer cells. J. Cell Sci. 2013, 126 Pt 20, 4647–4658. [Google Scholar]
- Hendrix, A.; Maynard, D.; Pauwels, P.; Braems, G.; Denys, H.; Van den Broecke, R.; Lambert, J.; Van Belle, S.; Cocquyt, V.; Gespach, C.; et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J. Natl. Cancer Inst. 2010, 102, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.A.; Kumar, R.; Hales, C.M.; Navarre, J.; Bhartur, S.G.; Burnette, J.O.; Provance, D.W., Jr.; Mercer, J.A.; Bähler, M.; Goldenring, J.R. Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 2001, 12, 1843–1857. [Google Scholar] [CrossRef] [PubMed]
- Welz, T.; Kerkhoff, E. Exploring the iceberg: Prospects of coordinated myosin V and actin assembly functions in transport processes. Small GTPases 2019, 10, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Howe, E.N.; Burnette, M.D.; Justice, M.E.; Schnepp, P.M.; Hedrick, V.; Clancy, J.W.; Guldner, I.H.; Lamere, A.T.; Li, J.; Aryal, U.K.; et al. Rab11b-mediated integrin recycling promotes brain metastatic adaptation and outgrowth. Nat. Commun. 2020, 11, 3017. [Google Scholar] [CrossRef] [PubMed]
- Ferro, E.; Bosia, C.; Campa, C.C. RAB11-mediated trafficking and human cancers: An updated review. Biology 2020, 10, 26. [Google Scholar] [CrossRef]
- Miserey-Lenkei, S.; Bousquet, H.; Pylypenko, O.; Bardin, S.; Dimitrov, A.; Bressanelli, G.; Bonifay, R.; Fraisier, V.; Guillou, C.; Bougeret, C.; et al. Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions. Nat. Commun. 2017, 8, 1254. [Google Scholar] [CrossRef]
- Ioannou, M.S.; McPherson, P.S. Regulation of Cancer Cell Behavior by the Small GTPase Rab13. J. Biol. Chem. 2016, 291, 9929–9937. [Google Scholar] [CrossRef]
- Neary, C.L.; Nesterova, M.; Cho, Y.S.; Cheadle, C.; Becker, K.G.; Cho-Chung, Y.S. Protein kinase A isozyme switching: Eliciting differential cAMP signaling and tumor reversion. Oncogene 2004, 23, 8847–8856. [Google Scholar] [CrossRef]
- Casalou, C.; Faustino, A.; Barral, D.C. Arf proteins in cancer cell migration. Small GTPases 2016, 7, 270–282. [Google Scholar] [CrossRef]
- Casalou, C.; Ferreira, A.; Barral, D.C. The Role of ARF Family Proteins and Their Regulators and Effectors in Cancer Progression: A Therapeutic Perspective. Front. Cell Dev. Biol. 2020, 8, 217. [Google Scholar] [CrossRef]
- Schlienger, S.; Campbell, S.; Claing, A. ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol. Biol. Cell 2014, 25, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tang, S.; Cai, Y.; Pi, W.; Deng, L.; Wu, G.; Chavanieu, A.; Teng, Y. Suppression of breast cancer metastasis through the inactivation of ADP-ribosylation factor 1. Oncotarget 2016, 7, 58111–58120. [Google Scholar] [CrossRef] [PubMed]
- Howley, B.V.; Link, L.A.; Grelet, S.; El-Sabban, M.; Howe, P.H. A CREB3-regulated ER-Golgi trafficking signature promotes metastatic progression in breast cancer. Oncogene 2018, 37, 1308–1325. [Google Scholar] [CrossRef] [PubMed]
- Howley, B.V.; Howe, P.H. Metastasis-associated upregulation of ER-Golgi trafficking kinetics: Regulation of cancer progression via the Golgi apparatus. Oncoscience 2018, 5, 142–143. [Google Scholar] [CrossRef]
- Ruggiero, C.; Grossi, M.; Fragassi, G.; Di Campli, A.; Di Ilio, C.; Luini, A.; Sallese, M. The KDEL receptor signalling cascade targets focal adhesion kinase on focal adhesions and invadopodia. Oncotarget 2018, 9, 10228–10246. [Google Scholar] [CrossRef]
- Bajaj, R.; Kundu, S.T.; Grzeskowiak, C.L.; Fradette, J.J.; Scott, K.L.; Creighton, C.J.; Gibbons, D.L. IMPAD1 and KDELR2 drive invasion and metastasis by enhancing Golgi-mediated secretion. Oncogene 2020, 39, 5979–5994. [Google Scholar] [CrossRef]
- Malsam, J.; Söllner, T.H. Organization of SNAREs within the Golgi stack. Cold Spring Harb Perspect. Biol. 2011, 3, a005249. [Google Scholar] [CrossRef]
- Sehgal, P.B.; Mukhopadhyay, S.; Xu, F.; Patel, K.; Shah, M. Dysfunction of Golgi tethers, SNAREs, and SNAPs in monocrotaline-induced pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1526–L1542. [Google Scholar] [CrossRef]
- Steffen, A.; Le Dez, G.; Poincloux, R.; Recchi, C.; Nassoy, P.; Rottner, K.; Galli, T.; Chavrier, P. MT1-MMP-Dependent Invasion Is Regulated by TI-VAMP/VAMP7. Curr. Biol. 2008, 18, 926–931. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J. Role of SNARE proteins in tumourigenesis and their potential as targets for novel anti-cancer therapeutics. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2015, 1856, 1–12. [Google Scholar] [CrossRef]
- Peak, T.C.; Su, Y.; Chapple, A.G.; Chyr, J.; Deep, G. Syntaxin 6: A novel predictive and prognostic biomarker in papillary renal cell carcinoma. Sci. Rep. 2019, 9, 3146. [Google Scholar] [CrossRef]
- Du, Y.; Shen, J.; Hsu, J.L.; Han, Z.; Hsu, M.C.; Yang, C.C.; Kuo, H.P.; Wang, Y.N.; Yamaguchi, H.; Miller, S.A.; et al. Syntaxin 6-mediated Golgi translocation plays an important role in nuclear functions of EGFR through microtubule-dependent trafficking. Oncogene 2014, 33, 756–770. [Google Scholar] [CrossRef]
- Moreno-Smith, M.; Halder, J.B.; Meltzer, P.S.; Gonda, T.A.; Mangala, L.S.; Rupaimoole, R.; Lu, C.; Nagaraja, A.S.; Gharpure, K.M.; Kang, Y.; et al. ATP11B mediates platinum resistance in ovarian cancer. J. Clin. Investig. 2013, 123, 2119–2130. [Google Scholar] [CrossRef]
- Boddul, S.V.; Meng, J.; Dolly, J.O.; Wang, J. SNAP-23 and VAMP-3 contribute to the release of IL-6 and TNFα from a human synovial sarcoma cell line. FEBS J. 2014, 281, 750–765. [Google Scholar] [CrossRef]
- Wang, L.; Brautigan, D.L. α-SNAP inhibits AMPK signaling to reduce mitochondrial biogenesis and dephosphorylates Thr172 in AMPKα in vitro. Nat. Commun. 2013, 4, 1559. [Google Scholar] [CrossRef]
- Naydenov, N.G.; Brown, B.; Harris, G.; Dohn, M.R.; Morales, V.M.; Baranwal, S.; Reynolds, A.B.; Ivanov, A.I. A membrane fusion protein αSNAP is a novel regulator of epithelial apical junctions. PLoS ONE 2012, 7, e34320. [Google Scholar] [CrossRef]
- Ravichandran, Y.; Goud, B.; Manneville, J.B. The Golgi apparatus and cell polarity: Roles of the cytoskeleton, the Golgi matrix, and Golgi membranes. Curr. Opin. Cell Biol. 2020, 62, 104–113. [Google Scholar] [CrossRef]
- Rizzo, R.; Russo, D.; Kurokawa, K.; Sahu, P.; Lombardi, B.; Supino, D.; Zhukovsky, M.A.; Vocat, A.; Pothukuchi, P.; Kunnathully, V.; et al. Golgi maturation-dependent glycoenzyme recycling controls glycosphingolipid biosynthesis and cell growth via GOLPH3. EMBO J. 2021, 40, e107238. [Google Scholar] [CrossRef]
- Kellokumpu, S. Golgi pH, Ion and Redox Homeostasis: How Much Do They Really Matter? Front. Cell Dev. Biol. 2019, 7, 93. [Google Scholar] [CrossRef]
- Mennerich, D.; Kellokumpu, S.; Kietzmann, T. Hypoxia and Reactive Oxygen Species as Modulators of Endoplasmic Reticulum and Golgi Homeostasis. Antioxid. Redox Signal. 2019, 30, 113–137. [Google Scholar] [CrossRef]
- Rankin, E.B.; Nam, J.M.; Giaccia, A.J. Hypoxia: Signaling the Metastatic Cascade. Trends Cancer 2016, 2, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, D.; Lucien, F.; Dubois, C.M. Hypoxia enhances cancer cell invasion through relocalization of the proprotein convertase furin from the trans-Golgi network to the cell surface. J. Cell. Physiol. 2012, 227, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Bensellam, M.; Maxwell, E.L.; Chan, J.Y.; Luzuriaga, J.; West, P.K.; Jonas, J.-C.; Gunton, J.E.; Laybutt, D.R. Hypoxia reduces ER-to-Golgi protein trafficking and increases cell death by inhibiting the adaptive unfolded protein response in mouse beta cells. Diabetologia 2016, 59, 1492–1502. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, L.; Mollica, M.; Re, A.T.; Wu, S.; Zuo, L. Nitric oxide in cancer metastasis. Cancer lett. 2014, 353, 1–7. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Satoh, A.; Chatterjee, S.; Toomre, D.K.; Chalouni, C.M.; Fulton, D.; Groszmann, R.J.; Shah, V.H.; Sessa, W.C. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc. Natl. Acad. Sci. USA 2006, 103, 19777–19782. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.J.; Allen, J.L.; Caswell, P.T. Vesicle trafficking pathways that direct cell migration in 3D matrices and in vivo. Traffic 2018, 19, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, A.; Louvard, D.; Singer, S.J. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc. Natl. Acad. Sci. USA 1982, 79, 2603–2607. [Google Scholar] [CrossRef]
- Mu, G.; Ding, Q.; Li, H.; Zhang, L.; Zhang, L.; He, K.; Wu, L.; Deng, Y.; Yang, D.; Wu, L.; et al. Gastrin stimulates pancreatic cancer cell directional migration by activating the Gz12/13-RhoA-ROCK signaling pathway. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Zhang, S.; Schafer-Hales, K.; Khuri, F.R.; Zhou, W.; Vertino, P.M.; Marcus, A.I. The tumor suppressor LKB1 regulates lung cancer cell polarity by mediating cdc42 recruitment and activity. Cell Tumor Stem. Cell Biol. 2008, 68, 740–748. [Google Scholar] [CrossRef]
- Xing, M.; Peterman, M.C.; Davis, R.L.; Oegema, K.; Shiau, A.K.; Field, S.J. GOLPH3 drives cell migration by promoting Golgi reorientation and directional trafficking to the leading edge. Mol. Biol. Cell 2016, 27, 3828–3840. [Google Scholar] [CrossRef]
- Baschieri, F.; Confalonieri, S.; Bertalot, G.; Di Fiore, P.P.; Dietmaier, W.; Leist, M.; Crespo, P.; Macara, I.G.; Farhan, H. Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis. Nat. Commun. 2014, 5, 4839. [Google Scholar] [CrossRef]
- Baschieri, F.; Farhan, H. Endomembrane control of cell polarity: Relevance to cancer. Small GTPases 2015, 6, 104–107. [Google Scholar] [CrossRef]
- Baschieri, F.; Uetz-von Allmen, E.; Legler, D.F.; Farhan, H. Loss of GM130 in breast cancer cells and its effects on cell migration, invasion and polarity. Cell Cycle 2015, 14, 1139–1147. [Google Scholar] [CrossRef]
- Dubois, F.; Alpha, K.; Turner, C.E. Paxillin regulates cell polarization and anterograde vesicle trafficking during cell migration. Mol. Biol. Cell 2017, 28, 3815–3831. [Google Scholar] [CrossRef]
- Mousson, A.; Legrand, M.; Steffan, T.; Vauchelles, R.; Carl, P.; Gies, J.P.; Lehmann, M.; Zuber, G.; De Mey, J.; Dujardin, D.; et al. Inhibiting FAK-Paxillin Interaction Reduces Migration and Invadopodia-Mediated Matrix Degradation in Metastatic Melanoma Cells. Cancers 2021, 13, 1871. [Google Scholar] [CrossRef]
- Yadav, S.; Puri, S.; Linstedt, A.D. A primary role for Golgi positioning in directed secretion, cell polarity and wound healing. Mol. Biol. Cell 2009, 20, 1728–1736. [Google Scholar] [CrossRef]
- Tan, X.; Banerjee, P.; Guo, H.F.; Ireland, S.; Pankova, D.; Ahn, Y.H.; Nikolaidis, I.M.; Liu, X.; Zhao, Y.; Xue, Y.; et al. Epithelial-to-mesenchymal transition drives a pro-metastatic Golgi compaction process through scaffolding protein PAQR11. J. Clin. investig. 2017, 127, 117–131. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Peng, D.H.; Ungewiss, C.; Tong, P.; Byers, L.A.; Wang, J.; Canales, J.R.; Villalobos, P.A.; Uraoka, N.; Mino, B.; Behrens, C.; et al. ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis. Oncogene 2017, 36, 1925–1938. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Lin, W.; Creighton, C.J.; Rizvi, Z.H.; Gregory, P.A.; Goodall, G.J.; Thilaganathan, N.; Du, L.; Zhang, Y.; Pertsemlidis, A.; et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009, 23, 2140–2151. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Ungewiss, C.; Rizvi, Z.H.; Roybal, J.D.; Peng, D.H.; Gold, K.A.; Shin, D.-H.; Creighton, C.J.; Gibbons, D.L. The microRNA-200/Zeb1 axis regulates ECM-dependent β1-integrin/FAK signaling, cancer cell invasion and metastasis through CRKL. Sci. Rep. 2016, 6, 18652. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-F.; Bota-Rabassedas, N.; Terajima, M.; Leticia Rodriguez, B.; Gibbons, D.L.; Chen, Y.; Banerjee, P.; Tsai, C.-L.; Tan, X.; Liu, X.; et al. A collagen glucosyltransferase drives lung adenocarcinoma progression in mice. Commun. Biol. 2021, 4, 482. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef]
- Sechi, S.; Frappaolo, A.; Karimpour-Ghahnavieh, A.; Piergentili, R.; Giansanti, M.G. Oncogenic Roles of GOLPH3 in the Physiopathology of Cancer. Int. J. Mol. Sci. 2020, 21, 933. [Google Scholar] [CrossRef]
- Rahajeng, J.; Kuna, R.S.; Makowski, S.L.; Tran, T.T.T.; Buschman, M.D.; Li, S.; Cheng, N.; Ng, M.M.; Field, S.J. Efficient Golgi Forward Trafficking Requires GOLPH3-Driven, PI4P-Dependent Membrane Curvature. Dev. Cell 2019, 50, 573–585.e575. [Google Scholar] [CrossRef]
- Scott, K.L.; Kabbarah, O.; Liang, M.C.; Ivanova, E.; Anagnostou, V.; Wu, J.; Dhakal, S.; Wu, M.; Chen, S.; Feinberg, T.; et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature 2009, 459, 1085–1090. [Google Scholar] [CrossRef]
- Kuna, R.S.; Field, S.J. GOLPH3: A Golgi phosphatidylinositol(4)phosphate effector that directs vesicle trafficking and drives cancer. J. Lipid Res. 2019, 60, 269–275. [Google Scholar] [CrossRef]
- Makhoul, C.; Gosavi, P.; Gleeson, P.A. Golgi Dynamics: The Morphology of the Mammalian Golgi Apparatus in Health and Disease. Front. Cell Dev. Biol. 2019, 7, 112. [Google Scholar] [CrossRef]
- Saraste, J.; Prydz, K. A New Look at the Functional Organization of the Golgi Ribbon. Front. Cell Dev. Biol. 2019, 7, 171. [Google Scholar] [CrossRef]
- Stow, J.L.; Murray, R.Z. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 2013, 24, 227–239. [Google Scholar] [CrossRef]
- Robbins, E.; Gonatas, N.K. The Ultrastructure of a Mammalian Cell during the Mitotic Cycle. J. Cell Biol. 1964, 21, 429–463. [Google Scholar] [CrossRef]
- Valente, C.; Colanzi, A. Mechanisms and Regulation of the Mitotic Inheritance of the Golgi Complex. Front. Cell Dev. Biol. 2015, 3, 79. [Google Scholar] [CrossRef]
- Petrosyan, A. Unlocking Golgi: Why Does Morphology Matter? Biochemistry 2019, 84, 1490–1501. [Google Scholar] [CrossRef]
- Petrosyan, A. Onco-Golgi: Is Fragmentation a Gate to Cancer Progression? Biochem. Mol. Biol. J. 2015, 1, 16. [Google Scholar] [CrossRef]
- Chia, J.; Goh, G.; Racine, V.; Ng, S.; Kumar, P.; Bard, F. RNAi screening reveals a large signaling network controlling the Golgi apparatus in human cells. Mol. Syst. Biol. 2012, 8, 629. [Google Scholar] [CrossRef]
- Lin, S.C.; Chien, C.W.; Lee, J.C.; Yeh, Y.C.; Hsu, K.F.; Lai, Y.Y.; Lin, S.C.; Tsai, S.J. Suppression of dual-specificity phosphatase-2 by hypoxia increases chemoresistance and malignancy in human cancer cells. J. Clin. Investig. 2011, 121, 1905–1916. [Google Scholar] [CrossRef]
- Lee, J.E.; Patel, K.; Almodóvar, S.; Tuder, R.M.; Flores, S.C.; Sehgal, P.B. Dependence of Golgi apparatus integrity on nitric oxide in vascular cells: Implications in pulmonary arterial hypertension. Am. J. Physiology. Heart Circ. Physiol. 2011, 300, H1141–H1158. [Google Scholar] [CrossRef]
- Lee, J.E.; Yuan, H.; Liang, F.X.; Sehgal, P.B. Nitric oxide scavenging causes remodeling of the endoplasmic reticulum, Golgi apparatus and mitochondria in pulmonary arterial endothelial cells. Nitric Oxide Biol. Chem. 2013, 33, 64–73. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Reviews. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Ferrer, L.; Legler, K.; Milde-Langosch, K. Role of protein glycosylation in cancer metastasis. Semin. Cancer Biol. 2017, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989, 52, 257–331. [Google Scholar]
- Hakomori, S.-I.; Cummings, R.D. Glycosylation effects on cancer development. Glycoconj. J. 2012, 29, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Nishikawa, A.; Ihara, Y.; Taniguchi, S.; Taniguchi, N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc. Natl. Acad. Sci. USA 1995, 92, 8754–8758. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Ihara, Y.; Matsuzawa, Y.; Taniguchi, N. Aberrant glycosylation of E-cadherin enhances cell-cell binding to suppress metastasis. J. Biol. Chem. 1996, 271, 13811–13815. [Google Scholar] [CrossRef]
- Kakugawa, Y.; Wada, T.; Yamaguchi, K.; Yamanami, H.; Ouchi, K.; Sato, I.; Miyagi, T. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc. Natl. Acad. Sci. USA 2002, 99, 10718–10723. [Google Scholar] [CrossRef]
- Rivinoja, A.; Pujol, F.M.; Hassinen, A.; Kellokumpu, S. Golgi pH, its regulation and roles in human disease. Ann. Med. 2010, 44, 542–554. [Google Scholar] [CrossRef]
- Julien, S.; Picco, G.; Sewell, R.; Vercoutter-Edouart, A.S.; Tarp, M.; Miles, D.; Clausen, H.; Taylor-Papadimitriou, J.; Burchell, J.M. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br. J. Cancer 2009, 100, 1746–1754. [Google Scholar] [CrossRef]
- Belo, A.I.; van Vliet, S.J.; Maus, A.; Laan, L.C.; Nauta, T.D.; Koolwijk, P.; Tefsen, B.; van Die, I. Hypoxia inducible factor 1α down regulates cell surface expression of α1,2-fucosylated glycans in human pancreatic adenocarcinoma cells. FEBS Lett. 2015, 589, 2359–2366. [Google Scholar] [CrossRef]
- Lu, H.H.; Lin, S.Y.; Weng, R.R.; Juan, Y.H.; Chen, Y.W.; Hou, H.H.; Hung, Z.C.; Oswita, G.A.; Huang, Y.J.; Guu, S.Y.; et al. Fucosyltransferase 4 shapes oncogenic glycoproteome to drive metastasis of lung adenocarcinoma. EBioMedicine 2020, 57, 102846. [Google Scholar] [CrossRef]
- Pothukuchi, P.; Agliarulo, I.; Pirozzi, M.; Rizzo, R.; Russo, D.; Turacchio, G.; Nüchel, J.; Yang, J.-S.; Gehin, C.; Capolupo, L.; et al. GRASP55 regulates intra-Golgi localization of glycosylation enzymes to control glycoshingolipid biosynthesis. EMBO J. 2021, 40, e107766. [Google Scholar] [CrossRef]
- Stevenson, N.L.; Bergen, D.J.M.; Skinner, R.E.H.; Kague, E.; Martin-Silverstone, E.; Brown, K.A.R.; Hammond, C.L.; Stephens, D.J. Giantin-knockout models reveal a feedback loop between Golgi function and glycosyltrasferase expression. J. Cell Sci. 2017, 130, 4132–4143. [Google Scholar]
- Conroy, L.R.; Stanback, A.E.; Young, L.E.A.; Clarke, H.A.; Austin, G.L.; Liu, J.; Allison, D.B.; Sun, R.C. In Situ analysis of N-linked glycans as potential biomarkers of clinical course in human prostate cancer. Mol. Cancer Res. 2021, 19, 1727–1738. [Google Scholar] [CrossRef]
- Langford, J.K.; Stanley, M.J.; Cao, D.; Sanderson, R.D. Multiple heparan sulfate chains are required for optimal syndecan-1 function. J. Biol. Chem. 1998, 273, 29965–29971. [Google Scholar] [CrossRef]
- Vicente, C.M.; da Silva, D.A.; Sartorio, P.V.; Silva, T.D.; Saad, S.S.; Nader, H.B.; Forones, N.M.; Toma, L. Heparan Sulfate Proteoglycans in Human Colorectal Cancer. Anal. Cell. Pathol. 2018, 2018, 8389595. [Google Scholar] [CrossRef]
- Pudełko, A.; Wisowski, G.; Olczyk, K.; Koźma, E.M. The dual role of the glycosaminoglycan chondroitin-6-sulfate in the development, progression and metastasis of cancer. FEBS J. 2019, 286, 1815–1837. [Google Scholar] [CrossRef]
- Jin, H.; Zangar, R.C. Protein modifications as potential biomarkers in breast cancer. Biomark. Insights 2009, 4, S2557. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Lee, Y.; Viswanathan, S.R.; El-Guindy, A.S.; Gerlach, J.; Nikiforow, S.; Shedd, D.; Gradoville, L.; Miller, G. N-linked glycosylation is required for optimal function of Kaposi’s sarcoma herpesvirus-encoded, but not cellular, interleukin 6. J. Exp. Med. 2004, 199, 503–514. [Google Scholar] [CrossRef]
- Zappa, F.; Failli, M.; De Matteis, M.A. The Golgi complex in disease and therapy. Curr. Opin. Cell Biol. 2018, 50, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Shi, L.; Banerjee, P.; Liu, X.; Guo, H.; Yu, J.; Bota-Rabassedas, N.; Rodriguez, B.L.; Gibbons, D.L.; Russell, W.K.; et al. A protumorigenic secretory pathway activated by p53 deficiency in lung adenocarcinoma. J. Clin. Investig. 2021, 131, e137186. [Google Scholar] [CrossRef] [PubMed]
- Rutaganira, F.U.; Fowler, M.L.; McPhail, J.A.; Gelman, M.A.; Nguyen, K.; Xiong, A.; Dornan, G.L.; Tavshanjian, B.; Glenn, J.S.; Shokat, K.M.; et al. Design and Structural Characterization of Potent and Selective Inhibitors of Phosphatidylinositol 4 Kinase IIIβ. J. Med. Chem. 2016, 59, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Shi, D.S.; Grossmann, A.H.; Odelberg, S.J.; Ostanin, K.; Li, D.Y. ARF6 is an actionable node that orchestrates oncogenic GNAQ signaling in uveal melanoma. Cancer Cell 2016, 29, 889–904. [Google Scholar] [CrossRef]
- Fu, D.; Roufogalis, B.D. Actin disruption inhibits endosomal traffic of P-glycoprotein-EGFP and resistance to daunorubicin accumulation. Am. J. Physiol. Cell Physiol. 2007, 292, C1543–C1552. [Google Scholar] [CrossRef]
- Drummond, A.H.; Beckett, P.; Brown, P.D.; Bone, E.A.; Davidson, A.H.; Galloway, W.A.; Gearing, A.J.H.; Huxley, P.; Laber, D.; McCourt, M.; et al. Preclinical and clinical studies of MMP inhibitors in cancer. Ann. N. Y. Acad. Sci. 2006, 878, 228–235. [Google Scholar] [CrossRef]
- Shah, M.A.; Starodub, A.; Sharma, S.; Silverman, J.A.; Lenz, H.; Bendell, J.C. Andecaliximab/GS-5745 alone and combined with mFOLFOX6 in advanced gastric and gastroesophageal junction adenocarcinoma: Results from a Phase I study. Clin. Cancer Res. 2018, 24, 3829–3837. [Google Scholar] [CrossRef]
- Huet, G.; Hennebicq-Reig, S.; Bolos, C.; Ulloa, F.; Lesuffleur, T.; Barbat, A.; Carriere, V.; Kim, I.; Real, F.X.; Delannoy, P.; et al. GalNAc-alpha-O-benzyl inhibits NeuAcalpha2-3 glycosylation and blocks the intracellular transport of apical glycoproteins and mucus in differentiated HT-29 cells. J. Cell Biol. 1998, 141, 1311–1322. [Google Scholar] [CrossRef]
- Song, L.; Linstedt, A.D. Inhibitor of ppGalNAc-T3-mediated O-glycosylation blocks cancer cell invasiveness and lowers FGF23 levels. Elife 2017, 6, e24051. [Google Scholar] [CrossRef]
- Madden, E.C.; Gorman, A.M.; Logue, S.E.; Samali, A. Tumour Cell Secretome in Chemoresistance and Tumour Recurrence. Trends Cancer 2020, 6, 489–505. [Google Scholar] [CrossRef]
- Grieve, A.G.; Rabouille, C. Golgi bypass: Skirting around the heart of classical secretion. Cold Spring Harb. Perspect. Biol. 2011, 3, a005298. [Google Scholar] [CrossRef]
- Almiron Bonnin, D.A.; Havrda, M.C.; Israel, M.A. Glioma Cell Secretion: A Driver of Tumor Progression and a Potential Therapeutic Target. Cancer Res. 2018, 78, 6031–6039. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajaj, R.; Warner, A.N.; Fradette, J.F.; Gibbons, D.L. Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis. Cells 2022, 11, 1484. https://doi.org/10.3390/cells11091484
Bajaj R, Warner AN, Fradette JF, Gibbons DL. Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis. Cells. 2022; 11(9):1484. https://doi.org/10.3390/cells11091484
Chicago/Turabian StyleBajaj, Rakhee, Amanda N. Warner, Jared F. Fradette, and Don L. Gibbons. 2022. "Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis" Cells 11, no. 9: 1484. https://doi.org/10.3390/cells11091484
APA StyleBajaj, R., Warner, A. N., Fradette, J. F., & Gibbons, D. L. (2022). Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis. Cells, 11(9), 1484. https://doi.org/10.3390/cells11091484





