Microbiota and Pain: Save Your Gut Feeling
Abstract
1. Introduction
2. The Pathway of Pain at a Glance
- TRP (transient receptor potential) family which includes different thermosensitive ion channels (TRPV1, TRPV2, TRPV3, TRPV4, TRPM8 and TRPA1) that are activated by distinct thermal thresholds [3,7]. TRPV1 is also a vanilloid receptor for capsaicin and protons (acid content), whilst TRPM8 and TRPA1 are cold- and menthol-sensitive channels [3].
- Two members of KCNK potassium channel family (KCNK2, also called TREK-1 and KCNK4 or TRAAK), which are expressed in a subset of C-fiber nociceptors. These receptors can be modulated by pressure, temperature and pharmacological stimuli and, at the same time, they can modulate nociceptor excitability [3,8].
- Acid-sensing ion channels (ASICs) which mediate acid or chemical stimulus [4].
- ○
- Aẟ fibers project to lamina I and deeper lamina V;
- ○
- Aβ fibers end at laminae III and IV;
- ○
- C fibers project to laminae I and II.
Pain Processing
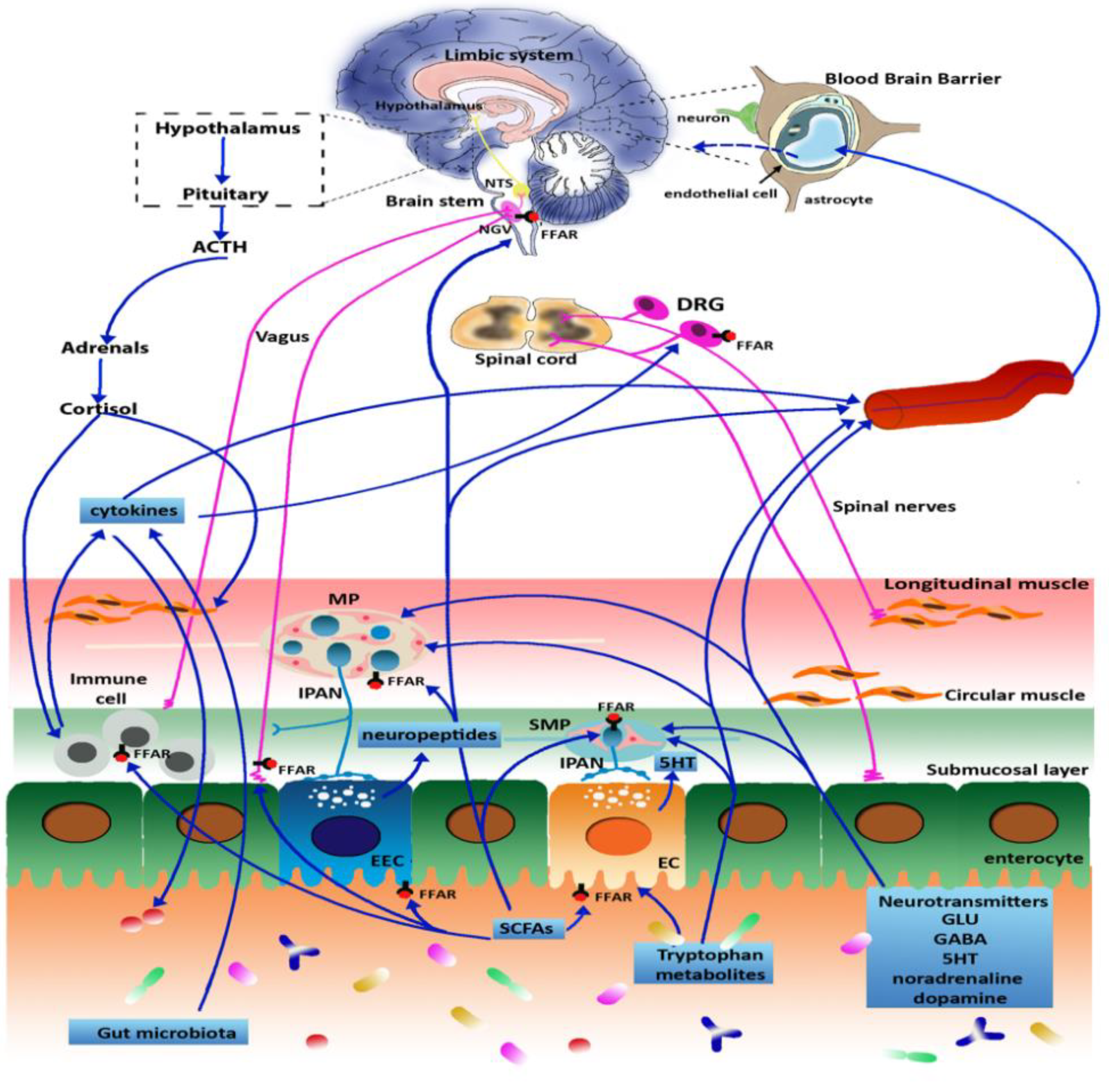
3. Microbiota and Gut-Brain Axis
4. Microbiota and Pain Regulation
4.1. Visceral Pain
4.2. Inflammatory Pain
5. Peripheral and Central Mechanism of Pain Regulation
6. Stress, Microbiota and Pain Amplification
7. Probiotics, Evidence of Efficacy in Pain Disorders
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garland, E.L. Pain Processing in the Human Nervous System: A Selective Review of Nociceptive and Biobehavioral Pathways. Prim. Care Clin. Off. Pract. 2012, 39, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Swieboda, P.; Filip, R.; Prystupa, A.; Drozd, M. Assessment of pain: Types, mechanism and treatment. Ann. Agric. Environ. Med. 2013. [Google Scholar]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, P.; Stillman, A.M. Pathogenesis of Pain. Semin. Pediatr. Neurol. 2016, 23, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Bishop, G.H.; Landau, W.M. Evidence for a Double Peripheral Pathway for Pain. Science 1958, 128, 712–713. [Google Scholar] [CrossRef]
- Leffler, A.; Linte, R.M.; Nau, C.; Reeh, P.; Babes, A. A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. Eur. J. Neurosci. 2007, 26, 12–22. [Google Scholar] [CrossRef]
- Qiu, Y.; Huang, L.; Fu, J.; Han, C.; Fang, J.; Liao, P.; Chen, Z.; Mo, Y.; Sun, P.; Liao, D.; et al. TREK Channel Family Activator with a Well-Defined Structure–Activation Relationship for Pain and Neurogenic Inflammation. J. Med. Chem. 2020, 63, 3665–3677. [Google Scholar] [CrossRef]
- Fenton, B.W.; Shih, E.; Zolton, J. The neurobiology of pain perception in normal and persistent pain. Pain Manag. 2015, 5, 297–317. [Google Scholar] [CrossRef]
- Price, D.D. Central Neural Mechanisms that Interrelate Sensory and Affective Dimensions of Pain. Mol. Interv. 2002, 2, 392–403. [Google Scholar] [CrossRef]
- Benarroch, E.E. Pain-autonomic interactions. Neurol Sci. 2006, 27 (Suppl. 2), S130–S133. [Google Scholar] [CrossRef] [PubMed]
- Taddio, A.; Shah, V.; Gilbert-MacLeod, C.; Katz, J. Conditioning and Hyperalgesia in Newborns Exposed to Repeated Heel Lances. JAMA 2002, 288, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.C.; Giaroni, C.; Baj, A. Impact of Microbial Metabolites on Microbiota–Gut–Brain Axis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef] [PubMed]
- Bistoletti, M.; Bosi, A.; Banfi, D.; Giaroni, C.; Baj, A. The Microbiota-Gut-Brain Axis: Focus on the Fundamental Communication Pathways. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2020; Volume 176, pp. 43–110. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. Microb. Endocrinol. 2014, 817, 39–71. [Google Scholar] [CrossRef]
- Furness, J.B.; Jones, C.; Nurgali, K.; Clerc, N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 2004, 72, 143–164. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Donovan, M.H.; Tecott, L.H. Serotonin and the regulation of mammalian energy balance. Front. Neurosci. 2013, 7, 36. [Google Scholar] [CrossRef]
- Baj, A.; Bistoletti, M.; Bosi, A.; Moro, E.; Giaroni, C.; Crema, F. Marine Toxins and Nociception: Potential Therapeutic Use in the Treatment of Visceral Pain Associated with Gastrointestinal Disorders. Toxins 2019, 11, 449. [Google Scholar] [CrossRef]
- Ye, L.; Liddle, R.A. Gastrointestinal hormones and the gut connectome. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 24, 9–14. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.; Pradhananga, S.; Sessenwein, J.L.; O’Malley, D. Bacterial modulation of visceral sensation: Mediators and mechanisms. Am. J. Physiol. Liver Physiol. 2019, 317, G363–G372. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2014, 113, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; Johnson, A.; Mahony, S.O.; Dinan, T.; Meerveld, B.G.-V.; Cryan, J.F. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2015, 22, 102–117. [Google Scholar] [CrossRef]
- Lista, G.; Meneghin, F.; Bresesti, I.; Castoldi, F.; Cavigioli, F. Functional nutrients in infants born by vaginal delivery or Cesarean section. La Pediatr. Med. e Chir. 2017, 39, 184. [Google Scholar] [CrossRef][Green Version]
- Bresesti, I.; Salvatore, S.; Valetti, G.; Baj, A.; Giaroni, C.; Agosti, M. The Microbiota-Gut Axis in Premature Infants: Physio-Pathological Implications. Cells 2022, 11, 379. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L., IV; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Marsilio, I.; Caputi, V.; Latorre, E.; Cerantola, S.; Paquola, A.; Alcalde, A.I.; Mesonero, J.E.; O’Mahony, S.M.; Bertazzo, A.; Giaroni, C.; et al. Oxidized phospholipids affect small intestine neuromuscular transmission and serotonergic pathways in juvenile mice. Neurogastroenterol. Motil. 2020, 33, e14036. [Google Scholar] [CrossRef]
- Cerantola, S.; Caputi, V.; Marsilio, I.; Ridolfi, M.; Faggin, S.; Bistoletti, M.; Giaroni, C.; Giron, M.C. Involvement of Enteric Glia in Small Intestine Neuromuscular Dysfunction of Toll-Like Receptor 4-Deficient Mice. Cells 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Caputi, V.; Marsilio, I.; Filpa, V.; Cerantola, S.; Orso, G.; Bistoletti, M.; Paccagnella, N.; DE Martin, S.; Montopoli, M.; Dall’Acqua, S.; et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. J. Cereb. Blood Flow Metab. 2017, 174, 3623–3639. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Giron, M.C.; Qesari, M.; Porzionato, A.; Caputi, V.; Zoppellaro, C.; Banzato, S.; Grillo, A.R.; Spagnol, L.; De Caro, R.; et al. Toll-Like Receptor 2 Regulates Intestinal Inflammation by Controlling Integrity of the Enteric Nervous System. Gastroenterology 2013, 145, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Buisman-Pijlman, F.; Hutchinson, M.R. Toll-like receptor 4: Innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front. Neurosci. 2014, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef] [PubMed]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan Res. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Varela, R.B.; Valvassori, S.S.; Lopes-Borges, J.; Mariot, E.; Dal-Pont, G.C.; Amboni, R.T.; Bianchini, G.; Quevedo, J. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J. Psychiatr. Res. 2015, 61, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Brooks, A.K.; Lawson, M.A.; Smith, R.A.; Janda, T.M.; Kelley, K.W.; McCusker, R.H. Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the Kynurenine Pathway in the mouse hippocampus. J. Neuroinflamm. 2016, 13, 98. [Google Scholar] [CrossRef]
- Yeung, A.W.; Terentis, A.C.; King, N.J.; Thomas, S.R. Role of indoleamine 2,3-dioxygenase in health and disease. Clin. Sci. 2015, 129, 601–672. [Google Scholar] [CrossRef]
- Waclawiková, B.; El Aidy, S. Role of Microbiota and Tryptophan Metabolites in the Remote Effect of Intestinal Inflammation on Brain and Depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Rodríguez-Sanoja, R.; Ramos, A.; Demain, A.L. Our microbes not only produce antibiotics, they also overproduce amino acids. J. Antibiot. 2017, 71, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Hashimoto, K.-I.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium glutamicum mechanosensitive channels: Towards unpuzzling “glutamate efflux” for amino acid production. Biophys. Rev. 2018, 10, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Zareian, M.; Ebrahimpour, A.; Bakar, F.A.; Mohamed, A.K.S.; Forghani, B.; Ab-Kadir, M.S.B.; Saari, M. A Glutamic Acid-Producing Lactic Acid Bacteria Isolated from Malaysian Fermented Foods. Int. J. Mol. Sci. 2012, 13, 5482–5497. [Google Scholar] [CrossRef] [PubMed]
- Ger, M.-F.; Rendon, G.; Tilson, J.L.; Jakobsson, E. Domain-Based Identification and Analysis of Glutamate Receptor Ion Channels and Their Relatives in Prokaryotes. PLoS ONE 2010, 5, e12827. [Google Scholar] [CrossRef]
- Genoux, D.; Montgomery, J.M. GLUTAMATE RECEPTOR PLASTICITY AT EXCITATORY SYNAPSES IN THE BRAIN. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1058–1063. [Google Scholar] [CrossRef]
- Giaroni, C.; Zanetti, E.; Giuliani, D.; Oldrini, R.; Marchet, S.; Moro, E.; Borroni, P.; Trinchera, M.; Crema, F.; Lecchini, S.; et al. Protein kinase c modulates NMDA receptors in the myenteric plexus of the guinea pig ileum during in vitro ischemia and reperfusion. Neurogastroenterol. Motil. 2010, 23, e91–e103. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Bai, T.; Xiang, X.; Qian, W.; Song, J.; Hou, X. EphrinB2/ephB2-mediated myenteric synaptic plasticity: Mechanisms underlying the persistent muscle hypercontractility and pain in postinfectious. FASEB J. 2019, 33, 13644–13659. [Google Scholar] [CrossRef]
- Liu, M.-T.; Rothstein, J.D.; Gershon, M.D.; Kirchgessner, A.L. Glutamatergic Enteric Neurons. J. Neurosci. 1997, 17, 4764–4784. [Google Scholar] [CrossRef]
- Neunlist, M.; Michel, K.; Reiche, D.; Dobreva, G.; Huber, K.; Schemann, M. Glycine activates myenteric neurones in adult guinea-pigs. J. Physiol. 2001, 536, 727–739. [Google Scholar] [CrossRef]
- Chen, A.; Chen, Y.; Tang, Y.; Bao, C.; Cui, Z.; Xiao, M.; Lin, C. Hippocampal AMPARs involve the central sensitization of rats with irritable bowel syndrome. Brain Behav. 2017, 7, e00650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lin, W.-J.; Salton, S.R. Role of a VGF/BDNF/TrkB Autoregulatory Feedback Loop in Rapid-Acting Antidepressant Efficacy. J. Mol. Neurosci. 2018, 68, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2010, 23, 255-e119. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Gronier, B.; Savignac, H.M.; Di Miceli, M.; Idriss, S.M.; Tzortzis, G.; Anthony, D.; Burnet, P.W. Increased cortical neuronal responses to NMDA and improved attentional set-shifting performance in rats following prebiotic (B-GOS®) ingestion. Eur. Neuropsychopharmacol. 2017, 28, 211–224. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. Gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pessione, E. The Neuro-endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Janik, R.; Thomason, L.A.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. NeuroImage 2016, 125, 988–995. [Google Scholar] [CrossRef]
- Perez-Berezo, T.; Pujo, J.; Martin, P.; Le Faouder, P.; Galano, J.-M.; Guy, A.; Knauf, C.; Tabet, J.C.; Tronnet, S.; Barreau, F.; et al. Identification of an analgesic lipopeptide produced by the probiotic Escherichia coli strain Nissle 1917. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Bjurstöm, H.; Wang, J.; Ericsson, I.; Bengtsson, M.; Liu, Y.; Mendu, S.K.; Issazadeh-Navikas, S.; Birnir, B. GABA, a natural immunomodulator of T lymphocytes. J. Neuroimmunol. 2008, 205, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Cho, K.-H.; Lee, D.-H.; Kwon, J.; Woo, J.; Kim, J.; Na, H.; Park, S.-H.; Kim, S.; Cho, M.-L. Oral Administration of Lactobacillus rhamnosus Ameliorates the Progression of Osteoarthritis by Inhibiting Joint Pain and Inflammation. Cells 2021, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Ruan, W.; Esparza, M.; Fultz, R.; Shi, Z.; Engevik, K.A.; Engevik, A.C.; Ihekweazu, F.D.; Visuthranukul, C.; Venable, S.; et al. Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites. Physiol. Rep. 2021, 9, e14719. [Google Scholar] [CrossRef] [PubMed]
- Klug, M.; Hill, R.; Choy, K.H.; Kyrios, M.; Hannan, A.; Buuse, M.V.D. Long-term behavioral and NMDA receptor effects of young-adult corticosterone treatment in BDNF heterozygous mice. Neurobiol. Dis. 2012, 46, 722–731. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Hosoi, T.; Okuma, Y.; Nomura, Y. Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R141–R147. [Google Scholar] [CrossRef]
- Alverdy, J.; Holbrook, C.; Rocha, F.; Seiden, L.; Wu, R.L.; Musch, M.; Chang, E.; Ohman, D.; Suh, S. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: Evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 2000, 232, 480–489. [Google Scholar] [CrossRef]
- Rolig, A.S.; Mittge, E.K.; Ganz, J.; Troll, J.V.; Melancon, E.; Wiles, T.J.; Alligood, K.; Stephens, W.Z.; Eisen, J.S.; Guillemin, K. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. 2017, 15, e2000689. [Google Scholar] [CrossRef]
- Friesen, C.; Colombo, J.M.; Deacy, A.; Schurman, J.V. An Update on the Assessment and Management of Pediatric Abdominal Pain. Pediatr. Health Med. Ther. 2021, 12, 373–393. [Google Scholar] [CrossRef]
- Mahony, S.O.; Felice, V.; Nally, K.; Savignac, H.; Claesson, M.; Scully, P.; Woznicki, J.; Hyland, N.; Shanahan, F.; Quigley, E.; et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Decision letter: Microbiota regulates visceral pain in the mouse. Elife 2017. [Google Scholar] [CrossRef]
- Egrenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10 (Suppl. 1), S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Alvarez, P.; Levine, J.D. A role for gut microbiota in early-life stress-induced widespread muscle pain in the adult rat. Mol. Pain 2021, 17. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Piscitelli, F.; De Filippis, F.; Paino, S.; Ricciardi, F.; Siniscalco, D.; Marabese, I.; et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav. Immun. 2019, 85, 128–141. [Google Scholar] [CrossRef]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.-A.; Fernández-Pena, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef]
- Husebye, E.; Hellström, P.M.; Midtvedt, T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig. Dis. Sci. 1994, 39, 946–956. [Google Scholar] [CrossRef]
- Moloney, R.D.; Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Early-life stress-induced visceral hypersensitivity and anxiety behavior is reversed by histone deacetylase inhibition. Neurogastroenterol. Motil. 2015, 27, 1831–1836. [Google Scholar] [CrossRef]
- Hong, S.; Zheng, G.; Wiley, J.W. Epigenetic Regulation of Genes That Modulate Chronic Stress-Induced Visceral Pain in the Peripheral Nervous System. Gastroenterology 2015, 148, 148–157.e7. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Chaloner, A.; Sawalha, A.; Van-Meerveld, B.G. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology 2013, 38, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Hao, H.; Yang, Y.; Huang, S.; Wang, C.; Gigout, S.; Ramli, R.; Li, X.; Jaworska, E.; Edwards, I.; et al. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J. Clin. Investig. 2017, 127, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- McRoberts, J.A.; Coutinho, S.V.; Marvizón, J.C.G.; Grady, E.F.; Tognetto, M.; Sengupta, J.N.; Ennes, H.S.; Chaban, V.V.; Amadesi, S.; Creminon, C.; et al. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology 2001, 120, 1737–1748. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernández, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef]
- De Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The microbiota-gut-brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J. Physiol. 2014, 592, 2989–2997. [Google Scholar] [CrossRef]
- Powell, N.D.; Sloan, E.K.; Bailey, M.T.; Arevalo, J.M.G.; Miller, G.E.; Chen, E.; Kobor, M.S.; Reader, B.F.; Sheridan, J.F.; Cole, S.W. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA 2013, 110, 16574–16579. [Google Scholar] [CrossRef]
- Salvatore, S.; Baldassarre, M.E.; Di Mauro, A.; Laforgia, N.; Tafuri, S.; Bianchi, F.P.; Dattoli, E.; Morando, L.; Pensabene, L.; Meneghin, F.; et al. Neonatal Antibiotics and Prematurity Are Associated with an Increased Risk of Functional Gastrointestinal Disorders in the First Year of Life. J. Pediatr. 2019, 212, 44–51. [Google Scholar] [CrossRef]
- McVey Neufeld, K.A.; Strain, C.R.; Pusceddu, M.M.; Waworuntu, R.V.; Manurung, S.; Gross, G.; Moloney, M.G.; Hoban, A.E.; Murphy, K.; Stanton, C.; et al. Lactobacillus rhamnosus GG soluble mediators ameliorate early life stress-induced visceral hypersensitivity and changes in spinal cord gene expression. Neuronal Signal. 2020, 4, Ns20200007. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Moayyedi, P. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Cuozzo, M.; Castelli, V.; Avagliano, C.; Cimini, A.; D’Angelo, M.; Cristiano, C.; Russo, R. Effects of Chronic Oral Probiotic Treatment in Paclitaxel-Induced Neuropathic Pain. Biomedicines 2021, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; You, Z.; Chen, Q.; Yang, L.; Doheny, J.; Zhou, X.; Li, N.; Wang, S.; Hu, K.; Chen, L.; et al. Gut Microbiota Influences Neuropathic Pain Through Modulating Proinflammatory and Anti-inflammatory T Cells. Anesth. Analg. 2020, 132, 1146–1155. [Google Scholar] [CrossRef]
- Ceuleers, H.; Hanning, N.; Heirbaut, J.; Van Remoortel, S.; Joossens, J.; Van Der Veken, P.; Francque, S.M.; De Bruyn, M.; Lambeir, A.-M.; De Man, J.G.; et al. Newly developed serine protease inhibitors decrease visceral hypersensitivity in a post-inflammatory rat model for irritable bowel syndrome. J. Cereb. Blood Flow Metab. 2018, 175, 3516–3533. [Google Scholar] [CrossRef]
- D’Auria, E.; Salvatore, S.; Pozzi, E.; Mantegazza, C.; Sartorio, M.U.A.; Pensabene, L.; Baldassarre, M.E.; Agosti, M.; Vandenplas, Y.; Zuccotti, G. Cow’s Milk Allergy: Immunomodulation by Dietary Intervention. Nutrients 2019, 11, 1399. [Google Scholar] [CrossRef]
- Zhao, K.; Yu, L.; Wang, X.; He, Y.; Lu, B. Clostridium butyricum regulates visceral hypersensitivity of irritable bowel syndrome by inhibiting colonic mucous low grade inflammation through its action on NLRP6. Acta Biochim. Biophys. Sin. 2018, 50, 216–223. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2018, 34, 1368–1376. [Google Scholar] [CrossRef]
- Benjak Horvat, I.; Gobin, I.; Kresović, A.; Hauser, G. How can probiotic improve irritable bowel syndrome symptoms? World J. Gastrointest. Surg. 2021, 13, 923–940. [Google Scholar] [CrossRef]
- Li, Y.-J.; Dai, C.; Jiang, M. Mechanisms of Probiotic VSL#3 in a Rat Model of Visceral Hypersensitivity Involves the Mast Cell-PAR2-TRPV1 Pathway. Am. J. Dig. Dis. 2018, 64, 1182–1192. [Google Scholar] [CrossRef]
- Kannampalli, P.; Pochiraju, S.; Chichlowski, M.; Berg, B.M.; Rudolph, C.; Bruckert, M.; Miranda, A.; Sengupta, J.N. ProbioticLactobacillus rhamnosusGG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol. Motil. 2014, 26, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Pensabene, L.; Borrelli, O.; Saps, M.; Thapar, N.; Concolino, D.; Staiano, A.; Vandenplas, Y. Mind the gut: Probiotics in paediatric neurogastroenterology. Benef. Microbes 2018, 9, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Miniello, V.; Magistà, A.M.; De Canio, A.; Bucci, N.; Gagliardi, F.; Lionetti, E.; Castellaneta, S.; Polimeno, L.; Peccarisi, L.; et al. A Randomized Controlled Trial of Lactobacillus GG in Children With Functional Abdominal Pain. Pediatrics 2010, 126, e1445–e1452. [Google Scholar] [CrossRef] [PubMed]
- Gawrońska, A.; Dziechciarz, P.; Horvath, A.; Szajewska, H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment. Pharmacol. Ther. 2006, 25, 177–184. [Google Scholar] [CrossRef]
- Guandalini, S.; Magazzù, G.; Chiaro, A.; La Balestra, V.; Di Nardo, G.; Gopalan, S.; Sibal, A.; Romano, C.; Canani, R.B.; Lionetti, P.; et al. VSL#3 Improves Symptoms in Children With Irritable Bowel Syndrome: A Multicenter, Randomized, Placebo-Controlled, Double-Blind, Crossover Study. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 24–30. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Theodorou, V.; Tompkins, T. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity Through Hypothalamic-Pituitary-Adrenal Axis Modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef]
- Ma, D.; Forsythe, P.; Bienenstock, J. Live Lactobacillus rhamnosus [corrected] is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect. Immun. 2004, 72, 5308–5314. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Indrio, F.; Bolio-Galvis, A.; Jiménez-Gutiérrez, C.; Jimenez-Escobar, I.; López-Velázquez, G. Efficacy of Lactobacillus reuteri DSM 17938 for infantile colic: Systematic review with network meta-analysis. Medicine (Baltimore) 2017, 96, e9375. [Google Scholar] [CrossRef]
- Trivić, I.; Niseteo, T.; Jadrešin, O.; Hojsak, I. Use of probiotics in the treatment of functional abdominal pain in children—systematic review and meta-analysis. Eur. J. Pediatr. 2020, 180, 339–351. [Google Scholar] [CrossRef]
- Su, G.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef]
- Bungau, S.G.; Behl, T.; Singh, A.; Sehgal, A.; Singh, S.; Chigurupati, S.; Vijayabalan, S.; Das, S.; Palanimuthu, V.R. Targeting Probiotics in Rheumatoid Arthritis. Nutrients 2021, 13, 3376. [Google Scholar] [CrossRef] [PubMed]
| Gut Microorganism | Molecules, Metabolites and Neurotransmitters Involved | Effects on Gut-Brain Axis |
|---|---|---|
| Bifidobacterium longum NCC3001 | ↑ BDNF | ↓ Anxiety and depressive behavior [30,58] ↑ Neuronal plasticity of ENS [58] |
| Bifidobacterium dentium | ↑ GABA | ↓ Visceral hypersensitivity [59] |
| Lacticaseibacillus rhamnosus JB-1 | ↑ GABA | ↓ Anxiety and depressive behavior [59] ↓ Intestinal damage and inflammation [64] |
| Escherichia coli Nissle 1917 | ↑ C12AsnGABAOH | ↓ Visceral hypersensitivity [61] ↑ Epithelial permeability of GABA [61] |
| Limosilactobacillus reuteri ATTC-PTA 6475 | ↑ Oxytocin | ↓ Restores social deficits of ASD [24] Promotes DC maturation and immune modulation via IL-10 [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morreale, C.; Bresesti, I.; Bosi, A.; Baj, A.; Giaroni, C.; Agosti, M.; Salvatore, S. Microbiota and Pain: Save Your Gut Feeling. Cells 2022, 11, 971. https://doi.org/10.3390/cells11060971
Morreale C, Bresesti I, Bosi A, Baj A, Giaroni C, Agosti M, Salvatore S. Microbiota and Pain: Save Your Gut Feeling. Cells. 2022; 11(6):971. https://doi.org/10.3390/cells11060971
Chicago/Turabian StyleMorreale, Chiara, Ilia Bresesti, Annalisa Bosi, Andreina Baj, Cristina Giaroni, Massimo Agosti, and Silvia Salvatore. 2022. "Microbiota and Pain: Save Your Gut Feeling" Cells 11, no. 6: 971. https://doi.org/10.3390/cells11060971
APA StyleMorreale, C., Bresesti, I., Bosi, A., Baj, A., Giaroni, C., Agosti, M., & Salvatore, S. (2022). Microbiota and Pain: Save Your Gut Feeling. Cells, 11(6), 971. https://doi.org/10.3390/cells11060971









