Respiratory Viral and Bacterial Exacerbations of COPD—The Role of the Airway Epithelium
Abstract
1. Introduction
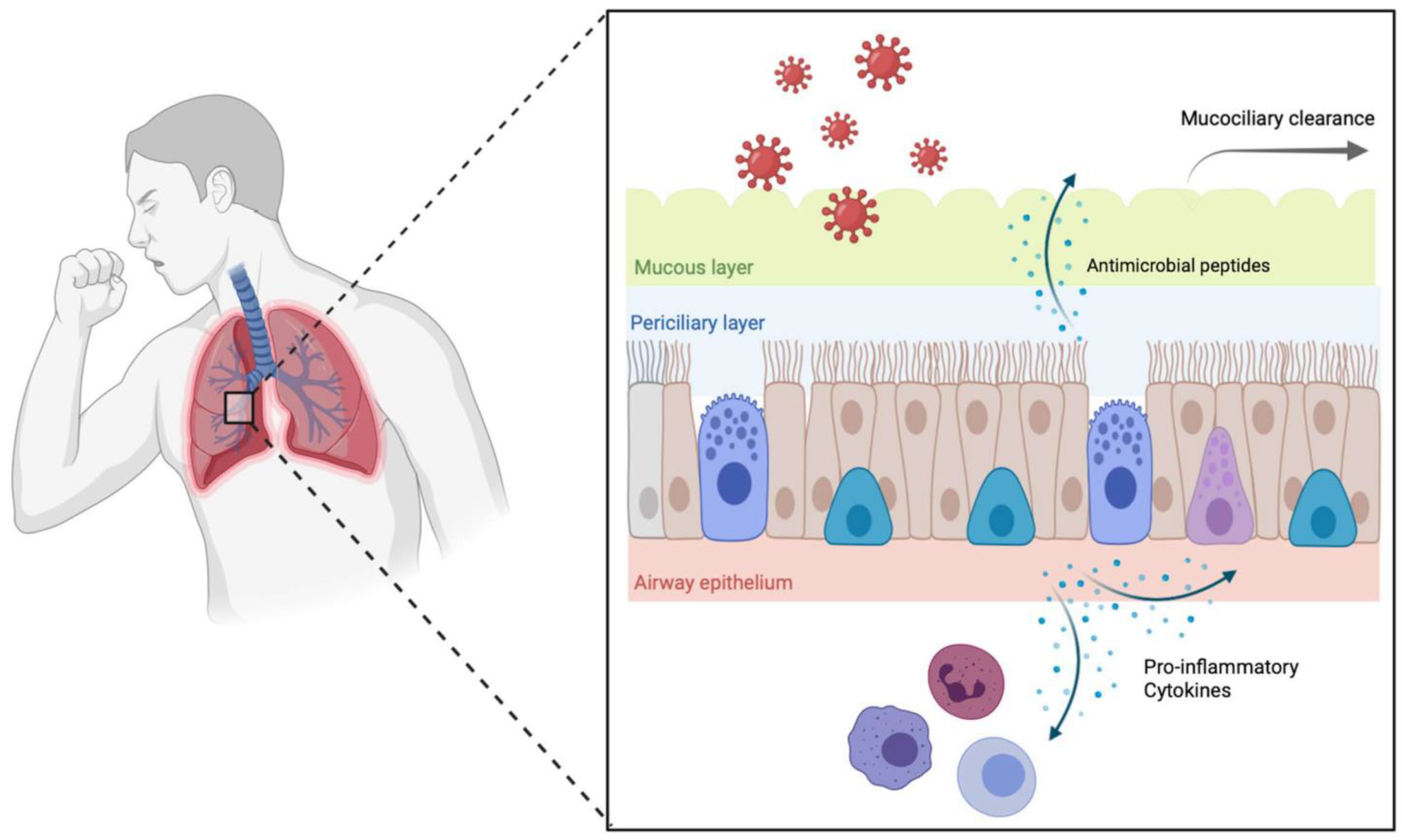
2. Role of the Epithelium in Host Defense
3. Cigarette Smoke Impairs Mucociliary Clearance and Barrier Function
4. Bacteria and COPD Exacerbations
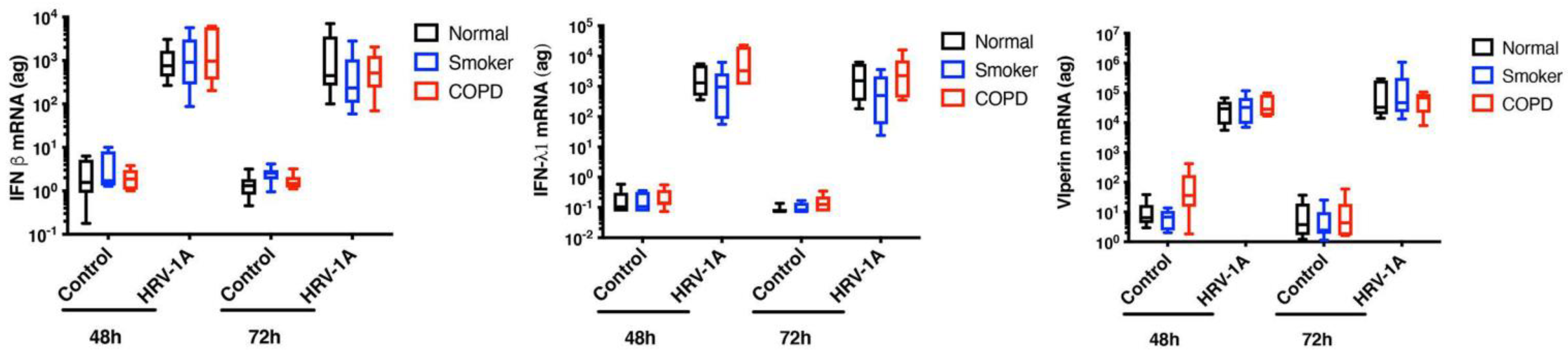
5. Viruses and COPD Exacerbations
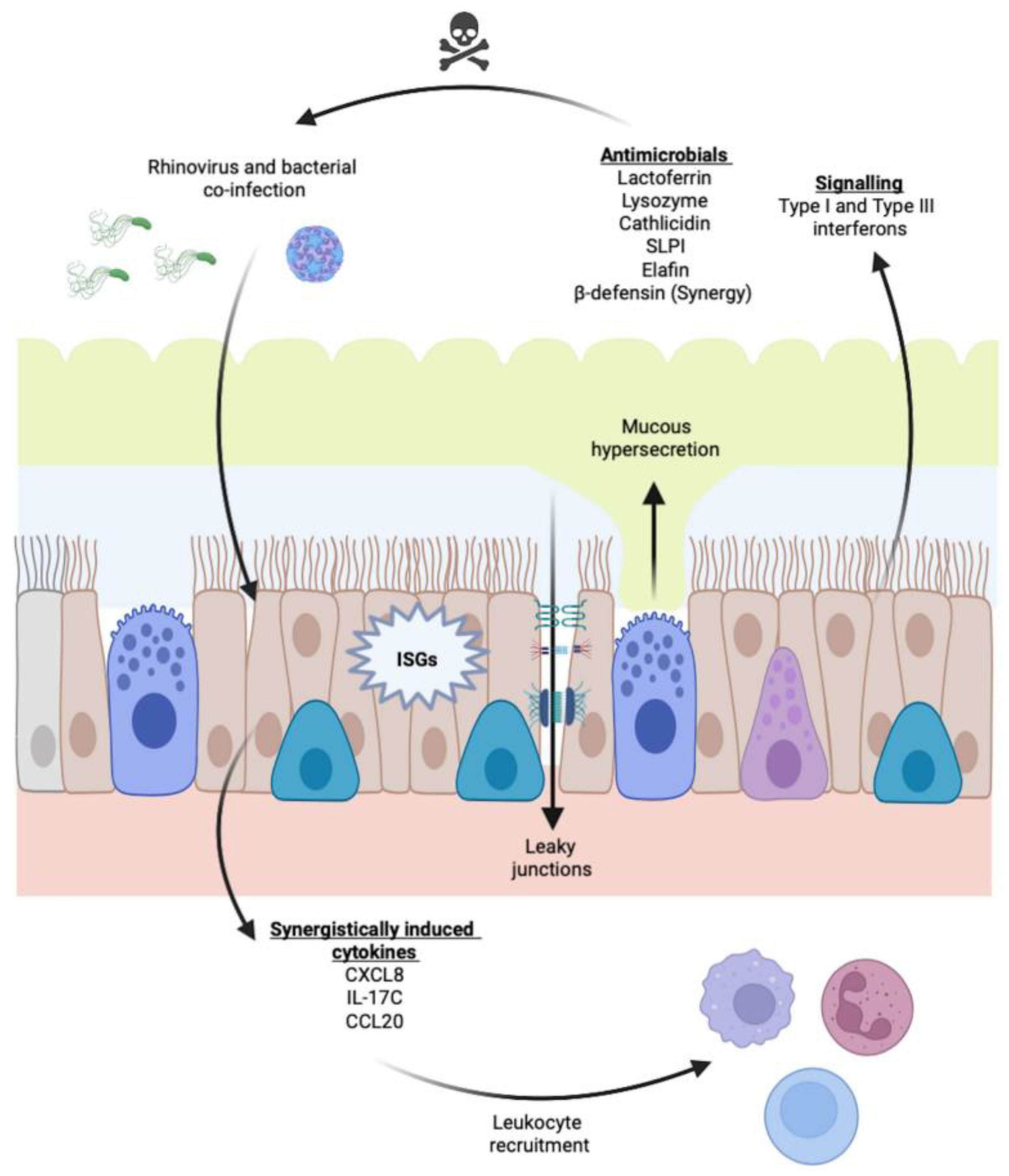
6. Viral–Bacterial Co-Infections
7. Current Treatment of COPD Exacerbations
Author Contributions
Funding
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease, Report. Global Strategy for Prevention, Diagnosis and Management of COPD: 2022 Report. Available online: https://goldcopd.org/2022-gold-reports-2/ (accessed on 28 March 2022).
- Halpin, D.M.G.; Celli, B.R.; Criner, G.J.; Frith, P.; López Varela, M.V.; Salvi, S.; Vogelmeier, C.F.; Chen, R.; Mortimer, K.; Montes de Oca, M.; et al. The GOLD summit on chronic obstructive pulmonary disease in low- and middle-income countries. Int. J. Tuberc. Lung Dis. 2019, 23, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.G.; Decramer, M.; Celli, B.R.; Mueller, A.; Metdorf, N.; Tashkin, D.P. Effect of a single exacerbation on decline in lung function in COPD. Respir. Med. 2017, 128, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Müllerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; MacNee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Seemungal, T.A.R.; Bhowmik, A.; Wedzicha, J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002, 57, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.R.; Donaldson, C.G.; Paul, E.A.; Bestall, J.C.; Jeffries, D.J.; Wedzicha, J.A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cataluña, J.J.; Martinez-Garcia, M.A.; Román-Sánchez, P.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Wedzicha, J.A. COPD exacerbations, 1: Epidemiology. Thorax 2006, 61, 164–168. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Ramsey, S.D.; Lee, T.A. The economic burden of COPD. Chest 2000, 117, 5S–9S. [Google Scholar] [CrossRef]
- Wouters, E.F.M. Economic analysis of the Confronting COPD survey: An overview of results. Respir. Med. 2003, 97, S3–S14. [Google Scholar] [CrossRef]
- Seemungal, T.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Wedzicha, J.A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med. 2000, 161, 1608–1613. [Google Scholar] [CrossRef]
- Cameron, R.J.; de Wit, D.; Welsh, T.N.; Ferguson, J.; Grissell, T.V.; Rye, P.J. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med. 2006, 32, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, J.; Tabor, D.E.; Silver, J.S.; Nair, V.; Tovchigrechko, A.; Griffin, M.P.; Esser, M.T.; Sellman, B.R.; Jin, H. Microbial burden and viral exacerbations in a longitudinal multicenter COPD cohort. Respir. Res. 2020, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.; Harper-Owen, R.; Bhowmik, A.; Moric, I.; Sanderson, G.; Message, S.; Maccallum, P.; Meade, T.W.; Jeffries, D.J.; Johnston, S.L.; et al. Respiratory viruses, symptoms and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med. 2001, 164, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Zwaans, W.A.R.; Mallia, P.; van Winden, M.E.C.; Rohde, G.G.U. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease-a systematic review. J. Clin. Virol. 2014, 61, 181–188. [Google Scholar] [CrossRef]
- Papi, A.; Bellettato, C.M.; Braccioni, F.; Romagnoli, M.; Casolari, P.; Caramori, G.; Fabbri, L.M.; Johnston, S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Wark, P.A.B.; Tooze, M.; Powell, H.; Parsons, K. Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission. Respirology 2013, 18, 996–1002. [Google Scholar] [CrossRef]
- Wilkinson, T.M.A.; Hurst, J.R.; Perera, W.R.; Wilks, M.; Donaldson, G.C.; Wedzicha, J.A. Effects of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006, 129, 317–324. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Kato, K.; Lu, W.; Kim, K.C. Cellular and molecular biology of airway mucins. Int. Rev. Cell Mol. Biol. 2013, 303, 139–202. [Google Scholar]
- Plotkowski, M.C.; Bajolet-Laudinet, O.; Puchelle, E. Cellular and molecular mechanisms of bacterial adhesion to respiratory mucosa. Eur. Respir. J. 1993, 6, 903–916. [Google Scholar]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell. Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006, 126, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Proud, D.; Leigh, R. Epithelial cells and airway diseases. Immunol. Rev. 2011, 242, 186–204. [Google Scholar] [CrossRef]
- Chapman, K.E.; Sinclair, S.E.; Zhuang, D.; Hassid, A.; Desai, L.P.; Waters, C.M. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L834–L841. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, P.; Lorentzen, D.; Excoffon, K.J.; Zabner, J.; McCray, P.B., Jr.; Nauseef, W.M.; Dupuy, C.; Bánfi, B. A novel host defense system of airways is defective in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 175, 174–183. [Google Scholar] [CrossRef]
- Bove, P.F.; van der Vliet, A. Nitric oxide and reactive nitrogen species in airway epithelial cell signaling and inflammation. Free Radic. Biol. Med. 2006, 41, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kubes, P. Nitric oxide as an antiinflammatory agent. Methods Enzymol. 1996, 269, 434–442. [Google Scholar]
- Sanders, S.P.; Siekierski, E.S.; Porter, J.D.; Richards, S.M.; Proud, D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J. Virol. 1998, 72, 934–942. [Google Scholar] [CrossRef]
- Ichinose, M.; Sugiura, H.; Yamagata, S.; Koarai, A.; Shirato, K. Increase in reactive oxygen species production in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 162, 701–706. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Caramori, G.; Ito, K.; Capelli, A.; Brun, P.; Abatangelo, G.; Papi, A.; Chung, K.F.; Adcock, I.; Barnes, P.J.; et al. Nitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2005, 116, 1028–1035. [Google Scholar] [CrossRef]
- Lumsden, A.B.; McLean, A.; Lamb, D. Goblet and Clara cells of human distal airways: Evidence of smoking induced changes in their numbers. Thorax 1984, 39, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Saetta, M.; Turato, G.; Baraldo, S.; Zanin, A.; Braccioni, F.; Mapp, C.E.; Maestrelli, P.; Cavallesco, G.; Papi, A.; Fabbri, L.M. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am. J. Respir. Crit. Care Med. 2000, 161, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Jeong, S.; Zhao, H.; Kesimer, M.; Boucher, R.C.; Wells, J.M.; Christenson, S.A.; Han, M.K.; Dransfield, M.; Paine, R.R.; et al. Current smoking with or without chronic bronchitis is independently associated with goblet cell hyperplasia in healthy smokers and COPD subjects. Sci. Rep. 2020, 10, 20133. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.Y.; Kaza, N.; Birket, S.E.; Kim, H.; Edwards, L.J.; LaFontaine, J.; Liu, L.; Mazur, M.; Byzek, S.A.; Hanes, J.; et al. Excess mucus viscosity and airway hydration impact COPD airway clearance. Eur. Respir. J. 2020, 55, 1900419. [Google Scholar] [CrossRef] [PubMed]
- Haswell, L.E.; Hewitt, K.; Thorne, D.; Richter, A.; Gaca, M.D. Cigarette smoke total particulate matter increases mucous secreting cell numbers in vitro: A potential model of goblet cell hyperpasia. Toxicol. In Vitro 2010, 24, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Gindele, J.A.; Kiechle, T.; Benediktus, K.; Birk, G.; Brendel, M.; Heinemann, F.; Wohnhaas, C.T.; LeBlanc, M.; Zhang, H.; Strulovici-Barel, Y.; et al. Intermittent exposure to whole cigarette smoke alters the differentiation of primary small airway epithelial cells in the air-liquid interface culture. Sci. Rep. 2020, 10, 6257. [Google Scholar] [CrossRef]
- Caramori, G.; Casolari, P.; Di Gregorio, C.; Saetta, M.; Baraldo, S.; Boschetto, P.; Ito, K.; Fabbri, L.M.; Barnes, P.J.; Adcock, I.M.; et al. MUC5AC expression is increased in bronchial submucosal glands of stable COPD patients. Histopathology 2009, 55, 321–331. [Google Scholar] [CrossRef]
- Vestbo, J.; Prescott, E.; Lange, P. Association of chronic mucus hypersecretion with FEV1 decine and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am. J. Respir. Crit. Care Med. 1996, 153, 1530–1535. [Google Scholar] [CrossRef]
- Kesimer, M.; Ford, A.A.; Ceppe, A.; Radicioni, G.; Cao, R.; Davis, C.W.; Doerschuk, C.M.; Alexis, N.E.; Anderson, W.H.; Henderson, A.G.; et al. Airway mucin concentration as a marker of chronic bronchitis. N. Engl. J. Med. 2017, 377, 911–922. [Google Scholar] [CrossRef]
- Kreindler, J.L.; Jackson, A.D.; Kemp, P.A.; Bridges, R.J.; Danahay, H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L894–L902. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hanrahan, J.W.; Bilodeau, G.; Ellis, L.; Dupuis, A.; Liao, J.; Zielenski, J.; Durie, P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am. J. Respir Crit. Care Med. 2006, 173, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Sun, A.; Chen, C.-S.; Mintz, A.J.; Chin, W.-C. Nicotine alters mucin rheological properties. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L149–L157. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, E.; Xiong, G.; Anselmo-Lima, W.T.; Kreindler, J.L.; Palmer, J.N.; Cohen, N.A. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am. J. Rhinol. Allergy 2009, 23, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Simet, S.M.; Sisson, J.H.; Pavlik, J.A.; Devasure, J.M.; Boyer, C.; Liu, X.; Kawasaki, S.; Sharp, J.G.; Rennard, S.I.; Wyatt, T.A. Long-term cigarette smoke exposure in a mouse model of ciliated epithelial function. Am. J. Respir. Cell Mol. Biol. 2010, 43, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Sisson, J.H.; Papi, A.; Beckmann, J.D.; Leise, K.L.; Wisecarver, J.; Broderson, B.W.; Kelling, C.L.; Spurzem, J.R.; Rennard, S.I. Smoke and viral infection cause cilia loss detectable by bronchoalveolar lavage cytology and dynein ELISA. Am. J. Respir. Crit. Care Med. 1994, 149, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Gattas, M.; Connor, G.E.; Fregien, N.L. Gefitinib, an EGFR tyrosine kinase inhibitor, prevents smoke-mediated ciliated airway epithelial cell loss and promoted their recovery. PLoS ONE 2016, 11, e0160216. [Google Scholar] [CrossRef] [PubMed]
- Leopold, P.L.; O’Mahoney, M.J.; Lian, X.J.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G. Smoking is associated with shortened airway cilia. PLoS ONE 2009, 4, e8157. [Google Scholar] [CrossRef]
- Hessel, J.; Heldrich, J.; Fuller, J.; Staudt, M.R.; Radisch, S.; Hollmann, C.; Harvey, B.-G.; Kaner, R.J.; Salit, J.; Yee-Levin, J.; et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS ONE 2014, 9, e85453. [Google Scholar] [CrossRef]
- Agius, A.M.; Smallman, L.A.; Pahor, A.L. Age, smoking and nasal ciliary beat frequency. Clin. Otolaryngol. Allied Sci. 1998, 23, 227–230. [Google Scholar] [CrossRef]
- Cohen, N.A.; Zhang, S.; Sharp, D.B.; Tamashiro, E.; Chen, B.; Sorscher, E.J.; Woodworth, B.A. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope 2009, 119, 2269–2274. [Google Scholar] [CrossRef]
- Ito, J.T.; Ramos, D.; Lima, F.; Rodrigues, F.M.M.; Gomes, P.R.; Moreira, G.L.; Macchione, M.; Toledo, A.C.; Ramos, E.M.C. Nasal mucociliary clearance in subjects with COPD after smoking cessation. Respir. Care 2015, 60, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.R.; Hosford, S.P.; Dunn, L.A.; Walker, D.C.; Hogg, J.C. Respiratory epithelial permeability after cigarette smoke exposure in guinea pigs. J. Appl. Physiol. 1989, 66, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, M.; Kan-O, K.; Ishii, Y.; Yamamoto, N.; Ogawa, T.; Fukuyama, S.; Ogawa, A.; Fujita, A.; Nakanishi, Y.; Matsumoto, K. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: Protective role of cathelicidin LL-37. Respir. Res. 2019, 20, 251. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Otaki, F.; Bonsu, P.; Dang, D.T.; Teater, M.; Stukovici-Barel, Y.; Salit, J.; Harvey, B.-G.; Crystal, R.G. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol. Life Sci. 2011, 68, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; Brandenburg, S.M.; Postma, D.S.; van Oosterhout, A.J.M. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur. Respir. J. 2012, 39, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Schamberger, A.C.; Mise, N.; Genoyer, E.; Yildirim, A.O.; Meiners, S.; Eickelberg, O. Cigarette smoke-induced disruption of bronchial epithelial tight junctions is prevented by transforming growth factor-β. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Oldenburger, A.; Poppinga, W.J.; Kos, F.; de Bruin, H.G.; Rijks, W.F.; Heijink, I.H.; Timens, W.; Meurs, H.; Maarsingh, H.; Schmidt, M. A-kinase anchoring proteins contribute to loss of E-cadherin and bronchial epithelial barrier by cigarette smoke. Am. J. Physiol. Cell Physiol. 2014, 306, C585–C597. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The microbiome and the respiratory tract. Annu Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef]
- Feng, Z.-H.; Li, Q.; Liu, S.-R.; Du, X.-N.; Wang, C.; Nie, X.-H.; Wang, W.; Ying, S. Comparison of composition and diversity of bacterial microbiome in human upper and lower respiratory tract. Chin. Med. J. 2017, 130, 1122–1124. [Google Scholar] [CrossRef]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef]
- Opron, K.; Begley, L.A.; Erb-Downward, J.R.; Freeman, C.; Madapoosi, S.; Alexis, N.E.; Barjaktarevic, I.; Barr, R.G.; Bleecker, E.R.; Bowler, R.P.; et al. Lung microbiota associations with clinical features of COPD in the SPIROMICS cohort. NPJ Biofilms Microbiomes 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Pragman, A.A.; Kim, H.B.; Reilly, C.S.; Wendt, C.; Isaacson, R.E. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS ONE 2012, 7, e47305. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Nuñez, M.; Millares, L.; Pomares, X.; Ferrari, R.; Pérez-Brocal, V.; Gallego, M.; Espasa, M.; Moya, A.; Monsó, E. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J. Clin. Microbiol. 2014, 52, 4217–4223. [Google Scholar] [CrossRef] [PubMed]
- Sze, M.A.; Dimitriu, P.A.; Suzuki, M.; McDonough, J.E.; Campbell, J.D.; Brothers, J.F.; Erb-Downward, J.R.; Huffnagle, G.B.; Hayashi, S.; Elliott, W.M.; et al. Host response to the lung microbiome in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 192, 438–445. [Google Scholar] [CrossRef]
- Zalacain, R.; Sobradillo, V.; Amilibia, J.; Barrón, J.; Achótegui, V.; Pijoan, J.I.; Llorente, J.L. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur. Respir. J. 1999, 13, 343–348. [Google Scholar] [CrossRef]
- Hill, A.T.; Campbell, E.J.; Hill, S.L.; Bayley, D.L.; Stockley, R.A. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am. J. Med. 2000, 109, 288–295. [Google Scholar] [CrossRef]
- Patel, I.S.; Seemungal, T.A.R.; Wilks, M.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wedzicha, J.A. Relationship between bacterial colonization and the frequency, character and severity of COPD exacerbations. Thorax 2002, 57, 759–764. [Google Scholar] [CrossRef]
- Wang, Z.; Maschera, B.; Lea, S.; Kolsum, U.; Michalovich, D.; Van Horn, S.; Traini, C.; Brown, J.R.; Hessel, E.M.; Singh, D. Airway-host microbiome interactions in chronic obstructive pulmonary disease. Respir. Res. 2019, 20, 113. [Google Scholar] [CrossRef]
- Singh, R.; Mackay, A.J.; Patel, A.R.C.; Garcha, D.S.; Kowlessar, B.S.; Brill, S.E.; Donnelly, L.E.; Barnes, P.J.; Donaldson, G.C.; Wedzicha, J.A. Inflammatory thresholds and the species-specific effects of colonizing bacteria in stable chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 114. [Google Scholar] [CrossRef]
- Wang, Z.; Singh, R.; Miller, B.E.; Tal-SInger, R.; Van Horn, S.; Tomsho, L.; Mackay, A.; Allinson, J.P.; Webb, A.J.; Brookes, A.J.; et al. Sputum microbiome temporal variability and dysbiosis in chronic obstructive pulmonary disease exacerbations: An analysis of the COPDMAP study. Thorax 2018, 73, 331–338. [Google Scholar] [CrossRef]
- Mayhew, D.; Devos, N.; Lambert, C.; Brown, J.R.; Clarke, S.C.; Kim, V.L.; Magid-Slav, M.; Miller, B.E.; Ostridge, K.K.; Patel, R.; et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax 2018, 73, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Sethi, R.; Eschberger, K.; Lobbins, P.; Cai, X.; Grant, B.J.B.; Murphy, T.F. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 176, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Evans, N.; Grant, B.J.; Murphy, T.F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 2002, 347, 465–471. [Google Scholar] [CrossRef]
- Murphy, T.F.; Brauer, A.L.; Grant, B.J.; Sethi, S. Morexella catarrhalis in chronic obstructive pulmonary disease: Burden of disease and immune response. Am. J. Respir Crit. Care Med. 2005, 172, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.F.; Brauer, A.L.; Eschberger, K.; Lobbins, P.; Grove, L.; Cai, X.; Sethi, S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.L.; Manzel, L.J.; Lehman, E.E.; Humlicek, A.L.; Shi, L.; Starner, T.D.; Denning, G.M.; Murphy, T.F.; Sethi, S.; Look, D.C. Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers. Am. J. Respir. Crit. Care Med. 2005, 172, 85–91. [Google Scholar] [CrossRef]
- Miravitlles, M.; Marín, A.; Monsó, E.; Vilà, S.; de la Roza, C.; Hervás, R.; Esquinas, C.; García, M.; Millares, L.; Morera, J.; et al. Efficacy of moxifloxacin in the treatment of bronchial colonization in COPD. Eur. Respir. J. 2009, 34, 1066–1071. [Google Scholar] [CrossRef]
- Fernaays, M.M.; Lesse, A.J.; Sethi, S.; Cai, X.; Murphy, T.F. Differential genome contents of nontypeable Haemophilus influenzae strains from adults with chronic obstructive pulmonary disease. Infect. Immun. 2006, 74, 3366–3374. [Google Scholar] [CrossRef][Green Version]
- Qiu, Y.; Bandi, V.; Atmar, R.L.; Hattotuwa, K.; Guntupalli, K.K.; Jeffery, P.K. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 168, 968–975. [Google Scholar] [CrossRef]
- Quint, J.K.; Wedzicha, J.A. The neutrophil in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2007, 119, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Mio, T.; Romberger, D.J.; Thompson, A.B.; Robbins, R.A.; Heires, A.; Rennard, S.I. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 1997, 155, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Hudy, M.H.; Traves, S.L.; Wiehler, S.; Proud, D. Cigarette smoke modulates rhinovirus-induced airway epithelial chemokine production. Eur. Respir. J. 2010, 35, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Moretto, N.; Bertolini, S.; Iadicicco, C.; Marchini, G.; Kaur, M.; Volpi, G.; Patacchini, R.; Singh, D.; Facchinetti, F. Cigarette smoke and its component acrolein augment IL-8/CXCL8 mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cells. Am. J. Physiol. Lung Cell. Mol. Biol. 2012, 303, L929–L938. [Google Scholar] [CrossRef]
- Herr, C.; Beisswenger, C.; Hess, C.; Kandler, K.; Suttorp, N.; Welte, T.; Schroeder, J.M.; Vogelmeier, C.; Bals, R. Suppression of pulmonary innate host defence in smokers. Thorax 2009, 64, 144–149. [Google Scholar] [CrossRef]
- Ram, F.S.F.; Rodriguez-Roisin, R.; Granados-Navarrete, A.; Garcia-Aymerich, J.; Barnes, N.C. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2006, 2, CD004403. [Google Scholar]
- Herath, S.C.; Normansell, R.; Maisey, S.; Poole, P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2018, 10, CD009764. [Google Scholar] [CrossRef]
- Martinez, F.J.; Curtis, J.L.; Albert, R. Role of macrolide therapy. in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct, Pulmon. Dis. 2008, 3, 331–350. [Google Scholar] [CrossRef]
- Seemungal, T.A.R.; Wilkinson, T.M.A.; Hurst, J.R.; Perera, W.R.; Sapsford, R.J.; Wedzicha, J.A. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am. J. Respir. Crit. Care Med. 2008, 178, 1139–1147. [Google Scholar] [CrossRef]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A.D.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C.; et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 365, 689–698. [Google Scholar] [CrossRef]
- Mallia, P.; Webber, J.; Gill, S.K.; Trujillo-Torralbo, M.-B.; Calderazzo, M.A.; Finney, L.; Bahksoliani, E.; Farne, H.; Singanayagam, A.; Footit, J.; et al. Role of airway glucose on bacetrial infections in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 142, 815–823.e6. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.R.; Harper-Owen, R.; Bhowmik, A.; Jeffries, D.J.; Wedzicha, J.A. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur. Respir. J. 2000, 16, 677–683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wedzicha, J.A. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2004, 1, 115–120. [Google Scholar] [CrossRef] [PubMed]
- McManus, T.E.; Marley, A.-M.; Baxter, N.; Christie, S.N.; O’Neill, H.J.; Elborn, J.S.; Coyle, P.V.; Kidney, J.C. Respiratory viral infection in exacerbations of COPD. Respir. Med. 2008, 102, 1575–1580. [Google Scholar] [CrossRef]
- Rohde, G.; Wiethege, A.; Borg, I.; Kauth, M.; Bauer, T.T.; Gillissen, A.; Bufe, A.; Schultze-Werninghaus, G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: A case-control study. Thorax 2003, 58, 37–42. [Google Scholar] [CrossRef]
- Mallia, P.; Message, S.D.; Gielen, V.; Contoli, M.; Gray, K.; Kebadze, T.; Aniscenko, J.; Laza-Stanca, V.; Edwards, M.R.; Slater, L.; et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am. J. Respir. Crit. Care Med. 2011, 183, 734–742. [Google Scholar] [CrossRef]
- Michi, A.N.; Love, M.E.; Proud, D. Rhinovirus-induced modulation of epithelial phenotype: Role in asthma. Viruses 2020, 12, 1328. [Google Scholar] [CrossRef]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef]
- Singanayagam, A.; Loo, S.-L.; Calderazzo, M.; Finney, L.J.; Trujillo Torralbo, M.-B.; Bakhsoliani, E.; Girkin, J.; Veerati, P.; Pathinayake, P.S.; Nichol, K.S.; et al. Antiviral immunity is impaired in COPD patients with frequent exacerbations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L893–L903. [Google Scholar] [CrossRef]
- Garcia-Valero, J.; Olloquequi, J.; Montes, J.F.; Rodríguez, E.; Martin-Satué, M.; Texidó, L.; Sancho, J.F. Deficient pulmonary IFN-β expression in COPD patients. PLoS ONE 2019, 14, e0217803. [Google Scholar]
- Schneider, D.; Ganesan, S.; Comstock, A.T.; Meldrum, C.A.; Mahidhara, R.; Goldsmith, A.M.; Curtis, J.L.; Martinez, F.J.; Hershenson, M.B.; Sajjan, U.S. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Baines, K.J.; Hsu, A.C.-Y.; Tooze, M.; Gunawardhana, L.P.; Gibson, P.G.; Wark, P.A.B. Novel immune genes associated with excessive inflammatory and antiviral responses to rhinovirus in COPD. Respir. Res. 2013, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Hilzendeger, C.; da Silva, J.; Henket, M.; Schleich, F.; Corhay, J.L.; Kebadze, T.; Edwards, M.R.; Mallia, P.; Johnston, S.L.; Louis, R. Reduced sputum expression of interferon-stimulated genes in severe COPD. Int. J. Chron. Obstruct Pulmon. Dis. 2016, 11, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Veerati, P.C.; Troy, N.M.; Reid, A.T.; Li, N.F.; Nichol, K.S.; Kaur, P.; Maltby, S.; Wark, P.A.B.; Knight, D.A.; Bosco, A.; et al. Airway epithelial cell immunity is delayed during rhinovirus infection in asthma and COPD. Front. Immunol. 2020, 11, 974. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Mordstein, M.; Kochs, G.; García-Sastre, A. Transcription factor redundancy ensures induction of the antiviral state. J. Biol. Chem. 2010, 285, 42013–42022. [Google Scholar] [CrossRef]
- Proud, D.; Hudy, M.H.; Wiehler, S.; Zaheer, R.S.; Amin, M.A.; Pelikan, J.B.; Tacon, C.E.; Tonsaker, T.O.; Walker, B.L.; Kooi, C.; et al. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS ONE 2012, 7, e40762. [Google Scholar] [CrossRef]
- Beekat-Berkani, R.; Wilkinson, T.; Buchy, P.; Dos Santos, G.; Stefanidis, D.; Devaster, J.-M.; Meyer, N. Seasonal influenza vaccination in patients with COPD: A systematic literature review. BMC Pulm. Med. 2017, 17, 79. [Google Scholar] [CrossRef]
- Modestou, M.A.; Manzel, L.J.; El-Mahdy, S.; Look, D.C. Inhibition of IFN-γ-dependent antiviral airway epithelial defense by cigarette smoke. Respir. Res. 2010, 11, 64. [Google Scholar] [CrossRef]
- Groskreutz, D.J.; Monick, M.M.; Babor, E.C.; Nyunoya, T.; Varga, S.M.; Look, D.C.; Hunninghake, G.W. Cigarette smoke alters respiratory syncytial virus-induced apoptosis and replication. Am. J. Respir. Cell Mol. Biol. 2009, 41, 189–198. [Google Scholar] [CrossRef]
- Foronjy, R.F.; Dabo, A.J.; Taggart, C.C.; Weldon, S.; Geraghty, P. Respiratory syncytial virus infections enhance cigarette smoke induced COPD in mice. PLoS ONE 2014, 9, e90567. [Google Scholar] [CrossRef]
- Maedel, C.; Kainz, K.; Frischer, T.; Reinweber, M.; Zacharasiewicz, A. Increased severity of respiratory syncytial virus airway infection due to passive smoke exposure. Pediatr. Pulmonol. 2018, 53, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Mallia, P.; Footitt, J.; Sotero, R.; Jepson, A.; Contoli, M.; Trujillo-Torralbo, M.-B.; Kebadze, T.; Aniscenko, J.; Oleszkiewicz, G.; Gray, K.; et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, P.L.; Mallia, P.; Cox, M.J.; Footitt, J.; Wiilis-Owen, S.A.G.; Homola, D.; Trujillo-Torralbo, M.-B.; Elkin, S.; Kon, O.M.; Cookson, W.O.C.; et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Chattoraj, S.S.; Ganesan, S.; Jones, A.M.; Helm, J.M.; Comstock, A.T.; Bright-Thomas, R.; LiPuma, J.J.; Hershenson, M.B.; Sajjan, U.S. Rhinovirus infection liberates planktonic bacteria from biofilm and increases chemokine responses in cystic fibrosis airway epithelial cells. Thorax 2011, 66, 333–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.H.; Kwon, H.J.; Jang, Y.J. Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope 2009, 119, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, U.; Wang, Q.; Zhao, Y.; Gruenert, D.C.; Hershenson, M.B. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am. J. Respir. Crit. Care Med. 2008, 178, 1271–1281. [Google Scholar] [CrossRef]
- Looi, K.; Buckley, A.G.; Rigby, P.J.; Garratt, L.W.; Iosifidis, T.; Zosky, G.R.; Larcombe, A.N.; Lannigan, F.J.; Ling, K.-M.; Martinovich, K.M.; et al. Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin. Exp. Allergy 2018, 48, 513–524. [Google Scholar] [CrossRef]
- Michi, A.N.; Yipp, B.G.; Dufour, A.; Lopes, F.; Proud, D. PGC-1α mediates a metabolic host defense response in human iarway epithelium during rhinovirus infections. Nat. Commun. 2021, 12, 3669. [Google Scholar] [CrossRef]
- Unger, B.L.; Faris, A.N.; Ganesan, S.; Comstock, A.T.; Hershenson, M.B.; Sajjan, U.S. Rhinovirus attenuates non-typeable Haemophilus influenza-stimulated IL-8 responses via TLR2-dependent degradation of IRAK-1. PLoS Pathog. 2012, 8, e1002969. [Google Scholar] [CrossRef]
- Maciejewski, B.A.; Jamieson, K.C.; Arnason, J.W.; Kooi, C.; Wiehler, S.; Traves, S.L.; Leigh, R.; Proud, D. Rhinovirus-bacteria coexposure synergistically induces CCL20 production from human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L731–L740. [Google Scholar] [CrossRef]
- Arnason, J.W.; Murphy, J.C.; Kooi, C.; Wiehler, S.; Traves, S.L.; Shelfoon, C.; Maciejewski, B.; Dumonceaux, C.J.; Lewenza, W.S.; Proud, D.; et al. Human β-defensin-2 production upon viral and bacterial co-infection is attenuated in COPD. PLoS ONE 2017, 12, e-175963. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, K.C.; Traves, S.L.; Kooi, C.; Wiehler, S.; Dumonceaux, C.J.; Maciejewski, B.A.; Arnason, J.W.; Michi, A.N.; Leigh, R.; Proud, D. Rhinovirus and bacteria synergistically induce IL-17C release from human airway epithelial cells to promote neutrophil recruitment. J. Immunol. 2019, 202, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.H.; Nielson, C.P.; Carvalho, P.; Charan, N.B.; Crowley, J.J. Controlled trial of oral prednisone in outparients with acute COPD exacerbations. Am. J. Respir. Crit. Care Med. 1996, 154, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Angus, R.M.; Calverley, P.M. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: A propsective randomized controlled trial. Lancet 1999, 354, 456–460. [Google Scholar] [CrossRef]
- Niewoehner, D.E.; Erbland, M.L.; Deupree, R.H.; Collins, D.; Gross, N.J.; Light, R.W.; Anderson, P.; Morgan, N. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N. Engl. J. Med. 1999, 340, 1941–1947. [Google Scholar] [CrossRef]
- Maltais, F.; Ostinelli, J.; Bourbeau, J.; Tonnal, A.B.; Jacquemet, N.; Haddon, J.; Rouleau, M.; Boukhana, M.; Martinot, J.B.; Duroux, P. Comparison of nebulized budesonide and oral prednisone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: A randomized control trial. Am. J. Respir. Crit. Care Med. 2002, 165, 698–703. [Google Scholar] [CrossRef]
- Sivapalan, P.; Ingebrigtsen, T.S.; Rasmussen, D.B.; Sørensen, R.; Rasmussen, C.M.; Jensen, C.B.; Allin, K.H.; Eklöf, J.; Seersholm, N.; Vestbo, J.; et al. COPD exacerbations: The impact of long versus short courses of oral corticosteroids on mortality and pneumonia: Nationwide data on 67,000 patients with COPD followed for 12 months. BMJ Open Respir Res. 2019, 6, e000407. [Google Scholar] [CrossRef]
- Calverley, P.M.; Anderson, J.A.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Jones, P.W.; Yates, J.C.; Vestbo, J. TORCH Investigators, Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 2007, 356, 775–789. [Google Scholar] [CrossRef]
- Calverley, P.M.A.; Tetzlaff, K.; Vogelmeier, C.; Fabbri, L.M.; Magnussen, H.; Wouters, E.F.M.; Mezzanotte, W.; Disse, B.; Finnigan, H.; Asijee, G.; et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017, 196, 1219–1221. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Palmenberg, A.C.; Rathe, J.A.; Liggett, S.B. Analysis of the complete genome sequences of human rhinovirus. J. Allergy Clin. Immunol. 2010, 125, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Royston, L.; Tapparel, C. Rhinoviruses and respiratory enteroviruses: Not as simple as ABC. Viruses 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- McLean, G.R. Vaccine strategies to induce broadly protective immunity to rhinovirus. Hum. Vaccin. Immunother. 2020, 16, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Buscho, R.F.; Perkins, J.C.; Knopf, H.L.; Kapikian, A.Z.; Chanock, R.M. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of inactivated rhinovirus 13 vaccine. J. Immunol. 1972, 108, 169–177. [Google Scholar]
- Choi, T.; Devries, M.; Bacharier, L.B.; Busse, W.; Carmargo, C.A.J.; Cohen, R.; Demuri, G.P.; Evans, M.D.; Fitzpatrick, A.M.; Gergen, P.J.; et al. Enhanced neutralizing antibody responses to rhinovirus C and age-dependent patterns of infection. Am. J. Respir. Crit. Care Med. 2021, 203, 822–830. [Google Scholar] [CrossRef]
- Barclay, W.S.; al-Nakib, W.; Higgins, P.G.; Tyrrell, D.A. The time course of the humoral immune response to rhinovirus infection. Epidemiol. Infect. 1989, 103, 659–669. [Google Scholar] [CrossRef]
- Gern, J.E.; Dick, E.C.; Kelley, E.A.B.; Vrtis, R.; Klein, B. Rhinovirus-specific T cells recognize both shared and serotype-restricted viral epitopes. J. Infect. Dis. 1997, 175, 1108–1114. [Google Scholar] [CrossRef]
- Warner, S.M.; Wiehler, S.; Michi, A.N.; Proud, D. Rhinovirus replication and innate immunity in highly differentiated human airway epithelial cells. Respir. Res. 2019, 20, 150. [Google Scholar] [CrossRef]
- Leigh, R.; Proud, D. Virus-induced modulation of lower airway diseases: Pathogenesis and pharmacologic approaches to treatment. Pharmacol. Ther. 2015, 148, 185–198. [Google Scholar] [CrossRef]
- Casanova, V.; Sousa, F.H.; Stevens, C.; Barlow, P.G. Antiviral therapeutic approaches for human rhinovirus infections. Future Virol. 2018, 13, 505–518. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Love, M.E.; Proud, D. Respiratory Viral and Bacterial Exacerbations of COPD—The Role of the Airway Epithelium. Cells 2022, 11, 1416. https://doi.org/10.3390/cells11091416
Love ME, Proud D. Respiratory Viral and Bacterial Exacerbations of COPD—The Role of the Airway Epithelium. Cells. 2022; 11(9):1416. https://doi.org/10.3390/cells11091416
Chicago/Turabian StyleLove, Michelle E., and David Proud. 2022. "Respiratory Viral and Bacterial Exacerbations of COPD—The Role of the Airway Epithelium" Cells 11, no. 9: 1416. https://doi.org/10.3390/cells11091416
APA StyleLove, M. E., & Proud, D. (2022). Respiratory Viral and Bacterial Exacerbations of COPD—The Role of the Airway Epithelium. Cells, 11(9), 1416. https://doi.org/10.3390/cells11091416





