Myasthenia Gravis: An Acquired Interferonopathy?
Abstract
1. Myasthenia Gravis
1.1. Generalities
1.2. Autoantibody Targets Defining MG Subtypes
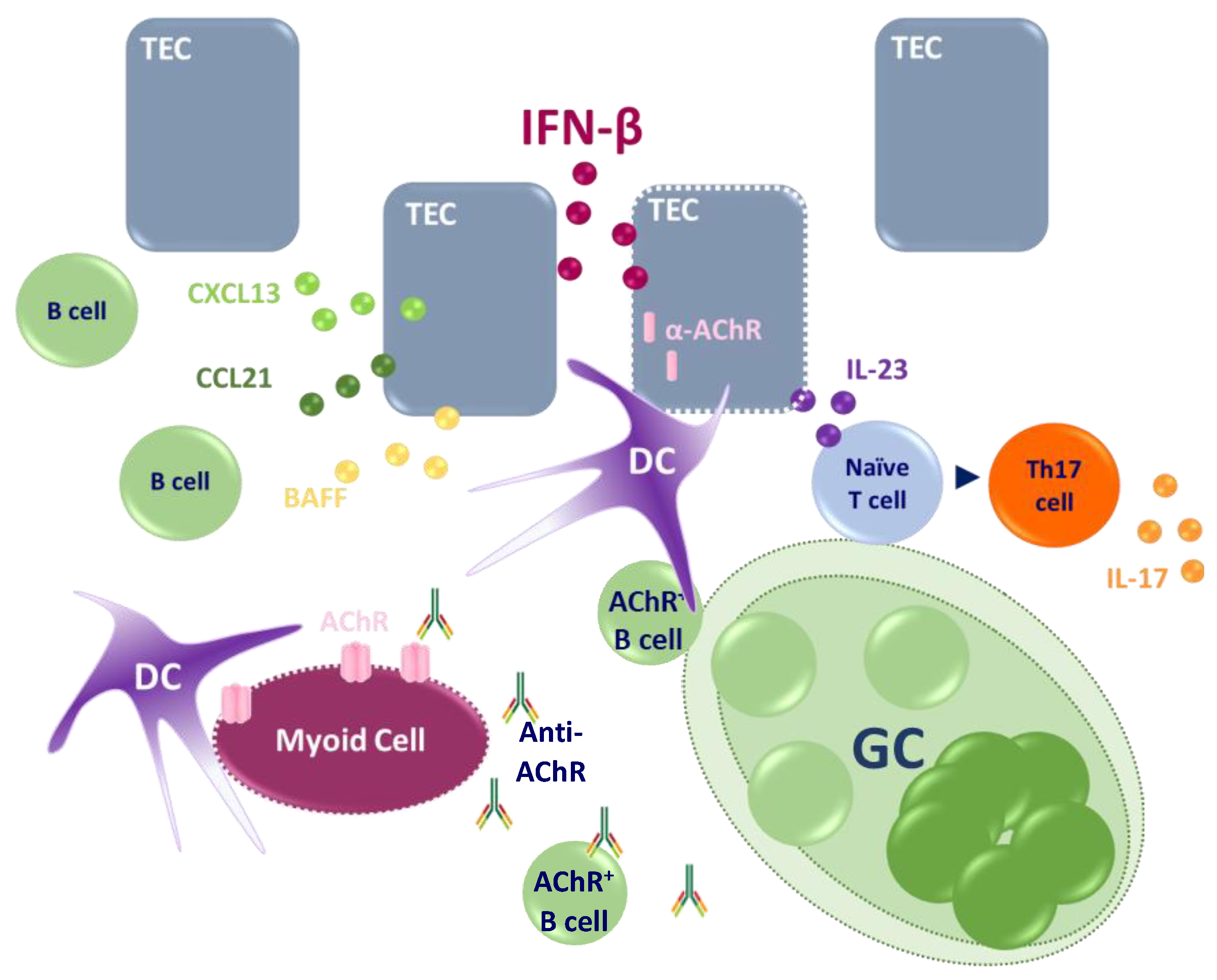
1.3. Implication of the Thymus in MG
1.3.1. Thymus of EOMG-AChR Patients
1.3.2. Thymomas
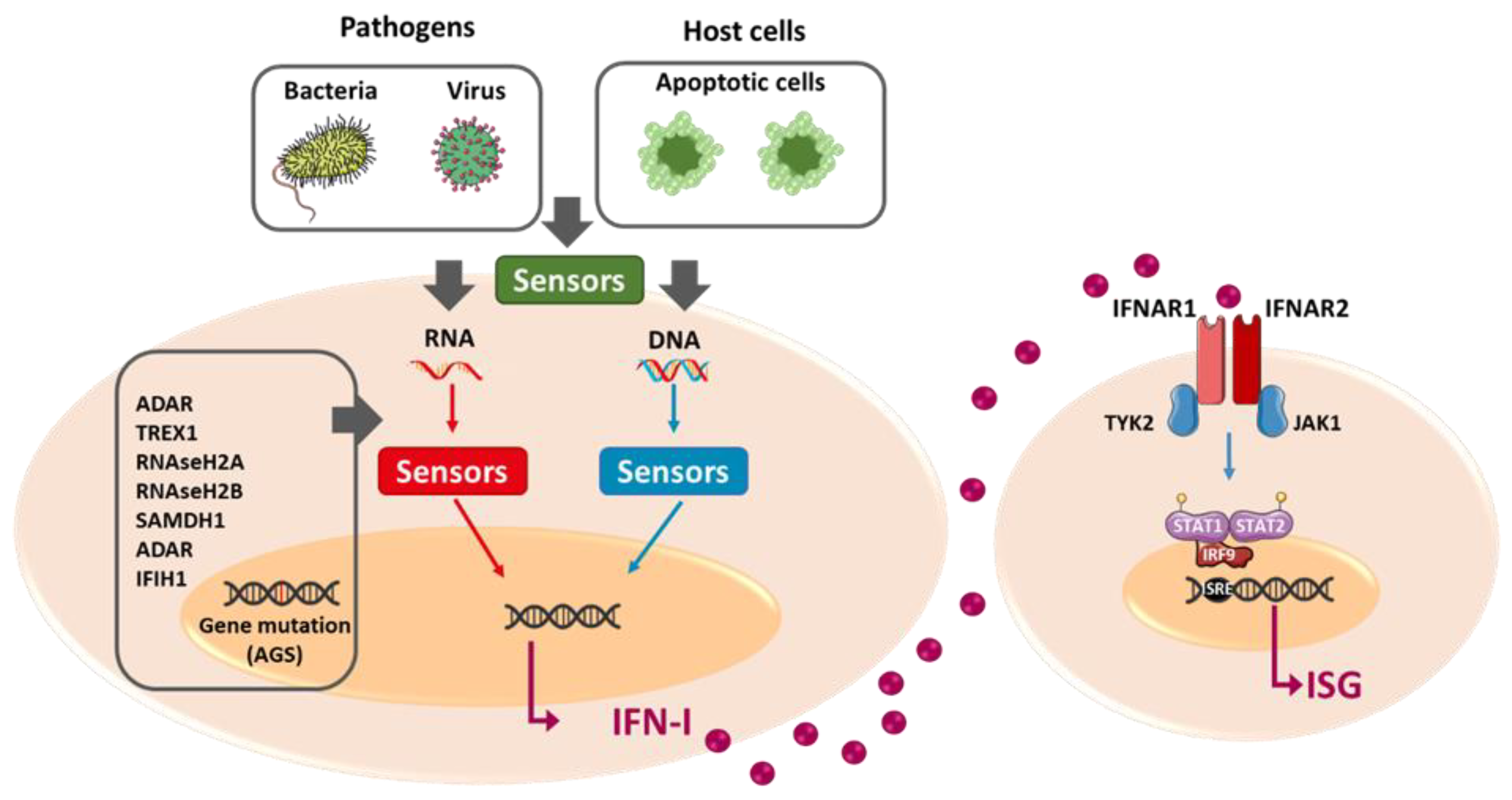
2. Interferon Type I
2.1. IFN-I Expression
2.2. IFN-I Signalization
2.3. Retro-Control Mechanisms of IFN-I Signaling
2.4. Role of IFN-I in Innate and Adaptative Immunity
3. Implication of IFN-I in Diseases
3.1. Type I Interferonopathies: Genetic Diseases
3.2. Acquired Interferonopathies: Autoimmune Diseases
3.3. Iatrogenic effect of IFN-I
4. Implication of IFN-I in Myasthenia Gravis
4.1. No Obvious IFN-I Signature in the Periphery or the Muscle of MG Patients
4.2. IFN-I in Early-Onset AChR-MG Patients
4.3. IFN-I in MG-Associated Thymoma
4.4. Impact of MG Treatments on the Thymic IFN-I Signature
4.5. Origins of IFN-I in MG Thymus
4.5.1. Implication of Viral Infection in IFN-I Signature
4.5.2. Activation of Innate Immune Signaling Pathways
4.5.3. Altered miRNA Expression
4.5.4. Potential Implication of Gene Polymorphisms in the Thymic IFN-I Signature in MG
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChR | Acetylcholine receptor |
| DAMP | Damage-associated molecular pattern |
| DC | Dendritic cell |
| EOMG | Early-onset myasthenia gravis |
| EBV | Epstein–Barr virus |
| GC | Germinal center |
| IFN | Interferon |
| IFNAR | Interferon-α/β receptor |
| IL | Interleukin |
| IRF | Interferon regulatory factor |
| ISG | Interferon-stimulated gene |
| JAK1 | Janus kinase 1 |
| LOMG | Late-onset myasthenia gravis |
| LRP4 | Low-density lipoprotein receptor-related protein 4 |
| MG | Myasthenia gravis |
| MGT | Thymoma-associated myasthenia gravis |
| miRNA | MicroRNA |
| MuSK | Muscle-specific kinase |
| PAMP | Pathogen-associated molecular pattern |
| PBMC | Peripheral mononuclear blood cell |
| RIG-1 | Retinoic acid-inducible gene I |
| SOCS | Suppressor of cytokine signaling |
| STAT | Signal transducer and activator of transcription |
| TEC | Thymic epithelial cell |
| Th17 cell | T helper 17 cell |
| TLR | Toll-like receptor |
| TSA | Tissue-specific antigen |
| USP18 | Ubiquitin specific peptidase 18 |
References
- Avidan, N.; Le Panse, R.; Berrih-Aknin, S.; Miller, A. Genetic Basis of Myasthenia Gravis—A Comprehensive Review. J. Autoimmun. 2014, 52, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia Gravis. Nat. Rev. Dis. Primers 2019, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vicente, E.; Álvarez-Velasco, R.; Segovia, S.; Paradas, C.; Casasnovas, C.; Guerrero-Sola, A.; Pardo, J.; Ramos-Fransi, A.; Sevilla, T.; de Munain, A.L.; et al. Clinical and Therapeutic Features of Myasthenia Gravis in Adults Based on Age at Onset. Neurology 2020, 94, e1171–e1180. [Google Scholar] [CrossRef] [PubMed]
- Aharonov, A.; Tarrab-Hazdai, R.; Abramsky, O.; Fuchs, S. Humoral Antibodies to Acetylcholine Receptor in Patients with Myastenia Gravis. Lancet 1975, 306, 340–342. [Google Scholar] [CrossRef]
- Cho, E.B.; Min, J.-H.; Lee, S.; Yoon, C.W.; Seok, J.M.; Cho, J.; Lee, H.L.; Kim, B.J. Late-Onset Non-Thymomatous Myasthenia Gravis: Comparison with Early-Onset and Very Late-Onset Myasthenia Gravis. Neurol. Asia 2017, 22, 123–131. [Google Scholar]
- Limburg, P.C.; The, T.H.; Hummel-Tappel, E.; Oosterhuis, H.J.G.H. Anti-Acetylcholine Receptor Antibodies in Myasthenia Gravis: Part 1. Relation to Clinical Parameters in 250 Patients. J. Neurol. Sci. 1983, 58, 357–370. [Google Scholar] [CrossRef]
- Hoch, W.; McConville, J.; Helms, S.; Newsom-Davis, J.; Melms, A.; Vincent, A. Auto-Antibodies to the Receptor Tyrosine Kinase MuSK in Patients with Myasthenia Gravis without Acetylcholine Receptor Antibodies. Nat. Med. 2001, 7, 365–368. [Google Scholar] [CrossRef]
- Higuchi, O.; Hamuro, J.; Motomura, M.; Yamanashi, Y. Autoantibodies to Low-Density Lipoprotein Receptor–Related Protein 4 in Myasthenia Gravis. Ann. Neurol. 2011, 69, 418–422. [Google Scholar] [CrossRef]
- Niks, E.H.; van Leeuwen, Y.; Leite, M.I.; Dekker, F.W.; Wintzen, A.R.; Wirtz, P.W.; Vincent, A.; van Tol, M.J.D.; Jol-van der Zijde, C.M.; Verschuuren, J.J.G.M. Clinical Fluctuations in MuSK Myasthenia Gravis Are Related to Antigen-Specific IgG4 Instead of IgG1. J. Neuroimmunol. 2008, 195, 151–156. [Google Scholar] [CrossRef]
- Zisimopoulou, P.; Evangelakou, P.; Tzartos, J.; Lazaridis, K.; Zouvelou, V.; Mantegazza, R.; Antozzi, C.; Andreetta, F.; Evoli, A.; Deymeer, F.; et al. A Comprehensive Analysis of the Epidemiology and Clinical Characteristics of Anti-LRP4 in Myasthenia Gravis. J. Autoimmun. 2014, 52, 139–145. [Google Scholar] [CrossRef]
- Lazaridis, K.; Tzartos, S.J. Autoantibody Specificities in Myasthenia Gravis; Implications for Improved Diagnostics and Therapeutics. Front. Immunol. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Takayanagi, H. Non-Epithelial Thymic Stromal Cells: Unsung Heroes in Thymus Organogenesis and T Cell Development. Front. Immunol. 2021, 11, 620894. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Kyewski, B.; Allen, P.M.; Hogquist, K.A. Positive and Negative Selection of the T Cell Repertoire: What Thymocytes See (and Don’t See). Nat. Rev. Immunol. 2014, 14, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Wakkach, A.; Guyon, T.; Bruand, C.; Tzartos, S.; Cohen-Kaminsky, S.; Berrih-Aknin, S. Expression of Acetylcholine Receptor Genes in Human Thymic Epithelial Cells: Implications for Myasthenia Gravis. J. Immunol. 1996, 157, 3752–3760. [Google Scholar] [PubMed]
- Mesnard-Rouiller, L.; Bismuth, J.; Wakkach, A.; Poëa-Guyon, S.; Berrih-Aknin, S. Thymic Myoid Cells Express High Levels of Muscle Genes. J. Neuroimmunol. 2004, 148, 97–105. [Google Scholar] [CrossRef]
- Leite, M.I.; Ströbel, P.; Jones, M.; Micklem, K.; Moritz, R.; Gold, R.; Niks, E.H.; Berrih-Aknin, S.; Scaravilli, F.; Canelhas, A.; et al. Fewer Thymic Changes in MuSK Antibody-Positive than in MuSK Antibody-Negative MG. Ann. Neurol. 2005, 57, 444–448. [Google Scholar] [CrossRef]
- Koneczny, I.; Cossins, J.; Waters, P.; Beeson, D.; Vincent, A. MuSK Myasthenia Gravis IgG4 Disrupts the Interaction of LRP4 with MuSK but Both IgG4 and IgG1-3 Can Disperse Preformed Agrin-Independent AChR Clusters. PLoS ONE 2013, 8, e80695. [Google Scholar] [CrossRef]
- Lynch, H.E.; Goldberg, G.L.; Chidgey, A.; Van den Brink, M.R.M.; Boyd, R.; Sempowski, G.D. Thymic Involution and Immune Reconstitution. Trends Immunol. 2009, 30, 366–373. [Google Scholar] [CrossRef]
- Bofill, M.; Janossy, G.; Willcox, N.; Chilosi, M.; Trejdosiewicz, L.K.; Newsom-Davis, J. Microenvironments in the Normal Thymus and the Thymus in Myasthenia Gravis. Am. J. Pathol. 1985, 119, 462–473. [Google Scholar]
- Berrih-Aknin, S.; Morel, E.; Raimond, F.; Safar, D.; Gaud, C.; Binet, J.P.; Levasseur, P.; Bach, J.F. The Role of the Thymus in Myasthenia Gravis: Immunohistological and Immunological Studies in 115 Casesa. Ann. N. Y. Acad. Sci. 1987, 505, 50–70. [Google Scholar] [CrossRef]
- Truffault, F.; de Montpreville, V.; Eymard, B.; Sharshar, T.; Le Panse, R.; Berrih-Aknin, S. Thymic Germinal Centers and Corticosteroids in Myasthenia Gravis: An Immunopathological Study in 1035 Cases and a Critical Review. Clin. Rev. Allergy Immunol. 2017, 52, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.I.; Jones, M.; Ströbel, P.; Marx, A.; Gold, R.; Niks, E.; Verschuuren, J.J.G.M.; Berrih-Aknin, S.; Scaravilli, F.; Canelhas, A.; et al. Myasthenia Gravis Thymus. Am. J. Pathol. 2007, 171, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Willcox, N.; Leite, M.I.; Kadota, Y.; Jones, M.; Meager, A.; Subrahmanyam, P.; Dasgupta, B.; Morgan, B.P.; Vincent, A. Autoimmunizing Mechanisms in Thymoma and Thymus. Ann. N. Y. Acad. Sci. 2008, 1132, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Cufi, P.; Bismuth, J.; Eymard, B.; Fadel, E.; Berrih-Aknin, S.; Le Panse, R. SDF-1/CXCL12 Recruits B Cells and Antigen-Presenting Cells to the Thymus of Autoimmune Myasthenia Gravis Patients. Immunobiology 2013, 218, 373–381. [Google Scholar] [CrossRef]
- Berrih-Aknin, S.; Ruhlmann, N.; Bismuth, J.; Cizeron-Clairac, G.; Zelman, E.; Shachar, I.; Dartevelle, P.; de Rosbo, N.K.; Panse, R.L. CCL21 Overexpressed on Lymphatic Vessels Drives Thymic Hyperplasia in Myasthenia. Ann. Neurol. 2009, 66, 521–531. [Google Scholar] [CrossRef]
- Cordiglieri, C.; Marolda, R.; Franzi, S.; Cappelletti, C.; Giardina, C.; Motta, T.; Baggi, F.; Bernasconi, P.; Mantegazza, R.; Cavalcante, P. Innate Immunity in Myasthenia Gravis Thymus: Pathogenic Effects of Toll-like Receptor 4 Signaling on Autoimmunity. J. Autoimmun. 2014, 52, 74–89. [Google Scholar] [CrossRef]
- Meraouna, A.; Cizeron-Clairac, G.; Panse, R.L.; Bismuth, J.; Truffault, F.; Tallaksen, C.; Berrih-Aknin, S. The Chemokine CXCL13 Is a Key Molecule in Autoimmune Myasthenia Gravis. Blood 2006, 108, 432–440. [Google Scholar] [CrossRef]
- Saito, R.; Onodera, H.; Tago, H.; Suzuki, Y.; Shimizu, M.; Matsumura, Y.; Kondo, T.; Itoyama, Y. Altered Expression of Chemokine Receptor CXCR5 on T Cells of Myasthenia Gravis Patients. J. Neuroimmunol. 2005, 170, 172–178. [Google Scholar] [CrossRef]
- Çebi, M.; Durmus, H.; Aysal, F.; Özkan, B.; Gül, G.E.; Çakar, A.; Hocaoglu, M.; Mercan, M.; Yentür, S.P.; Tütüncü, M.; et al. CD4+ T Cells of Myasthenia Gravis Patients Are Characterized by Increased IL-21, IL-4, and IL-17A Productions and Higher Presence of PD-1 and ICOS. Front. Immunol. 2020, 11, 809. [Google Scholar] [CrossRef]
- Feferman, T.; Maiti, P.K.; Berrih-Aknin, S.; Bismuth, J.; Bidault, J.; Fuchs, S.; Souroujon, M.C. Overexpression of IFN-Induced Protein 10 and Its Receptor CXCR3 in Myasthenia Gravis. J. Immunol. 2005, 174, 5324–5331. [Google Scholar] [CrossRef]
- Suster, S.; Moran, C.A. Thymoma Classification: Current Status and Future Trends. Am. J. Clin. Pathol. 2006, 125, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Galassi, C.; Ruffini, E.; Margaritora, S.; Bertolaccini, L.; Casadio, C.; Anile, M.; Venuta, F. Thymoma and the Increased Risk of Developing Extrathymic Malignancies: A Multicentre Study. Eur. J. Cardiothorac. Surg. 2013, 44, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Romi, F. Thymoma in Myasthenia Gravis: From Diagnosis to Treatment. Autoimmune Dis. 2011, 2011, 474512. [Google Scholar] [CrossRef] [PubMed]
- Marx, A.; Hohenberger, P.; Hoffmann, H.; Pfannschmidt, J.; Schnabel, P.; Hofmann, H.-S.; Wiebe, K.; Schalke, B.; Nix, W.; Gold, R.; et al. The Autoimmune Regulator AIRE in Thymoma Biology: Autoimmunity and Beyond. J. Thorac. Oncol. 2010, 5, S266–S272. [Google Scholar] [CrossRef] [PubMed]
- Meager, A.; Wadhwa, M.; Dilger, P.; Bird, C.; Thorpe, R.; Newsom-Davis, J.; Willcox, N. Anti-Cytokine Autoantibodies in Autoimmunity: Preponderance of Neutralizing Autoantibodies against Interferon-Alpha, Interferon-Omega and Interleukin-12 in Patients with Thymoma and/or Myasthenia Gravis. Clin. Exp. Immunol. 2003, 132, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Shiono, H.; Wong, Y.L.; Matthews, I.; Liu, J.-L.; Zhang, W.; Sims, G.; Meager, A.; Beeson, D.; Vincent, A.; Willcox, N. Spontaneous Production of Anti-IFN-α and Anti-IL-12 Autoantibodies by Thymoma Cells from Myasthenia Gravis Patients Suggests Autoimmunization in the Tumor. Int. Immunol. 2003, 15, 903–913. [Google Scholar] [CrossRef]
- Ströbel, P.; Rosenwald, A.; Beyersdorf, N.; Kerkau, T.; Elert, O.; Murumägi, A.; Sillanpää, N.; Peterson, P.; Hummel, V.; Rieckmann, P.; et al. Selective Loss of Regulatory T Cells in Thymomas. Ann. Neurol. 2004, 56, 901–904. [Google Scholar] [CrossRef]
- Lefeuvre, C.M.J.; Payet, C.A.; Fayet, O.-M.; Maillard, S.; Truffault, F.; Bondet, V.; Duffy, D.; de Montpreville, V.; Ghigna, M.-R.; Fadel, E.; et al. Risk Factors Associated with Myasthenia Gravis in Thymoma Patients: The Potential Role of Thymic Germinal Centers. J. Autoimmun. 2020, 106, 102337. [Google Scholar] [CrossRef]
- Fountain, J.W.; Karayiorgou, M.; Taruscio, D.; Graw, S.L.; Buckler, A.J.; Ward, D.C.; Dracopoli, N.C.; Housman, D.E. Genetic and Physical Map of the Interferon Region on Chromosome 9p. Genomics 1992, 14, 105–112. [Google Scholar] [CrossRef]
- Borden, E.C.; Sen, G.C.; Uze, G.; Silverman, R.H.; Ransohoff, R.M.; Foster, G.R.; Stark, G.R. Interferons at Age 50: Past, Current and Future Impact on Biomedicine. Nat. Rev. Drug Discov. 2007, 6, 975–990. [Google Scholar] [CrossRef]
- Michael Roberts, R.; Liu, L.; Alexenko, A. New and Atypical Families of Type I Interferons in Mammals: Comparative Functions, Structures, and Evolutionary Relationships1. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 56, pp. 287–325. ISBN 978-0-12-540056-5. [Google Scholar]
- de Maeyer, E.; de Maeyer-Guignard, J. Type I Interferons. In Cytokines and Cytokine Receptors; CRC Press: London, UK, 2000; ISBN 978-0-429-18280-8. [Google Scholar]
- Cella, M.; Jarrossay, D.; Facchetti, F.; Alebardi, O.; Nakajima, H.; Lanzavecchia, A.; Colonna, M. Plasmacytoid Monocytes Migrate to Inflamed Lymph Nodes and Produce Large Amounts of Type I Interferon. Nat. Med. 1999, 5, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. Type I Interferons: Diversity of Sources, Production Pathways and Effects on Immune Responses. Curr. Opin. Virol. 2011, 1, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mann-Nüttel, R.; Schulze, A.; Richter, L.; Alferink, J.; Scheu, S. Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver’s Seat. Front. Immunol. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-Sensing Receptors in Sterile Inflammation and Inflammatory Diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Rodero, M.P.; Decalf, J.; Bondet, V.; Hunt, D.; Rice, G.I.; Werneke, S.; McGlasson, S.L.; Alyanakian, M.-A.; Bader-Meunier, B.; Barnerias, C.; et al. Detection of Interferon Alpha Protein Reveals Differential Levels and Cellular Sources in Disease. J. Exp. Med. 2017, 214, 1547–1555. [Google Scholar] [CrossRef]
- Uzé, G.; Schreiber, G.; Piehler, J.; Pellegrini, S. The Receptor of the Type I Interferon Family. In Interferon: The 50th Anniversary; Current Topics in Microbiology and, Immunology; Pitha, P.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 71–95. ISBN 978-3-540-71329-6. [Google Scholar]
- Piehler, J.; Thomas, C.; Garcia, K.C.; Schreiber, G. Structural and Dynamic Determinants of Type I Interferon Receptor Assembly and Their Functional Interpretation. Immunol. Rev. 2012, 250, 317–334. [Google Scholar] [CrossRef]
- Zanin, N.; Viaris de Lesegno, C.; Lamaze, C.; Blouin, C.M. Interferon Receptor Trafficking and Signaling: Journey to the Cross Roads. Front. Immunol. 2021, 11, 615603. [Google Scholar] [CrossRef]
- Honke, N.; Shaabani, N.; Zhang, D.-E.; Hardt, C.; Lang, K.S. Multiple Functions of USP18. Cell Death Dis. 2016, 7, e2444. [Google Scholar] [CrossRef]
- François-Newton, V.; de Freitas Almeida, G.M.; Payelle-Brogard, B.; Monneron, D.; Pichard-Garcia, L.; Piehler, J.; Pellegrini, S.; Uzé, G. USP18-Based Negative Feedback Control Is Induced by Type I and Type III Interferons and Specifically Inactivates Interferon α Response. PLoS ONE 2011, 6, e22200. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.C.; Tate, M.D.; Hertzog, P.J. MicroRNA as Type I Interferon-Regulated Transcripts and Modulators of the Innate Immune Response. Front. Immunol. 2015, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.S.; Dooley, J.; Linterman, M.A.; Pierson, W.; Ucar, O.; Kyewski, B.; Zuklys, S.; Hollander, G.A.; Matthys, P.; Gray, D.H.D.; et al. The Thymic Epithelial MicroRNA Network Elevates the Threshold for Infection-Associated Thymic Involution via MiR-29a Mediated Suppression of the IFN-α Receptor. Nat. Immunol. 2012, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Bisceglie, A.M.D.; Ray, R.B. Hepatitis C Virus-Mediated Enhancement of MicroRNA MiR-373 Impairs the JAK/STAT Signaling Pathway. J. Virol. 2015, 89, 3356–3365. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, X.; Cui, H.; Ni, X.; Yuan, M.; Guo, Y.; Huang, X.; Zhou, H.; de Vries, N.; Tak, P.P.; et al. MicroRNA-146A Contributes to Abnormal Activation of the Type I Interferon Pathway in Human Lupus by Targeting the Key Signaling Proteins. Arthritis Rheum. 2009, 60, 1065–1075. [Google Scholar] [CrossRef]
- Wang, P.; Hou, J.; Lin, L.; Wang, C.; Liu, X.; Li, D.; Ma, F.; Wang, Z.; Cao, X. Inducible MicroRNA-155 Feedback Promotes Type I IFN Signaling in Antiviral Innate Immunity by Targeting Suppressor of Cytokine Signaling 1. J. Immunol. 2010, 185, 6226–6233. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do. Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Der, S.D.; Zhou, A.; Williams, B.R.G.; Silverman, R.H. Identification of Genes Differentially Regulated by Interferon α, β, or γ Using Oligonucleotide Arrays. Proc. Natl. Acad. Sci. USA 1998, 95, 15623–15628. [Google Scholar] [CrossRef]
- de Veer, M.J.; Holko, M.; Frevel, M.; Walker, E.; Der, S.; Paranjape, J.M.; Silverman, R.H.; Williams, B.R.G. Functional Classification of Interferon-Stimulated Genes Identified Using Microarrays. J. Leukoc. Biol. 2001, 69, 912–920. [Google Scholar] [CrossRef]
- Trinchieri, G.; Santoli, D. Anti-Viral Activity Induced by Culturing Lymphocytes with Tumor-Derived or Virus-Transformed Cells. Enhancement of Human Natural Killer Cell Activity by Interferon and Antagonistic Inhibition of Susceptibility of Target Cells to Lysis. J. Exp. Med. 1978, 147, 1314–1333. [Google Scholar] [CrossRef]
- Liang, S.; Wei, H.; Sun, R.; Tian, Z. IFNα Regulates NK Cell Cytotoxicity through STAT1 Pathway. Cytokine 2003, 23, 190–199. [Google Scholar] [CrossRef]
- Nakano, M.; Fujii, T.; Hashimoto, M.; Yukawa, N.; Yoshifuji, H.; Ohmura, K.; Nakaizumi, A.; Mimori, T. Type I Interferon Induces CX3CL1 (Fractalkine) and CCL5 (RANTES) Production in Human Pulmonary Vascular Endothelial Cells. Clin. Exp. Immunol. 2012, 170, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cepok, S.; Schreiber, H.; Hoffmann, S.; Zhou, D.; Neuhaus, O.; von Geldern, G.; Hochgesand, S.; Nessler, S.; Rothhammer, V.; Lang, M.; et al. Enhancement of Chemokine Expression by Interferon Beta Therapy in Patients with Multiple Sclerosis. Arch. Neurol. 2009, 66, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.; Gresser, I.; Leary, P.; Tovey, M. Interferon Treatment of Mice: Enhanced Expression of Histocompatibility Antigens on Lymphoid Cells. Proc. Natl. Acad. Sci. USA 1976, 73, 1284–1287. [Google Scholar] [CrossRef]
- Kolumam, G.A.; Thomas, S.; Thompson, L.J.; Sprent, J.; Murali-Krishna, K. Type I Interferons Act Directly on CD8 T Cells to Allow Clonal Expansion and Memory Formation in Response to Viral Infection. J. Exp. Med. 2005, 202, 637–650. [Google Scholar] [CrossRef]
- Marrack, P.; Kappler, J.; Mitchell, T. Type I Interferons Keep Activated T Cells Alive. J. Exp. Med. 1999, 189, 521–530. [Google Scholar] [CrossRef]
- Lin, Q.; Dong, C.; Cooper, M.D. Impairment of T and B Cell Development by Treatment with a Type I Interferon. J. Exp. Med. 1998, 187, 79–87. [Google Scholar] [CrossRef]
- Le Bon, A.; Schiavoni, G.; D’Agostino, G.; Gresser, I.; Belardelli, F.; Tough, D.F. Type I Interferons Potently Enhance Humoral Immunity and Can Promote Isotype Switching by Stimulating Dendritic Cells In Vivo. Immunity 2001, 14, 461–470. [Google Scholar] [CrossRef]
- Crow, Y.J. Type I Interferonopathies: A Novel Set of Inborn Errors of Immunity. Ann. N. Y. Acad. Sci. 2011, 1238, 91–98. [Google Scholar] [CrossRef]
- Aicardi, J.; Goutières, F. A Progressive Familial Encephalopathy in Infancy with Calcifications of the Basal Ganglia and Chronic Cerebrospinal Fluid Lymphocytosis. Ann. Neurol. 1984, 15, 49–54. [Google Scholar] [CrossRef]
- Yu, Z.-X.; Song, H.-M. Toward a Better Understanding of Type I Interferonopathies: A Brief Summary, Update and Beyond. World J. Pediatr. 2020, 16, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lebon, P.; Badoual, J.; Ponsot, G.; Goutières, F.; Hémeury-Cukier, F.; Aicardi, J. Intrathecal Synthesis of Interferon-Alpha in Infants with Progressive Familial Encephalopathy. J. Neurol. Sci. 1988, 84, 201–208. [Google Scholar] [CrossRef]
- Rice, G.I.; Forte, G.M.A.; Szynkiewicz, M.; Chase, D.S.; Aeby, A.; Abdel-Hamid, M.S.; Ackroyd, S.; Allcock, R.; Bailey, K.M.; Balottin, U.; et al. Assessment of Interferon-Related Biomarkers in Aicardi-Goutières Syndrome Associated with Mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: A Case-Control Study. Lancet Neurol. 2013, 12, 1159–1169. [Google Scholar] [CrossRef]
- Lebon, P.; Meritet, J.F.; Krivine, A.; Rozenberg, F. Interferon and Aicardi-Goutières Syndrome. Eur. J. Paediatr. Neurol. 2002, 6, A47–A53. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.; Palucka, A.K.; Arce, E.; Cantrell, V.; Borvak, J.; Banchereau, J.; Pascual, V. Interferon and Granulopoiesis Signatures in Systemic Lupus Erythematosus Blood. J. Exp. Med. 2003, 197, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sheng, Y.; Zhang, X. Genetic Susceptibility to SLE: Recent Progress from GWAS. J. Autoimmun. 2013, 41, 25–33. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Z.; Jallal, B.; Shen, N.; Rönnblom, L. Type I Interferons in Sjögren’s Syndrome. Autoimmun. Rev. 2013, 12, 558–566. [Google Scholar] [CrossRef]
- Lübbers, J.; Brink, M.; van de Stadt, L.A.; Vosslamber, S.; Wesseling, J.G.; van Schaardenburg, D.; Rantapää-Dahlqvist, S.; Verweij, C.L. The Type I IFN Signature as a Biomarker of Preclinical Rheumatoid Arthritis. Ann. Rheum. Dis. 2013, 72, 776–780. [Google Scholar] [CrossRef]
- Roelofs, M.F.; Wenink, M.H.; Brentano, F.; Abdollahi-Roodsaz, S.; Oppers-Walgreen, B.; Barrera, P.; van Riel, P.L.C.M.; Joosten, L.A.B.; Kyburz, D.; van den Berg, W.B.; et al. Type I Interferons Might Form the Link between Toll-like Receptor (TLR) 3/7 and TLR4-Mediated Synovial Inflammation in Rheumatoid Arthritis (RA). Ann. Rheum. Dis. 2009, 68, 1486–1493. [Google Scholar] [CrossRef]
- Greenberg, S.A.; Pinkus, J.L.; Pinkus, G.S.; Burleson, T.; Sanoudou, D.; Tawil, R.; Barohn, R.J.; Saperstein, D.S.; Briemberg, H.R.; Ericsson, M.; et al. Interferon-α/β–Mediated Innate Immune Mechanisms in Dermatomyositis. Ann. Neurol. 2005, 57, 664–678. [Google Scholar] [CrossRef]
- Bahri, D.M.; Khiari, H.; Essouri, A.; Laadhar, L.; Zaraa, I.; Mrabet, A.; Meddeb, N.; Sellami, S. Systemic Lupus Erythematosus Induced by Interferon Β1 Therapy in a Patient with Multiple Sclerosis. Fundam. Clin. Pharmacol. 2012, 26, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Nousari, H.C.; Kimyai-Asadi, A.; Tausk, F.A. Subacute Cutaneous Lupus Erythematosus Associated with Interferon Beta-1a. Lancet 1998, 352, 1825–1826. [Google Scholar] [CrossRef]
- Baik, S.J.; Kim, T.H.; Kim, H.I.; Rhie, J.Y. Myasthenia Crisis Induced by Pegylated-Interferon in Patient with Chronic Hepatitis C. Medicine 2016, 95, e3782. [Google Scholar] [CrossRef] [PubMed]
- Dionisiotis, J.; Zoukos, Y.; Thomaides, T. Development of Myasthenia Gravis in Two Patients with Multiple Sclerosis Following Interferon β Treatment. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1079. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gurtubay, I.G.; Morales, G.; Aréchaga, O.; Gállego, J. Development of Myasthenia Gravis after Interferon Alpha Therapy. Electromyogr. Clin. Neurophysiol. 1999, 39, 75–78. [Google Scholar] [PubMed]
- Jankovic, S.M. Injectable Interferon Beta-1b for the Treatment of Relapsing Forms of Multiple Sclerosis. J. Inflamm. Res. 2010, 3, 25–31. [Google Scholar] [CrossRef][Green Version]
- Barzago, C.; Lum, J.; Cavalcante, P.; Srinivasan, K.G.; Faggiani, E.; Camera, G.; Bonanno, S.; Andreetta, F.; Antozzi, C.; Baggi, F.; et al. A Novel Infection- and Inflammation-Associated Molecular Signature in Peripheral Blood of Myasthenia Gravis Patients. Immunobiology 2016, 221, 1227–1236. [Google Scholar] [CrossRef]
- Vilquin, J.-T.; Bayer, A.C.; Le Panse, R.; Berrih-Aknin, S. The Muscle Is Not a Passive Target in Myasthenia Gravis. Front. Neurol. 2019, 10, 1343. [Google Scholar] [CrossRef]
- Poëa-Guyon, S.; Christadoss, P.; Panse, R.L.; Guyon, T.; Baets, M.D.; Wakkach, A.; Bidault, J.; Tzartos, S.; Berrih-Aknin, S. Effects of Cytokines on Acetylcholine Receptor Expression: Implications for Myasthenia Gravis. J. Immunol. 2005, 174, 5941–5949. [Google Scholar] [CrossRef]
- Cufi, P.; Dragin, N.; Weiss, J.M.; Martinez-Martinez, P.; De Baets, M.H.; Roussin, R.; Fadel, E.; Berrih-Aknin, S.; Le Panse, R. Implication of Double-Stranded RNA Signaling in the Etiology of Autoimmune Myasthenia Gravis. Ann. Neurol. 2013, 73, 281–293. [Google Scholar] [CrossRef]
- Gradolatto, A.; Nazzal, D.; Truffault, F.; Bismuth, J.; Fadel, E.; Foti, M.; Berrih-Aknin, S. Both Treg Cells and Tconv Cells Are Defective in the Myasthenia Gravis Thymus: Roles of IL-17 and TNF-α. J. Autoimmun. 2014, 52, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Cufi, P.; Dragin, N.; Ruhlmann, N.; Weiss, J.M.; Fadel, E.; Serraf, A.; Berrih-Aknin, S.; Le Panse, R. Central Role of Interferon-Beta in Thymic Events Leading to Myasthenia Gravis. J. Autoimmun. 2014, 52, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Aime, C.; Cohen-Kaminsky, S.; Berrih-Aknin, S. In Vitro Interleukin-1 (IL-1) Production in Thymic Hyperplasia and Thymoma from Patients with Myasthenia Gravis. J. Clin. Immunol. 1991, 11, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.A.; Bayer, A.C.; Ider, K.; Bismuth, J.; Truffault, F.; Roussin, R.; Santelmo, N.; Le Panse, R.; Berrih-Aknin, S.; Dragin, N. Il-23/Th17 Cell Pathway: A Promising Target to Alleviate Thymic Inflammation Maintenance in Myasthenia Gravis. J. Autoimmun. 2019, 98, 59–73. [Google Scholar] [CrossRef]
- Cufi, P.; Soussan, P.; Truffault, F.; Fetouchi, R.; Robinet, M.; Fadel, E.; Berrih-Aknin, S.; Le Panse, R. Thymoma-Associated Myasthenia Gravis: On the Search for a Pathogen Signature. J. Autoimmun. 2014, 52, 29–35. [Google Scholar] [CrossRef]
- Flammer, J.R.; Dobrovolna, J.; Kennedy, M.A.; Chinenov, Y.; Glass, C.K.; Ivashkiv, L.B.; Rogatsky, I. The Type I Interferon Signaling Pathway Is a Target for Glucocorticoid Inhibition. Mol. Cell. Biol. 2010, 30, 4564–4574. [Google Scholar] [CrossRef]
- Cizeron-Clairac, G.; Le Panse, R.; Frenkian-Cuvelier, M.; Meraouna, A.; Truffault, F.; Bismuth, J.; Mussot, S.; Kerlero de Rosbo, N.; Berrih-Aknin, S. Thymus and Myasthenia Gravis: What Can We Learn from DNA Microarrays. J. Neuroimmunol. 2008, 201–202, 57–63. [Google Scholar] [CrossRef]
- Wolfe, G.I.; Kaminski, H.J.; Aban, I.B.; Minisman, G.; Kuo, H.-C.; Marx, A.; Ströbel, P.; Mazia, C.; Oger, J.; Cea, J.G.; et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 511–522. [Google Scholar] [CrossRef]
- Jiang, R.; Hoehn, K.B.; Lee, C.S.; Pham, M.C.; Homer, R.J.; Detterbeck, F.C.; Aban, I.; Jacobson, L.; Vincent, A.; Nowak, R.J.; et al. Thymus-Derived B Cell Clones Persist in the Circulation after Thymectomy in Myasthenia Gravis. Proc. Natl. Acad. Sci. USA 2020, 117, 30649–30660. [Google Scholar] [CrossRef]
- Xu, D.; Staedman, A.; Zhang, L. CD20 Antibody Primes B Lymphocytes for Type I Interferon Production. PLoS ONE 2013, 8, e67900. [Google Scholar] [CrossRef]
- Annane, D.; Heming, N.; Grimaldi-Bensouda, L.; Frémeaux-Bacchi, V.; Vigan, M.; Roux, A.-L.; Marchal, A.; Michelon, H.; Rottman, M.; Moine, P. Eculizumab as an Emergency Treatment for Adult Patients with Severe COVID-19 in the Intensive Care Unit: A Proof-of-Concept Study. eClinicalMedicine 2020, 28, 100590. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.-F. Infections and Autoimmune Diseases. J. Autoimmun. 2005, 25, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Leopardi, V.; Chang, Y.-M.; Pham, A.; Luo, J.; Garden, O.A. A Systematic Review of the Potential Implication of Infectious Agents in Myasthenia Gravis. Front. Neurol. 2021, 12, 618021. [Google Scholar] [CrossRef] [PubMed]
- Niller, H.H.; Wolf, H.; Minarovits, J. Regulation and Dysregulation of Epstein–Barr Virus Latency: Implications for the Development of Autoimmune Diseases. Autoimmunity 2008, 41, 298–328. [Google Scholar] [CrossRef]
- Savino, W. The Thymus Is a Common Target Organ in Infectious Diseases. PLoS Pathog. 2006, 2, e62. [Google Scholar] [CrossRef]
- McGuire, L.J.; Huang, D.P.; Teoh, R.; Arnold, M.; Wong, K.; Lee, J.C. Epstein-Barr Virus Genome in Thymoma and Thymic Lymphoid Hyperplasia. Am. J. Pathol. 1988, 131, 385–390. [Google Scholar]
- Cavalcante, P.; Serafini, B.; Rosicarelli, B.; Maggi, L.; Barberis, M.; Antozzi, C.; Berrih-Aknin, S.; Bernasconi, P.; Aloisi, F.; Mantegazza, R. Epstein-Barr Virus Persistence and Reactivation in Myasthenia Gravis Thymus. Ann. Neurol. 2010, 67, 726–738. [Google Scholar] [CrossRef]
- Kakalacheva, K.; Maurer, M.A.; Tackenberg, B.; Münz, C.; Willcox, N.; Lünemann, J.D. Intrathymic Epstein-Barr Virus Infection Is Not a Prominent Feature of Myasthenia Gravis. Ann. Neurol. 2011, 70, 508–514. [Google Scholar] [CrossRef]
- Meyer, M.; Höls, A.-K.; Liersch, B.; Leistner, R.; Gellert, K.; Schalke, B.; Marx, A.; Niedobitek, G. Lack of Evidence for Epstein-Barr Virus Infection in Myasthenia Gravis Thymus. Ann. Neurol. 2011, 70, 515–518. [Google Scholar] [CrossRef]
- Csuka, D.; Banati, M.; Rozsa, C.; Füst, G.; Illes, Z. High Anti-EBNA-1 IgG Levels Are Associated with Early-Onset Myasthenia Gravis. Eur. J. Neurol. 2012, 19, 842–846. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Z.; Shen, G.; Chai, Y.; Liang, C. Association between Epstein-Barr Virus and Thymic Epithelial Tumors: A Systematic Review. Infect. Agents Cancer 2019, 14, 32. [Google Scholar] [CrossRef]
- Cavalcante, P.; Marcuzzo, S.; Franzi, S.; Galbardi, B.; Maggi, L.; Motta, T.; Ghislandi, R.; Buzzi, A.; Spinelli, L.; Novellino, L.; et al. Epstein-Barr Virus in Tumor-Infiltrating B Cells of Myasthenia Gravis Thymoma: An Innocent Bystander or an Autoimmunity Mediator. Oncotarget 2017, 8, 95432–95449. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.L.; Mirzaei, F.; Ascherio, A. Infectious Mononucleosis and Risk for Multiple Sclerosis: A Meta-Analysis. Ann. Neurol. 2006, 59, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, P.; Barberis, M.; Cannone, M.; Baggi, F.; Antozzi, C.; Maggi, L.; Cornelio, F.; Barbi, M.; Dido, P.; Berrih-Aknin, S.; et al. Detection of Poliovirus-Infected Macrophages in Thymus of Patients with Myasthenia Gravis. Neurology 2010, 74, 1118–1126. [Google Scholar] [CrossRef]
- Münz, C.; Lünemann, J.D.; Getts, M.T.; Miller, S.D. Antiviral Immune Responses: Triggers of or Triggered by Autoimmunity. Nat. Rev. Immunol. 2009, 9, 246–258. [Google Scholar] [CrossRef]
- Bernasconi, P.; Barberis, M.; Baggi, F.; Passerini, L.; Cannone, M.; Arnoldi, E.; Novellino, L.; Cornelio, F.; Mantegazza, R. Increased Toll-like Receptor 4 Expression in Thymus of Myasthenic Patients with Thymitis and Thymic Involution. Am. J. Pathol. 2005, 167, 129–139. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef]
- Robinet, M.; Villeret, B.; Maillard, S.; Cron, M.A.; Berrih-Aknin, S.; Le Panse, R. Use of Toll-Like Receptor Agonists to Induce Ectopic Lymphoid Structures in Myasthenia Gravis Mouse Models. Front. Immunol. 2017, 8, 1029. [Google Scholar] [CrossRef]
- Cron, M.A.; Maillard, S.; Villegas, J.; Truffault, F.; Sudres, M.; Dragin, N.; Berrih-Aknin, S.; Le Panse, R. Thymus Involvement in Early-Onset Myasthenia Gravis: Thymic Changes in Autoimmune Myasthenia Gravis. Ann. N. Y. Acad. Sci. 2018, 1412, 137–145. [Google Scholar] [CrossRef]
- Sengupta, M.; Wang, B.-D.; Lee, N.H.; Marx, A.; Kusner, L.L.; Kaminski, H.J. MicroRNA and MRNA Expression Associated with Ectopic Germinal Centers in Thymus of Myasthenia Gravis. PLoS ONE 2018, 13, e0205464. [Google Scholar] [CrossRef] [PubMed]
- Bortone, F.; Scandiffio, L.; Marcuzzo, S.; Bonanno, S.; Frangiamore, R.; Motta, T.; Antozzi, C.; Mantegazza, R.; Cavalcante, P.; Bernasconi, P. MiR-146a in Myasthenia Gravis Thymus Bridges Innate Immunity with Autoimmunity and Is Linked to Therapeutic Effects of Corticosteroids. Front. Immunol. 2020, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Tian, F.-F.; Yan, M.; Zhang, J.-M.; Liu, Q.; Lu, J.-Y.; Zhou, W.-B.; Yang, H.; Li, J. Delivery of an MiR155 Inhibitor by Anti-CD20 Single-Chain Antibody into B Cells Reduces the Acetylcholine Receptor-Specific Autoantibodies and Ameliorates Experimental Autoimmune Myasthenia Gravis. Clin. Exp. Immunol. 2014, 176, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Grillo, A.R.; Scarpa, M.; Brun, P.; D’Incà, R.; Nai, L.; Banerjee, A.; Cavallo, D.; Barzon, L.; Palù, G.; et al. MiR-155 Modulates the Inflammatory Phenotype of Intestinal Myofibroblasts by Targeting SOCS1 in Ulcerative Colitis. Exp. Mol. Med. 2015, 47, e164. [Google Scholar] [CrossRef]
- Cron, M.A.; Payet, C.A.; Fayet, O.-M.; Maillard, S.; Truffault, F.; Fadel, E.; Guihaire, J.; Berrih-Aknin, S.; Liston, A.; Le Panse, R. Decreased Expression of MiR-29 Family Associated with Autoimmune Myasthenia Gravis. J. Neuroinflamm. 2020, 17, 294. [Google Scholar] [CrossRef]
- Xiong, X.; Xiang, M.; Cheng, X.; Huang, Y. PTPN22 R620W Polymorphism Is Associated with Myasthenia Gravis Risk: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2015, 21, 2567–2571. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Shaked, I.; Stanford, S.M.; Zhou, W.; Curtsinger, J.M.; Mikulski, Z.; Shaheen, Z.R.; Cheng, G.; Sawatzke, K.; Campbell, A.M.; et al. The Autoimmunity-Associated Gene PTPN22 Potentiates Toll-like Receptor-Driven, Type 1 Interferon-Dependent Immunity. Immunity 2013, 39, 111–122. [Google Scholar] [CrossRef]
- Gregersen, P.K.; Kosoy, R.; Lee, A.T.; Lamb, J.; Sussman, J.; McKee, D.; Simpfendorfer, K.R.; Pirskanen-Matell, R.; Piehl, F.; Pan-Hammarstrom, Q.; et al. Risk for Myasthenia Gravis Maps to a 151Pro→Ala Change in TNIP1 and to Human Leukocyte Antigen-B*08. Ann. Neurol. 2012, 72, 927–935. [Google Scholar] [CrossRef]
- Shamilov, R.; Aneskievich, B.J. TNIP1 in Autoimmune Diseases: Regulation of Toll-like Receptor Signaling. J. Immunol. Res. 2018, 2018, e3491269. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payet, C.A.; You, A.; Fayet, O.-M.; Dragin, N.; Berrih-Aknin, S.; Le Panse, R. Myasthenia Gravis: An Acquired Interferonopathy? Cells 2022, 11, 1218. https://doi.org/10.3390/cells11071218
Payet CA, You A, Fayet O-M, Dragin N, Berrih-Aknin S, Le Panse R. Myasthenia Gravis: An Acquired Interferonopathy? Cells. 2022; 11(7):1218. https://doi.org/10.3390/cells11071218
Chicago/Turabian StylePayet, Cloé A., Axel You, Odessa-Maud Fayet, Nadine Dragin, Sonia Berrih-Aknin, and Rozen Le Panse. 2022. "Myasthenia Gravis: An Acquired Interferonopathy?" Cells 11, no. 7: 1218. https://doi.org/10.3390/cells11071218
APA StylePayet, C. A., You, A., Fayet, O.-M., Dragin, N., Berrih-Aknin, S., & Le Panse, R. (2022). Myasthenia Gravis: An Acquired Interferonopathy? Cells, 11(7), 1218. https://doi.org/10.3390/cells11071218





