Aquaporin-3 and Aquaporin-5 Facilitate Migration and Cell–Cell Adhesion in Pancreatic Cancer by Modulating Cell Biomechanical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Transfection of BxPC-3 Cells
2.3. RNA Isolation and RT-PCR
2.4. Water and Glycerol Permeability
2.5. Hydrogen Peroxide Influx
2.6. Cell Migration
2.7. Membrane Fluidity
2.8. Cell Elasticity
2.9. AFM Imaging
2.10. Cell–Cell Adhesion
2.11. Cell Apoptosis
2.12. Statistical Analysis
3. Results
3.1. AQP3 and AQP5 Are the Most Abundant Aquaporins in BxPC3 Cells
3.2. Effect of AQP3- and AQP5-Silencing in Cell Migration
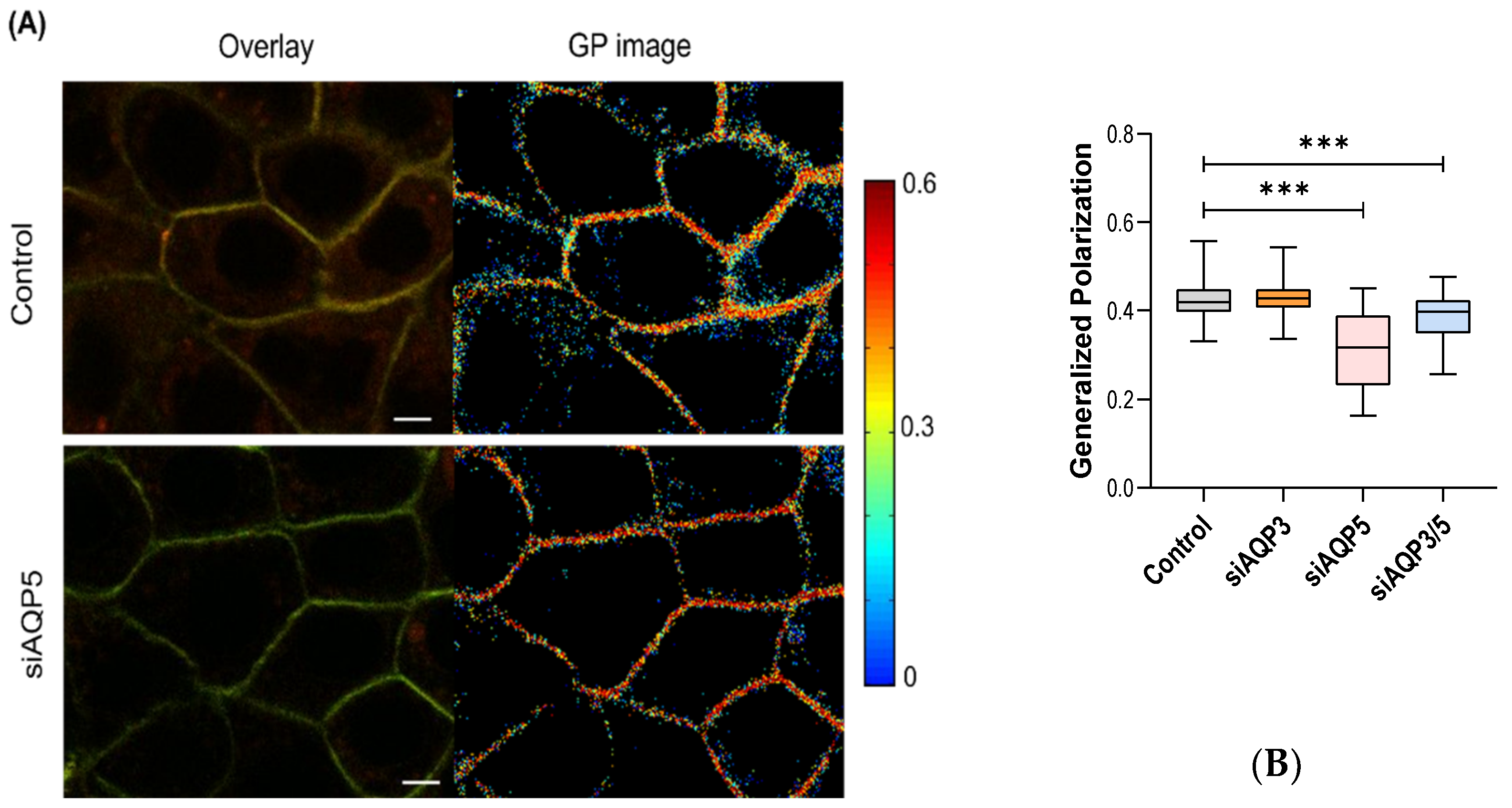
3.3. AQP5 Influences Membrane Fluidity
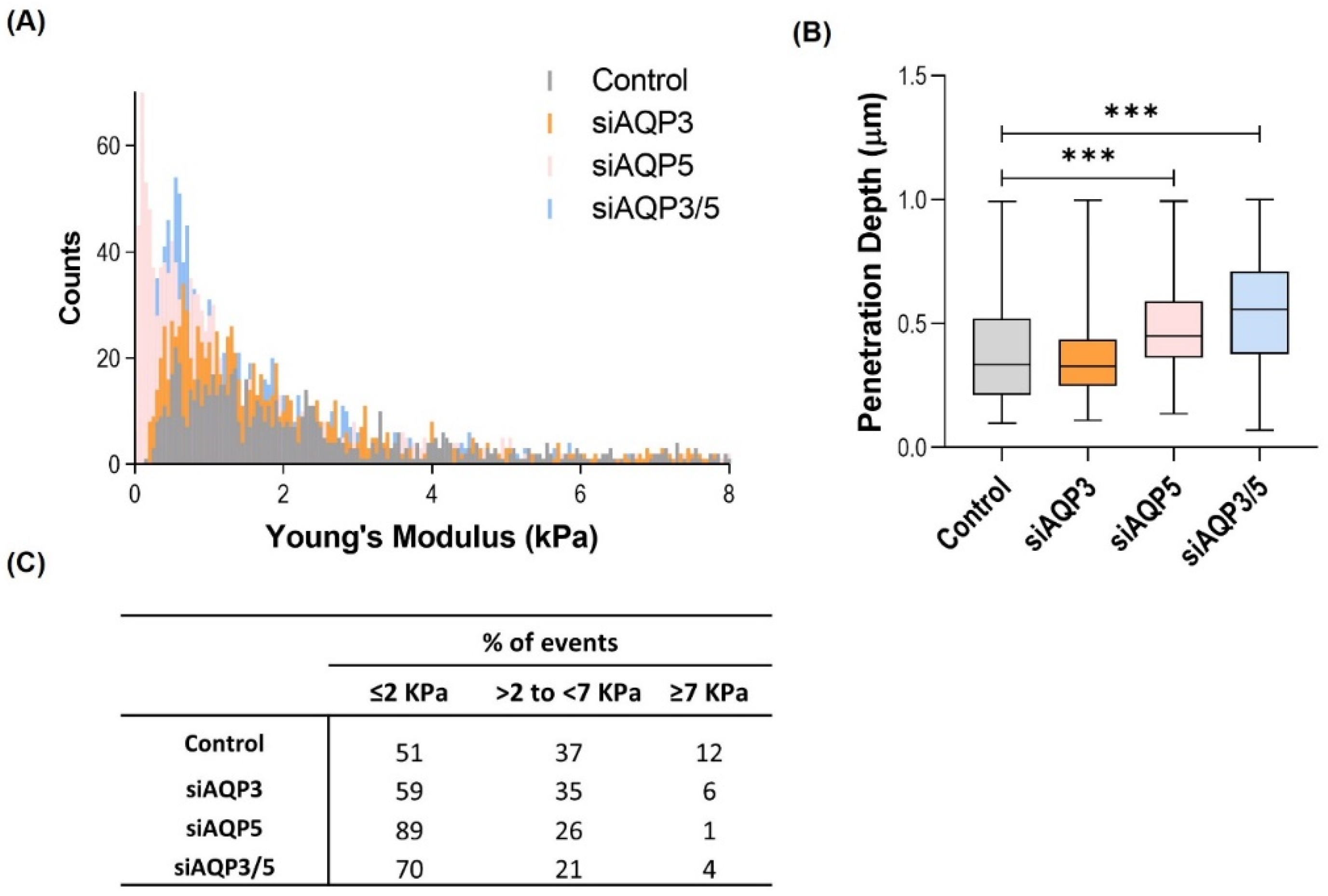
3.4. AQP5 Promotes Changes in the Cell Biomechanical and Morphological Properties
3.5. AQP3 and AQP5 Play an Important Role in Cell–Cell Adhesion
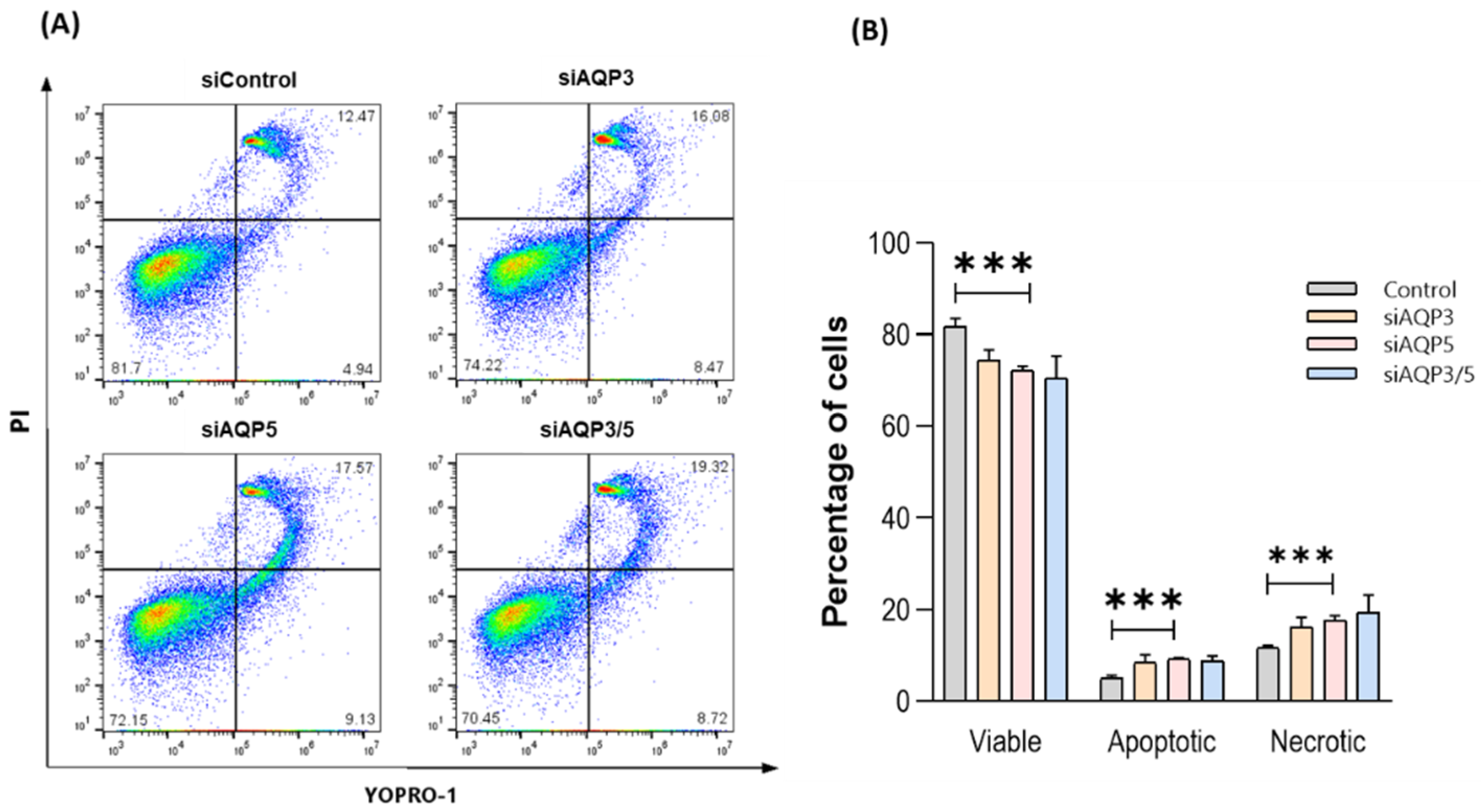
3.6. Silencing AQP5 Induces Cell Apoptosis and Necrosis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verkman, A.S. Aquaporins in Clinical Medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kruse, E.; Uehlein, N.; Kaldenhoff, R. The aquaporins. Genome Biol. 2006, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Nielsen, S.; Casini, A. Aquaporins in Health and Disease; Soveral, G., Nielsen, S., Casini, A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351229159. [Google Scholar]
- Direito, I.; Madeira, A.; Brito, M.A.; Soveral, G. Aquaporin-5: From structure to function and dysfunction in cancer. Cell. Mol. Life Sci. 2016, 73, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.V.; Soveral, G. Aquaporins in Immune Cells and Inflammation: New Targets for Drug Development. Int. J. Mol. Sci. 2021, 22, 1845. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Moura, T.F.; Soveral, G. Detecting Aquaporin Function and Regulation. Front. Chem. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Bestetti, S.; García-Manteiga, J.M.; Medraño-Fernandez, I.; Dal Mas, A.; Malosio, M.L.; Sitia, R. Tyrosine Kinase signal modulation: A matter of H2O2 membrane permeability? Antioxid. Redox Signal. 2013, 19, 1447–1451. [Google Scholar] [CrossRef]
- Prata, C.; Hrelia, S.; Fiorentini, D. Peroxiporins in cancer. Int. J. Mol. Sci. 2019, 20, 1371. [Google Scholar] [CrossRef]
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Taniyama, Y.; Griendling, K.K. Reactive Oxygen Species in the Vasculature. Hypertension 2003, 42, 1075–1081. [Google Scholar] [CrossRef]
- Rodrigues, C.; Tartaro Bujak, I.; Mihaljević, B.; Soveral, G.; Cipak Gasparovic, A. Yeast aquaporin regulation by 4-hydroxynonenal is implicated in oxidative stress response. IUBMB Life 2017, 69, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Kuroczycki-Saniutycz, S.; Grzeszczuk, A.; Zwierz, Z.W.; Kołodziejczyk, P.; Szczesiul, J.; Zalewska-Szajda, B.; Ościłowicz, K.; Waszkiewicz, N.; Zwierz, K.; Dariusz Szajda, S. Prevention of pancreatic cancer. Współczesna Onkol. 2017, 1, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Schwarz, L.; Borbath, I.; Henry, A.; Van Laethem, J.-L.; Malka, D.; Ducreux, M.; Conroy, T. An update on treatment options for pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 2019, 11, 175883591987556. [Google Scholar] [CrossRef] [PubMed]
- Direito, I.; Paulino, J.; Vigia, E.; Brito, M.A.; Soveral, G. Differential expression of aquaporin-3 and aquaporin-5 in pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2017, 115, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Bill, R.M.; Hedfalk, K. Aquaporins—Expression, purification and characterization. Biochim. Biophys. Acta 2021, 1863, 183650. [Google Scholar] [CrossRef] [PubMed]
- Milković, L.; Čipak Gašparović, A. AQP3 and AQP5—Potential Regulators of Redox Status in Breast Cancer. Molecules 2021, 26, 2613. [Google Scholar] [CrossRef]
- Chen, J.; Wang, T.; Zhou, Y.-C.; Gao, F.; Zhang, Z.-H.; Xu, H.; Wang, S.-L.; Shen, L.-Z. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J. Exp. Clin. Cancer Res. 2014, 33, 38. [Google Scholar] [CrossRef]
- Arsenijevic, T.; Perret, J.; Van Laethem, J.-L.; Delporte, C. Aquaporins Involvement in Pancreas Physiology and in Pancreatic Diseases. Int. J. Mol. Sci. 2019, 20, 5052. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta 2015, 1848, 2576–2583. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Serna, A.; Galán-Cobo, A.; Rodrigues, C.; Sánchez-Gomar, I.; Toledo-Aral, J.J.; Moura, T.F.; Casini, A.; Soveral, G.; Echevarría, M. Functional Inhibition of Aquaporin-3 With a Gold-Based Compound Induces Blockage of Cell Proliferation. J. Cell. Physiol. 2014, 229, 1787–1801. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef]
- da Silva, I.V.; Cardoso, C.; Martínez-Banaclocha, H.; Casini, A.; Pelegrín, P.; Soveral, G. Aquaporin-3 is involved in NLRP3-inflammasome activation contributing to the setting of inflammatory response. Cell. Mol. Life Sci. 2021, 78, 3073–3085. [Google Scholar] [CrossRef]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Galán Cobo, A.; Echevarría, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting Aquaporin Function: Potent Inhibition of Aquaglyceroporin-3 by a Gold-Based Compound. PLoS ONE 2012, 7, e37435. [Google Scholar] [CrossRef]
- Madeira, A.; Camps, M.; Zorzano, A.; Moura, T.F.; Soveral, G. Biophysical Assessment of Human Aquaporin-7 as a Water and Glycerol Channel in 3T3-L1 Adipocytes. PLoS ONE 2013, 8, e83442. [Google Scholar] [CrossRef]
- Noronha, H.; Araújo, D.; Conde, C.; Martins, A.P.; Soveral, G.; Chaumont, F.; Delrot, S.; Gerós, H. The Grapevine Uncharacterized Intrinsic Protein 1 (VvXIP1) Is Regulated by Drought Stress and Transports Glycerol, Hydrogen Peroxide, Heavy Metals but Not Water. PLoS ONE 2016, 11, e0160976. [Google Scholar] [CrossRef]
- Pinho, J.O.; da Silva, I.V.; Amaral, J.D.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Therapeutic potential of a copper complex loaded in pH-sensitive long circulating liposomes for colon cancer management. Int. J. Pharm. 2021, 599, 120463. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Connell, S.; Miltenberger-Miltenyi, G.; Pereira, S.V.; Tavares, A.; Ariëns, R.a.S.; Santos, N.C. Atomic force microscopy-based molecular recognition of a fibrinogen receptor on human erythrocytes. ACS Nano 2010, 4, 4609–4620. [Google Scholar] [CrossRef]
- Guedes, A.F.; Moreira, C.; Nogueira, J.B.; Santos, N.C.; Carvalho, F.A. Fibrinogen-erythrocyte binding and hemorheology measurements in the assessment of essential arterial hypertension patients. Nanoscale 2019, 11, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.F.; Carvalho, F.A.; Malho, I.; Lousada, N.; Sargento, L.; Santos, N.C. Atomic force microscopy as a tool to evaluate the risk of cardiovascular diseases in patients. Nat. Nanotechnol. 2016, 11, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Santos, N.C. Atomic force microscopy-based force spectroscopy—Biological and biomedical applications. IUBMB Life 2012, 64, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, N.; Abreu, S.; Carvalho, F.A.; Fernandes, F.; Santos, N.C.; Fialho, A.M. Modulation of membrane properties of lung cancer cells by azurin enhances the sensitivity to EGFR-targeted therapy and decreased β1 integrin-mediated adhesion. Cell Cycle 2016, 15, 1415–1424. [Google Scholar] [CrossRef]
- Guedes, A.F.; Carvalho, F.A.; Moreira, C.; Nogueira, J.B.; Santos, N.C. Essential arterial hypertension patients present higher cell adhesion forces, contributing to fibrinogen-dependent cardiovascular risk. Nanoscale 2017, 9, 14897–14906. [Google Scholar] [CrossRef]
- Gaus, K.; Zech, T.; Harder, T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol. Membr. Biol. 2006, 23, 41–48. [Google Scholar] [CrossRef]
- Pinto, S.N.; Fernandes, F.; Fedorov, A.; Futerman, A.H.; Silva, L.C.; Prieto, M. A combined fluorescence spectroscopy, confocal and 2-photon microscopy approach to re-evaluate the properties of sphingolipid domains. Biochim. Biophys. Acta 2013, 1828, 2099–2110. [Google Scholar] [CrossRef]
- Sariisik, E.; Popov, C.; Müller, J.P.; Docheva, D.; Clausen-Schaumann, H.; Benoit, M. Decoding Cytoskeleton-Anchored and Non-Anchored Receptors from Single-Cell Adhesion Force Data. Biophys. J. 2015, 109, 1330–1333. [Google Scholar] [CrossRef]
- Shan, T.; Chen, S.; Chen, X.; Kong, D.; Lin, W.; Li, W.; Ma, J.; Li, Y.; Cui, X.; Kang, Y. Impact of AQP-5 on the growth of colorectal cancer cells and the underlying mechanism. Int. J. Clin. Exp. Pathol. 2018, 11, 58–67. [Google Scholar]
- da Silva, I.; Barroso, M.; Moura, T.; Castro, R.; Soveral, G. Endothelial Aquaporins and Hypomethylation: Potential Implications for Atherosclerosis and Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 130. [Google Scholar] [CrossRef]
- Pimpão, C.; da Silva, I.V.; Mósca, A.F.; Pinho, J.O.; Gaspar, M.M.; Gumerova, N.I.; Rompel, A.; Aureliano, M.; Soveral, G. The Aquaporin-3-Inhibiting Potential of Polyoxotungstates. Int. J. Mol. Sci. 2020, 21, 2467. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Nowak, N.J.; Loor, R.; Ochi, H.; Sandberg, A.A.; Lopez, C.; Pickren, J.W.; Berjian, R.; Douglass, H.O.; Chu, T.M. Characterization of a new primary human pancreatic tumor line. Cancer Investig. 1986, 4, 15–23. [Google Scholar] [CrossRef]
- McLaughlin, P.J.; Zagon, I.S. Gastrin regulates growth of human pancreatic cancer in a tonic and autocrine fashion. Am. J. Physiol. 1996, 270. [Google Scholar] [CrossRef]
- Delesque, N.; Buscail, L.; Estève, J.P.; Saint-Laurent, N.; Müller, C.; Weckbecker, G.; Bruns, C.; Vaysse, N.; Susini, C. sst2 somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Res. 1997, 57, 956–962. [Google Scholar]
- Cole, J.M.; Simmons, K.; Prosperi, J.R. Effect of Adenomatous Polyposis Coli Loss on Tumorigenic Potential in Pancreatic Ductal Adenocarcinoma. Cells 2019, 8, 1084. [Google Scholar] [CrossRef]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Ollila, S.; Hyvönen, M.T.; Vattulainen, I. Polyunsaturation in lipid membranes: Dynamic properties and lateral pressure profiles. J. Phys. Chem. B 2007, 111, 3139–3150. [Google Scholar] [CrossRef]
- Tzoneva, R.; Stoyanova, T.; Petrich, A.; Popova, D.; Uzunova, V.; Momchilova, A.; Chiantia, S. Effect of Erufosine on Membrane Lipid Order in Breast Cancer Cell Models. Biomolecules 2020, 10, 802. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Keely, P.J. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 2009, 28, 4326–4343. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, S.; Wang, X.; Xia, D.; Li, Q. Alteration of nanomechanical properties of pancreatic cancer cells through anticancer drug treatment revealed by atomic force microscopy. Beilstein J. Nanotechnol. 2021, 12, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.F.; Carvalho, F.A.; Domingues, M.M.; Macrae, F.L.; McPherson, H.R.; Santos, N.C.; Ariёns, R.A.S. Sensing adhesion forces between erythrocytes and γ’ fibrinogen, modulating fibrin clot architecture and function. Nanomedicine 2018, 14, 909–918. [Google Scholar] [CrossRef]
- Sidhaye, V.K.; Chau, E.; Srivastava, V.; Sirimalle, S.; Balabhadrapatruni, C.; Aggarwal, N.R.; D’Alessio, F.R.; Robinson, D.N.; King, L.S. A Novel Role for Aquaporin-5 in Enhancing Microtubule Organization and Stability. PLoS ONE 2012, 7, e38717. [Google Scholar] [CrossRef]
- Cross, S.E.; Jin, Y.-S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Cross, S.E.; Jin, Y.-S.; Tondre, J.; Wong, R.; Rao, J.; Gimzewski, J.K. AFM-based analysis of human metastatic cancer cells. Nanotechnology 2008, 19, 384003. [Google Scholar] [CrossRef]
- Hung, R.W.Y.; Chow, A.W. Dissecting the “end game”: Clinical relevance, molecular mechanisms and laboratory assessment of apoptosis. Clin. Investig. Med. 2004, 27, 324–344. [Google Scholar]
- Bortner, C.D.; Cidlowski, J.A. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflug. Arch. Eur. J. Physiol. 2004, 448, 313–318. [Google Scholar] [CrossRef]
- Jablonski, E.M.; Adrian Mattocks, M.; Sokolov, E.; Koniaris, L.G.; Hughes, F.M.; Fausto, N.; Pierce, R.H.; McKillop, I.H. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007, 250, 36–46. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.N.; Chen, W.L.; Wang, G.S.; Mao, Q.; Li, S.Q.; Xiong, W.H.; Lin, Y.Y.; Ge, J.W.; Li, X.X.; et al. Effects of AQP5 gene silencing on proliferation, migration and apoptosis of human glioma cells through regulating EGFR/ERK/ p38 MAPK signaling pathway. Oncotarget 2017, 8, 38444–38455. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.M.; da Silva, I.V.; Sarmento, M.J.; Silva, Í.C.; Carvalho, F.A.; Soveral, G.; Santos, N.C. Aquaporin-3 and Aquaporin-5 Facilitate Migration and Cell–Cell Adhesion in Pancreatic Cancer by Modulating Cell Biomechanical Properties. Cells 2022, 11, 1308. https://doi.org/10.3390/cells11081308
Silva PM, da Silva IV, Sarmento MJ, Silva ÍC, Carvalho FA, Soveral G, Santos NC. Aquaporin-3 and Aquaporin-5 Facilitate Migration and Cell–Cell Adhesion in Pancreatic Cancer by Modulating Cell Biomechanical Properties. Cells. 2022; 11(8):1308. https://doi.org/10.3390/cells11081308
Chicago/Turabian StyleSilva, Patrícia M., Inês V. da Silva, Maria J. Sarmento, Ítala C. Silva, Filomena A. Carvalho, Graça Soveral, and Nuno C. Santos. 2022. "Aquaporin-3 and Aquaporin-5 Facilitate Migration and Cell–Cell Adhesion in Pancreatic Cancer by Modulating Cell Biomechanical Properties" Cells 11, no. 8: 1308. https://doi.org/10.3390/cells11081308
APA StyleSilva, P. M., da Silva, I. V., Sarmento, M. J., Silva, Í. C., Carvalho, F. A., Soveral, G., & Santos, N. C. (2022). Aquaporin-3 and Aquaporin-5 Facilitate Migration and Cell–Cell Adhesion in Pancreatic Cancer by Modulating Cell Biomechanical Properties. Cells, 11(8), 1308. https://doi.org/10.3390/cells11081308








