Increased Frequency of CD4+ Follicular Helper T and CD8+ Follicular T Cells in Human Lymph Node Biopsies during the Earliest Stages of Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Sample Processing and Cell Culture
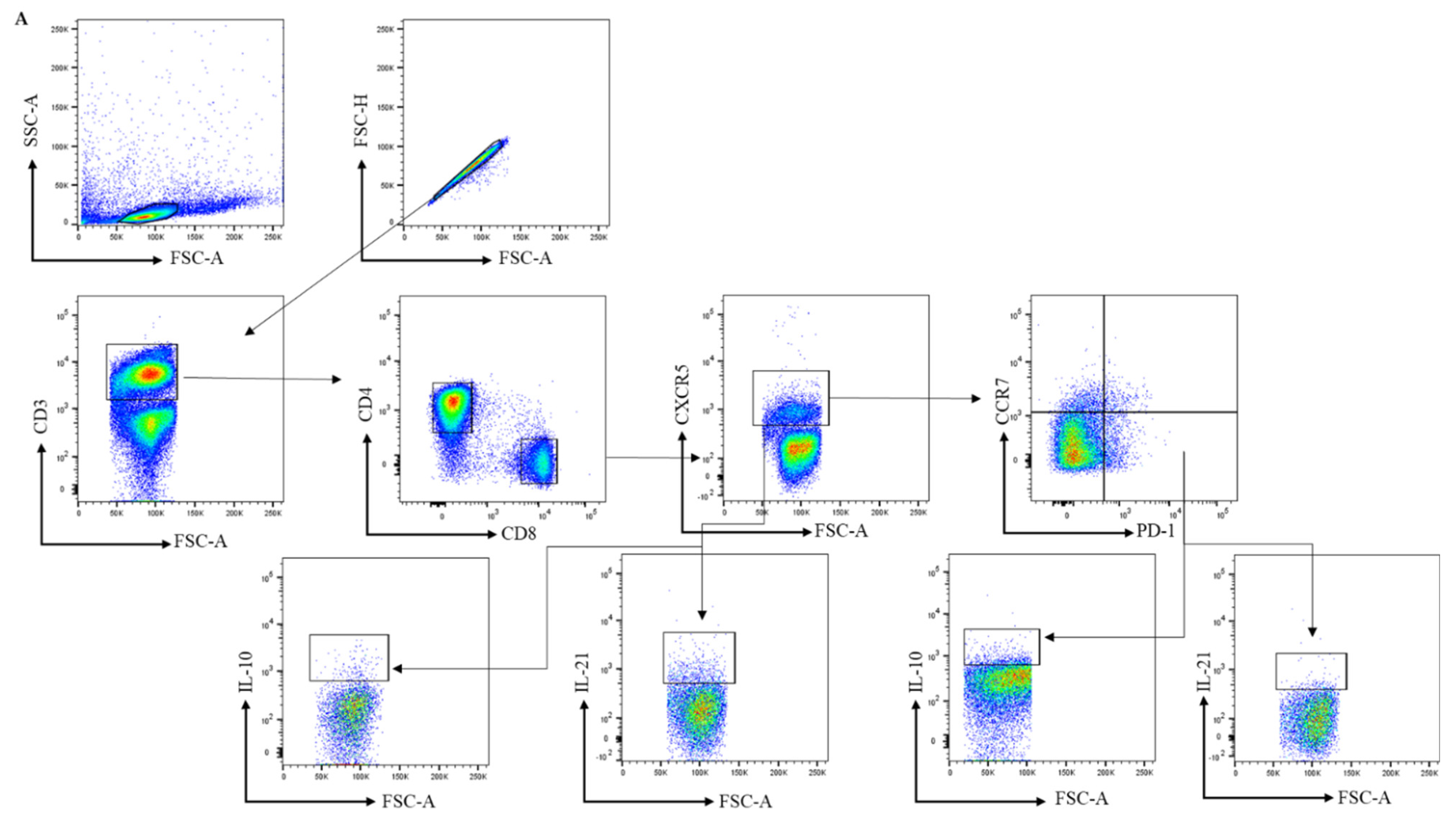
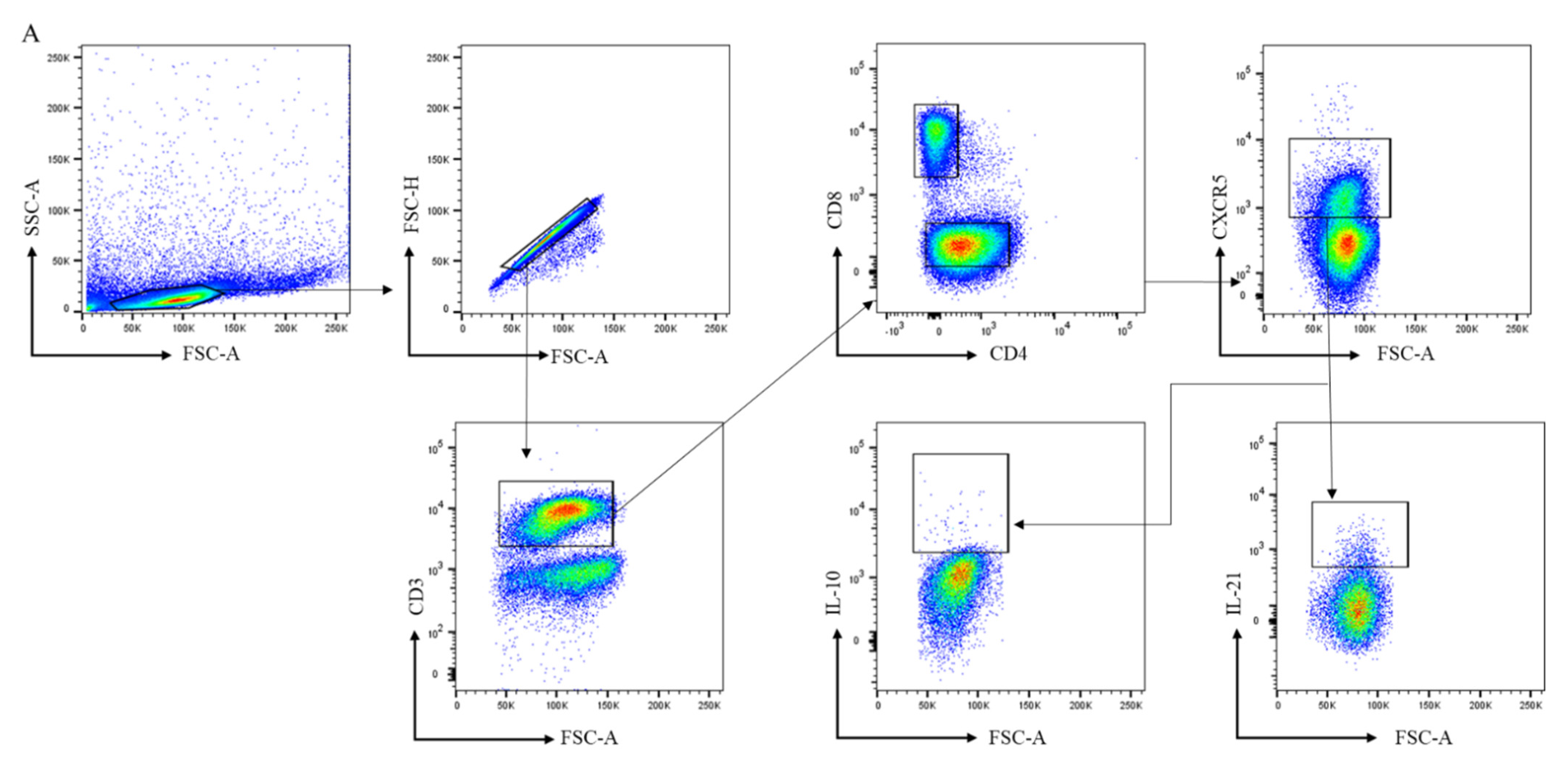
2.3. Antibodies and Flow Cytometry Analysis
2.4. Immunofluorescent Microscopy
2.5. Statistical Analysis
3. Results
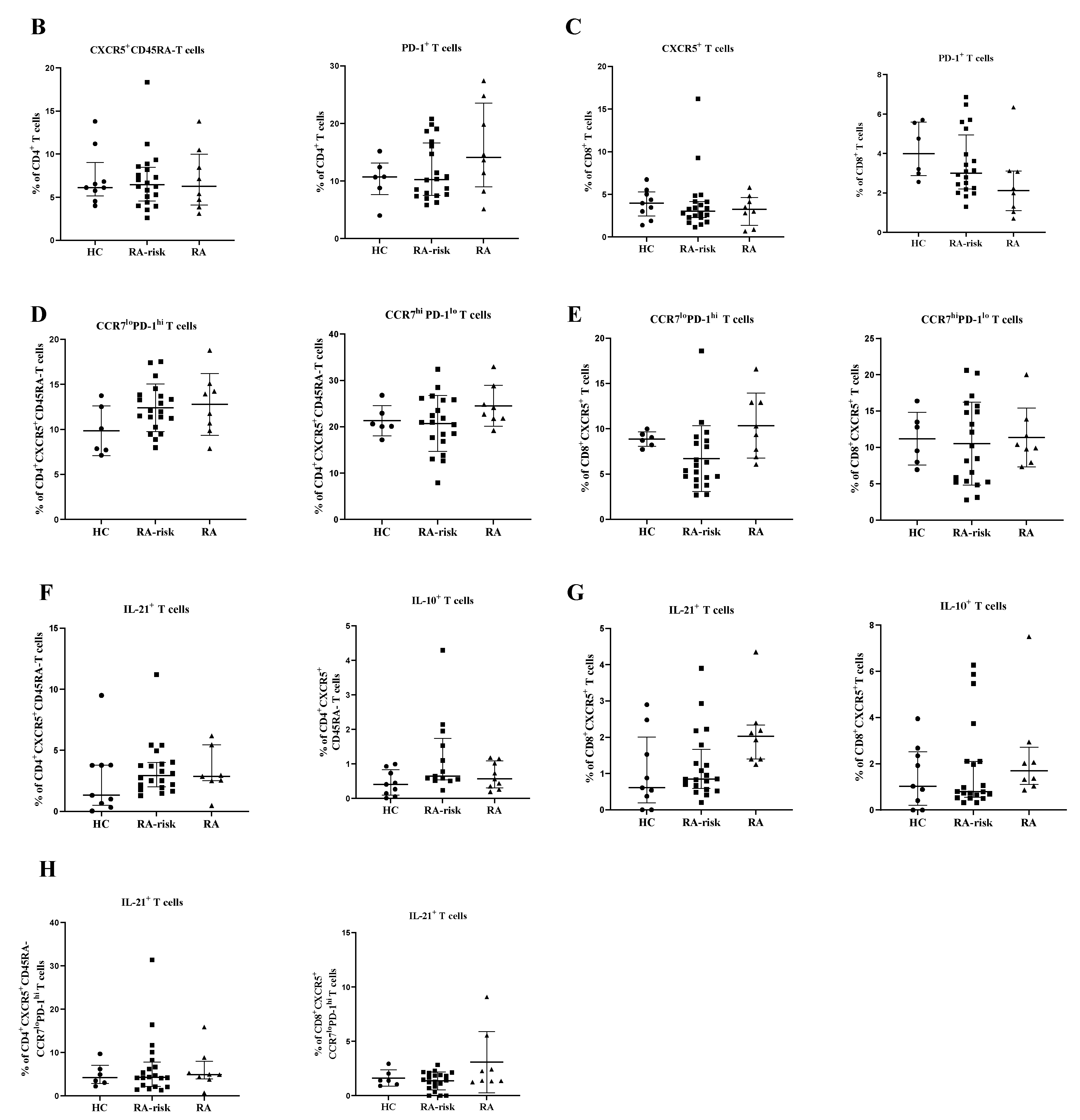
3.1. The Frequency of Peripheral Blood CD4+ Circulating Follicular Helper T and Circulating CD8+ Follicular T Cells Is Comparable between Healthy Controls, RA-Risk Individuals, and Early RA Patients
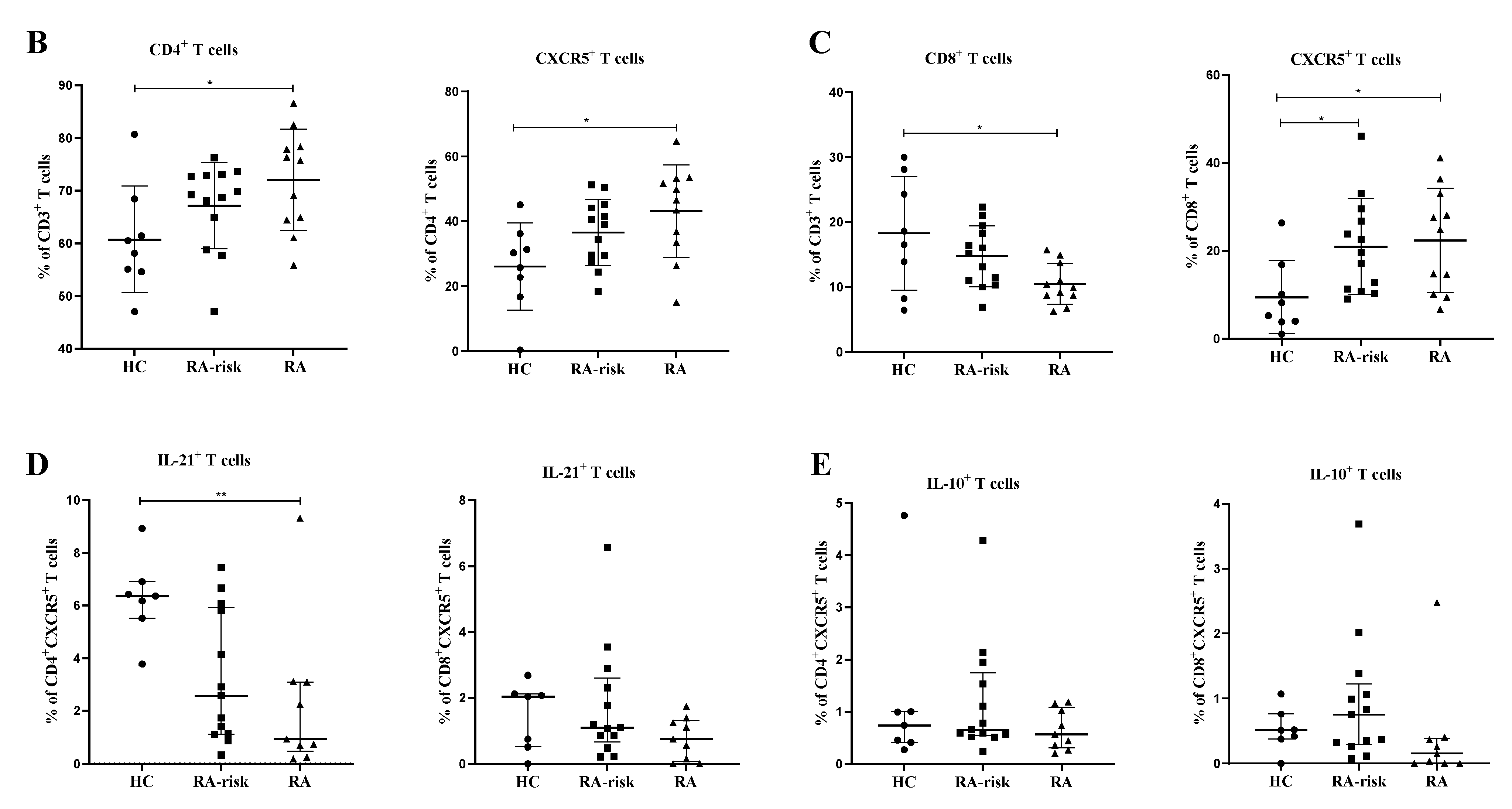
3.2. CD4+ Follicular Helper T and CD8+ Follicular T Cells Are Increased in Lymphoid Tissue of RA Patients
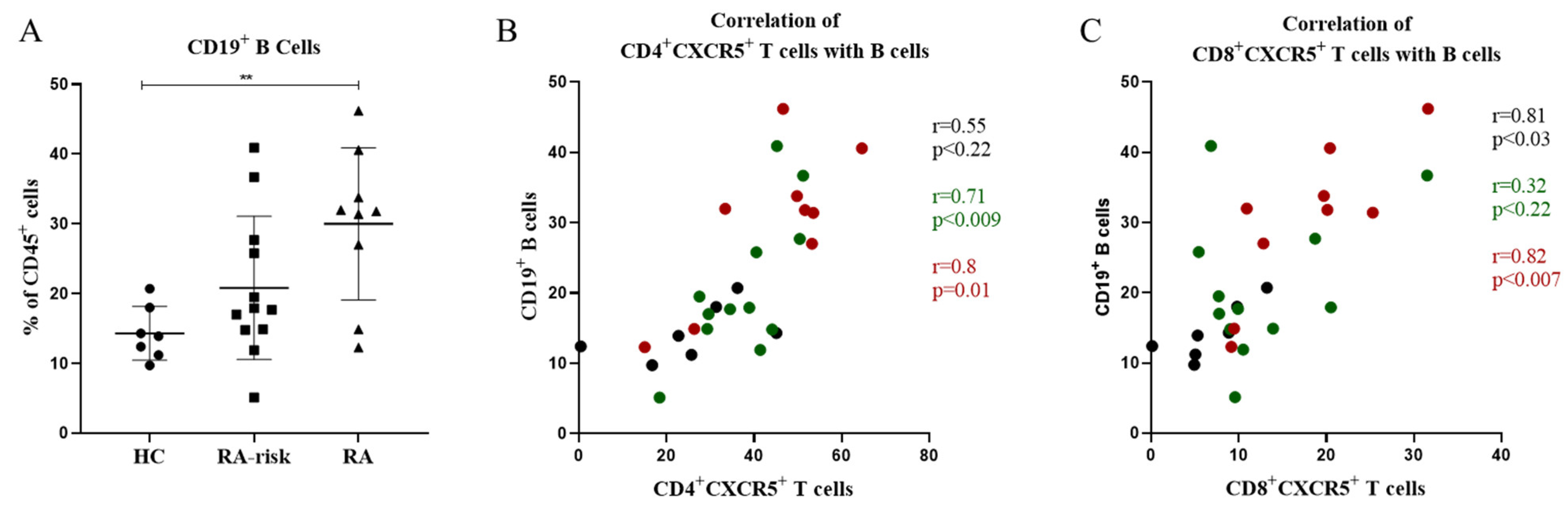
3.3. In Lymphoid Tissue, the Frequencies of CD4+ Follicular Helper T Cells and CD8+ Follicular T Cells Correlate with the Frequencies of CD19+ B Cells
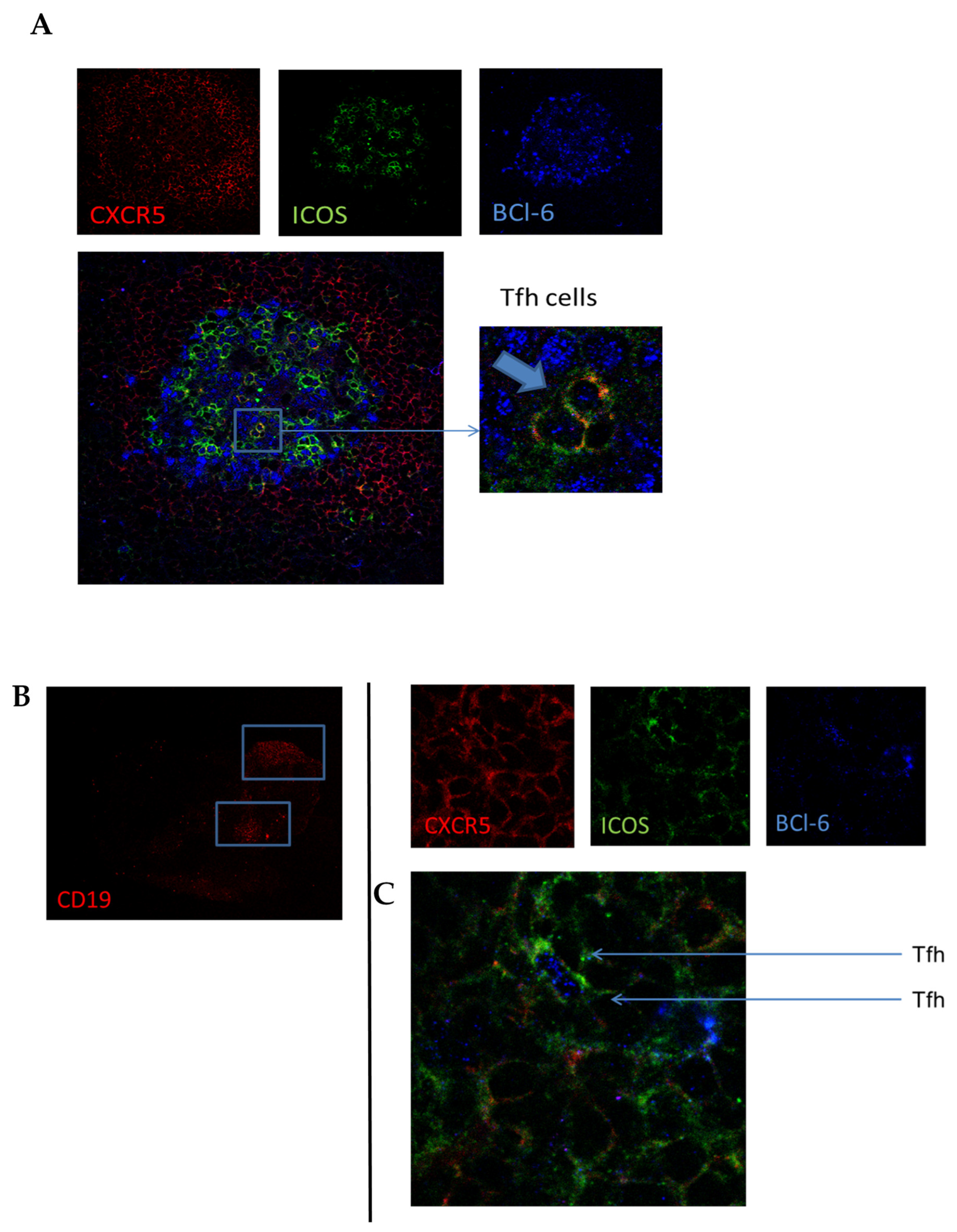
3.4. In Lymphoid and Synovial Tissue, Follicular T Cells Are Located in B-Cell Areas
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rantapaa-Dahlqvist, S.; de Jong, B.A.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Patakas, A.; Platt, A.M.; Butcher, J.P.; Maffia, P.; McInnes, I.B.; Brewer, J.M.; Garside, P.; Benson, R.A. Putative existence of reciprocal dialogue between Tfh and B cells and its impact on infectious and autoimmune disease. Immunol. Lett. 2011, 138, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Anang, D.C.; Balzaretti, G.; van Kampen, A.; de Vries, N.; Klarenbeek, P.L. The Germinal Center Milieu in Rheumatoid Arthritis: The Immunological Drummer or Dancer? Int. J. Mol. Sci. 2021, 22, 10514. [Google Scholar] [PubMed]
- Alexander, C.M.; Tygrett, L.T.; Boyden, A.W.; Wolniak, K.L.; Legge, K.L.; Waldschmidt, T.J. T regulatory cells participate in the control of germinal centre reactions. Immunology 2011, 133, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Cook, M.C.; Angelucci, C.; Athanasopoulos, V.; Rui, L.; Hill, K.M.; Yu, D.; Domaschenz, H.; Whittle, B.; Lambe, T.; et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 2005, 435, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, J.; Cui, X.; Zuo, Y.; Zhang, Z.; Li, Y.; Tao, R.; Li, Y.; Pang, J. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell. Immunol. 2015, 295, 46–51. [Google Scholar] [CrossRef]
- Zhang, X.; Lindwall, E.; Gauthier, C.; Lyman, J.; Spencer, N.; Alarakhia, A.; Fraser, A.; Ing, S.; Chen, M.; Webb-Detiege, T.; et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 2015, 24, 909–917. [Google Scholar] [CrossRef]
- Hutloff, A.; Buchner, K.; Reiter, K.; Baelde, H.J.; Odendahl, M.; Jacobi, A.; Dorner, T.; Kroczek, R.A. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum. 2004, 50, 3211–3220. [Google Scholar] [CrossRef]
- Simpson, N.; Gatenby, P.A.; Wilson, A.; Malik, S.; Fulcher, D.A.; Tangye, S.G.; Manku, H.; Vyse, T.J.; Roncador, G.; Huttley, G.A.; et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 234–244. [Google Scholar] [CrossRef]
- Arroyo-Villa, I.; Bautista-Caro, M.B.; Balsa, A.; Aguado-Acin, P.; Bonilla-Hernan, M.G.; Plasencia, C.; Villalba, A.; Nuno, L.; Puig-Kroger, A.; Martin-Mola, E.; et al. Constitutively altered frequencies of circulating follicullar helper T cell counterparts and their subsets in rheumatoid arthritis. Arthritis Res. Ther. 2014, 16, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, R.C.; Simons, H.Z.; Thompson, W.S.; Cutler, A.J.; Dopico, X.C.; Smyth, D.J.; Mashar, M.; Schuilenburg, H.; Walker, N.M.; Dunger, D.B.; et al. IL-21 production by CD4+ effector T cells and frequency of circulating follicular helper T cells are increased in type 1 diabetes patients. Diabetologia 2015, 58, 781–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Zhu, C.; Ma, B.; Tian, J.; Baidoo, S.E.; Mao, C.; Wu, W.; Chen, J.; Tong, J.; Yang, M.; et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2012, 2012, 827480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Tsai, L.M.; Leong, Y.A.; Hu, X.; Ma, C.S.; Chevalier, N.; Sun, X.; Vandenberg, K.; Rockman, S.; Ding, Y.; et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013, 39, 770–781. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wu, Q.; Su, D.; Che, N.; Chen, H.; Geng, L.; Chen, J.; Chen, W.; Li, X.; Sun, L. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R255. [Google Scholar] [CrossRef] [Green Version]
- Quigley, M.F.; Gonzalez, V.D.; Granath, A.; Andersson, J.; Sandberg, J.K. CXCR5+ CCR7− CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur. J. Immunol. 2007, 37, 3352–3362. [Google Scholar] [CrossRef]
- He, R.; Hou, S.; Liu, C.; Zhang, A.; Bai, Q.; Han, M.; Yang, Y.; Wei, G.; Shen, T.; Yang, X.; et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 2016, 537, 412–428. [Google Scholar] [CrossRef]
- Leong, Y.A.; Chen, Y.; Ong, H.S.; Wu, D.; Man, K.; Deleage, C.; Minnich, M.; Meckiff, B.J.; Wei, Y.; Hou, Z.; et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol. 2016, 17, 1187–1196. [Google Scholar] [CrossRef]
- Miles, B.; Miller, S.M.; Folkvord, J.M.; Levy, D.N.; Rakasz, E.G.; Skinner, P.J.; Connick, E. Follicular Regulatory CD8 T Cells Impair the Germinal Center Response in SIV and Ex Vivo HIV Infection. PLoS Pathog. 2016, 12, e1005924. [Google Scholar] [CrossRef] [Green Version]
- Valentine, K.M.; Davini, D.; Lawrence, T.J.; Mullins, G.N.; Manansala, M.; Al-Kuhlani, M.; Pinney, J.M.; Davis, J.K.; Beaudin, A.E.; Sindi, S.S.; et al. CD8 Follicular T Cells Promote B Cell Antibody Class Switch in Autoimmune Disease. J. Immunol. 2018, 201, 31–40. [Google Scholar] [CrossRef]
- Raza, K.; Falciani, F.; Curnow, S.J.; Ross, E.J.; Lee, C.Y.; Akbar, A.N.; Lord, J.M.; Gordon, C.; Buckley, C.D.; Salmon, M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 2005, 7, R784–R795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cope, A.P. T cells in rheumatoid arthritis. Arthritis Res. Ther. 2008, 10 (Suppl. S1), 1–12. [Google Scholar] [CrossRef] [Green Version]
- Alzabin, S.; Williams, R.O. Effector T cells in rheumatoid arthritis: Lessons from animal models. FEBS Lett. 2011, 585, 3649–3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeets, T.J.; Dolhain, R.; Miltenburg, A.M.; de Kuiper, R.; Breedveld, F.C.; Tak, P.P. Poor expression of T cell-derived cytokines and activation and proliferation markers in early rheumatoid synovial tissue. Clin. Immunol. Immunopathol. 1998, 88, 84–90. [Google Scholar] [CrossRef]
- Ueno, H. T follicular helper cells in human autoimmunity. Curr. Opin. Immunol. 2016, 43, 24–31. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef]
- Su, R.; Wang, Y.; Hu, F.; Li, B.; Guo, Q.; Zheng, X.; Liu, Y.; Gao, C.; Li, X.; Wang, C. Altered Distribution of Circulating T Follicular Helper-Like Cell Subsets in Rheumatoid Arthritis Patients. Front. Med. 2021, 8, 1024. [Google Scholar] [CrossRef]
- de Hair, M.J.; Zijlstra, I.A.; Boumans, M.J.; van de Sande, M.G.; Maas, M.; Gerlag, D.M.; Tak, P.P. Hunting for the pathogenesis of rheumatoid arthritis: Core-needle biopsy of inguinal lymph nodes as a new research tool. Ann. Rheum. Dis. 2012, 71, 1911–1912. [Google Scholar] [CrossRef]
- Van Baarsen, L.G.; de Hair, M.J.; Ramwadhdoebe, T.H.; Zijlstra, I.J.; Maas, M.; Gerlag, D.M.; Tak, P.P. The cellular composition of lymph nodes in the earliest phase of inflammatory arthritis. Ann. Rheum. Dis. 2013, 72, 1420–1424. [Google Scholar] [CrossRef]
- Gerlag, D.M.; Raza, K.; van Baarsen, L.G.; Brouwer, E.; Buckley, C.D.; Burmester, G.R.; Gabay, C.; Catrina, A.I.; Cope, A.P.; Cornelis, F.; et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: Report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann. Rheum. Dis. 2012, 71, 638–641. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51 (Suppl. S6), vi5–vi9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Van de Sande, M.G.; Gerlag, D.M.; Lodde, B.M.; van Baarsen, L.G.; Alivernini, S.; Codullo, V.; Felea, I.; Vieira-Sousa, E.; Fearon, U.; Reece, R.; et al. Evaluating antirheumatic treatments using synovial biopsy: A recommendation for standardisation to be used in clinical trials. Ann. Rheum. Dis. 2011, 70, 423–427. [Google Scholar] [CrossRef]
- Tak, P.P.; van der Lubbe, P.A.; Cauli, A.; Daha, M.R.; Smeets, T.J.; Kluin, P.M.; Meinders, A.E.; Yanni, G.; Panayi, G.S.; Breedveld, F.C. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum. 1995, 38, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuzin, I.; Moshkani, S.; Proulx, S.T.; Xing, L.; Skrombolas, D.; Dunn, R.; Sanz, I.; Schwarz, E.M.; Bottaro, A. Expanded CD23(+)/CD21(hi) B cells in inflamed lymph nodes are associated with the onset of inflammatory-erosive arthritis in TNF-transgenic mice and are targets of anti-CD20 therapy. J. Immunol. 2010, 184, 6142–6150. [Google Scholar] [CrossRef] [Green Version]
- Suan, D.; Nguyen, A.; Moran, I.; Bourne, K.; Hermes, J.R.; Arshi, M.; Hampton, H.R.; Tomura, M.; Miwa, Y.; Kelleher, A.D.; et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity 2015, 42, 704–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, L.M.; Yu, D. Follicular helper T-cell memory: Establishing new frontiers during antibody response. Immunol. Cell Biol. 2014, 92, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.N.; Gebhardt, T.; Carbone, F.R.; Heath, W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013, 31, 137–161. [Google Scholar] [CrossRef]
- Fukazawa, Y.; Lum, R.; Okoye, A.A.; Park, H.; Matsuda, K.; Bae, J.Y.; Hagen, S.I.; Shoemaker, R.; Deleage, C.; Lucero, C.; et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 2015, 21, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Zhang, X.; Wagner, U.; Yang, H.; Beckenbaugh, R.; Kurtin, P.; Goronzy, J.; Weyand, C. CD8 T Cells Are Required for the Formation of Ectopic Germinal Centers in Rheumatoid Synovitis. J. Exp. Med. 2002, 195, 1325–1336. [Google Scholar] [CrossRef] [Green Version]
- Thurlings, R.M.; Wijbrandts, C.A.; Mebius, R.E.; Cantaert, T.; Dinant, H.J.; van der Pouw-Kraan, T.C.; Verweij, C.L.; Baeten, D.; Tak, P.P. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum. 2008, 58, 1582–1589. [Google Scholar] [CrossRef]
- Ramos-Amaya, A.; Rodriguez-Bayona, B.; Lopez-Blanco, R.; Andujar, E.; Perez-Alegre, M.; Campos-Caro, A.; Brieva, J.A. Survival of human circulating antigen-induced plasma cells is supported by plasma cell-niche cytokines and T follicular helper lymphocytes. J. Immunol. 2015, 194, 1031–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Wang, F.; Zhou, M.; Chen, L.; Lu, Y. A preliminary study on the characterization of follicular helper T (Tfh) cells in rheumatoid arthritis synovium. Acta Histochem. 2014, 116, 539–543. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Rao, S.; Tsai, L.M.; Lee, S.K.; He, Y.; Sutcliffe, E.L.; Srivastava, M.; Linterman, M.; Zheng, L.; Simpson, N.; et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009, 31, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Ise, W.; Inoue, T.; McLachlan, J.B.; Kometani, K.; Kubo, M.; Okada, T.; Kurosaki, T. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc. Natl. Acad. Sci. USA 2014, 111, 11792–11797. [Google Scholar] [CrossRef] [Green Version]
- Ramwadhdoebe, T.H.; Hahnlein, J.; Maijer, K.I.; van Boven, L.J.; Gerlag, D.M.; Tak, P.P.; van Baarsen, L.G. Lymph node biopsy analysis reveals an altered immunoregulatory balance already during the at-risk phase of autoantibody positive rheumatoid arthritis. Eur. J. Immunol. 2016, 46, 2812–2821. [Google Scholar] [CrossRef]
- Hahnlein, J.S.; Nadafi, R.; de Jong, T.; Ramwadhdoebe, T.H.; Semmelink, J.F.; Maijer, K.I.; Zijlstra, I.A.; Maas, M.; Gerlag, D.M.; Geijtenbeek, T.B.H.; et al. Impaired lymph node stromal cell function during the earliest phases of rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 35. [Google Scholar] [CrossRef] [Green Version]
- Schietinger, A.; Greenberg, P.D. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol. 2014, 35, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Spaan, M.; Kreefft, K.; de Graav, G.N.; Brouwer, W.P.; de Knegt, R.J.; ten Kate, F.J.; Baan, C.C.; Vanwolleghem, T.; Janssen, H.L.; Boonstra, A. CD4+ CXCR5+ T cells in chronic HCV infection produce less IL-21, yet are efficient at supporting B cell responses. J. Hepatol. 2015, 62, 303–310. [Google Scholar] [CrossRef]
- Fairfax, K.C.; Everts, B.; Amiel, E.; Smith, A.M.; Schramm, G.; Haas, H.; Randolph, G.J.; Taylor, J.J.; Pearce, E.J. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J. Immunol. 2015, 194, 2999–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| HC n = 17 | RA-Risk n = 24 | Early RA n = 16 | |
|---|---|---|---|
| Sex, female (%) | 11 (65) | 20 (83) | 9 (56) |
| Age (years) # | 38.0 (15.91) | 48.0 (12.9) | 52.0 (14.1) |
| IgM-RF positive (n (%)) | 0 (0) | 11 (46) | 15 (94) |
| IgM-RF level (kU/L) * | 3.3 (1.0–15.0) | 21.0 (6.3–190.3) | 312.0 (230.0–405.5) |
| ACPA positive (n (%)) | 0 (0) | 15 (63) | 15 (94) |
| ACPA level (kAU/L) * | 4.0 (2.0–9.0) | 45.0 (4.3–176.5) | 388.0 (103.5–1529.5) |
| IgM-RF and ACPA both pos. (n (%)) | 0 (0) | 2 (8) | 14 (88) |
| ESR (mm/h) * | nd | 7.5 (2.0–12.0) | 13.5 (5.0–27.5) |
| CRP (mg/L) * | 0.9 (0.5–2.3) | 1.9 (0.9–3.8) | 7.7 (4.5–13.2) |
| 68 TJC (n) * | 0 (0) | 2.0 (1.0–3.8) | 15.0 (9.8–21.8) |
| 66 SJC (n) * | 0 (0) | 0 (0) | 10.0 (5.0–13.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anang, D.C.; Ramwadhdoebe, T.H.; Hähnlein, J.S.; van Kuijk, B.; Smits, N.; van Lienden, K.P.; Maas, M.; Gerlag, D.M.; Tak, P.P.; de Vries, N.; et al. Increased Frequency of CD4+ Follicular Helper T and CD8+ Follicular T Cells in Human Lymph Node Biopsies during the Earliest Stages of Rheumatoid Arthritis. Cells 2022, 11, 1104. https://doi.org/10.3390/cells11071104
Anang DC, Ramwadhdoebe TH, Hähnlein JS, van Kuijk B, Smits N, van Lienden KP, Maas M, Gerlag DM, Tak PP, de Vries N, et al. Increased Frequency of CD4+ Follicular Helper T and CD8+ Follicular T Cells in Human Lymph Node Biopsies during the Earliest Stages of Rheumatoid Arthritis. Cells. 2022; 11(7):1104. https://doi.org/10.3390/cells11071104
Chicago/Turabian StyleAnang, Dornatien Chuo, Tamara H. Ramwadhdoebe, Janine S. Hähnlein, Bo van Kuijk, Noortje Smits, Krijn P. van Lienden, Mario Maas, Daniëlle M. Gerlag, Paul P. Tak, Niek de Vries, and et al. 2022. "Increased Frequency of CD4+ Follicular Helper T and CD8+ Follicular T Cells in Human Lymph Node Biopsies during the Earliest Stages of Rheumatoid Arthritis" Cells 11, no. 7: 1104. https://doi.org/10.3390/cells11071104
APA StyleAnang, D. C., Ramwadhdoebe, T. H., Hähnlein, J. S., van Kuijk, B., Smits, N., van Lienden, K. P., Maas, M., Gerlag, D. M., Tak, P. P., de Vries, N., & van Baarsen, L. G. M. (2022). Increased Frequency of CD4+ Follicular Helper T and CD8+ Follicular T Cells in Human Lymph Node Biopsies during the Earliest Stages of Rheumatoid Arthritis. Cells, 11(7), 1104. https://doi.org/10.3390/cells11071104







