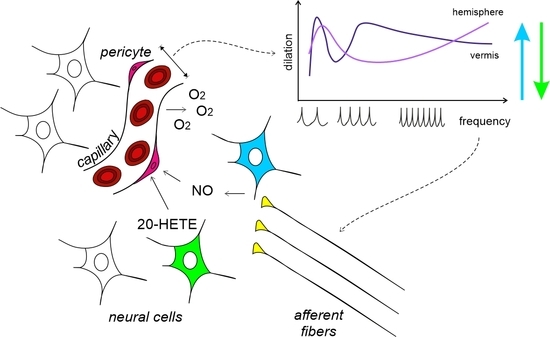
Non-Linear Frequency Dependence of Neurovascular Coupling in the Cerebellar Cortex Implies Vasodilation–Vasoconstriction Competition
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Acute Cerebellar Slices
2.2. Immunofluorescence Staining
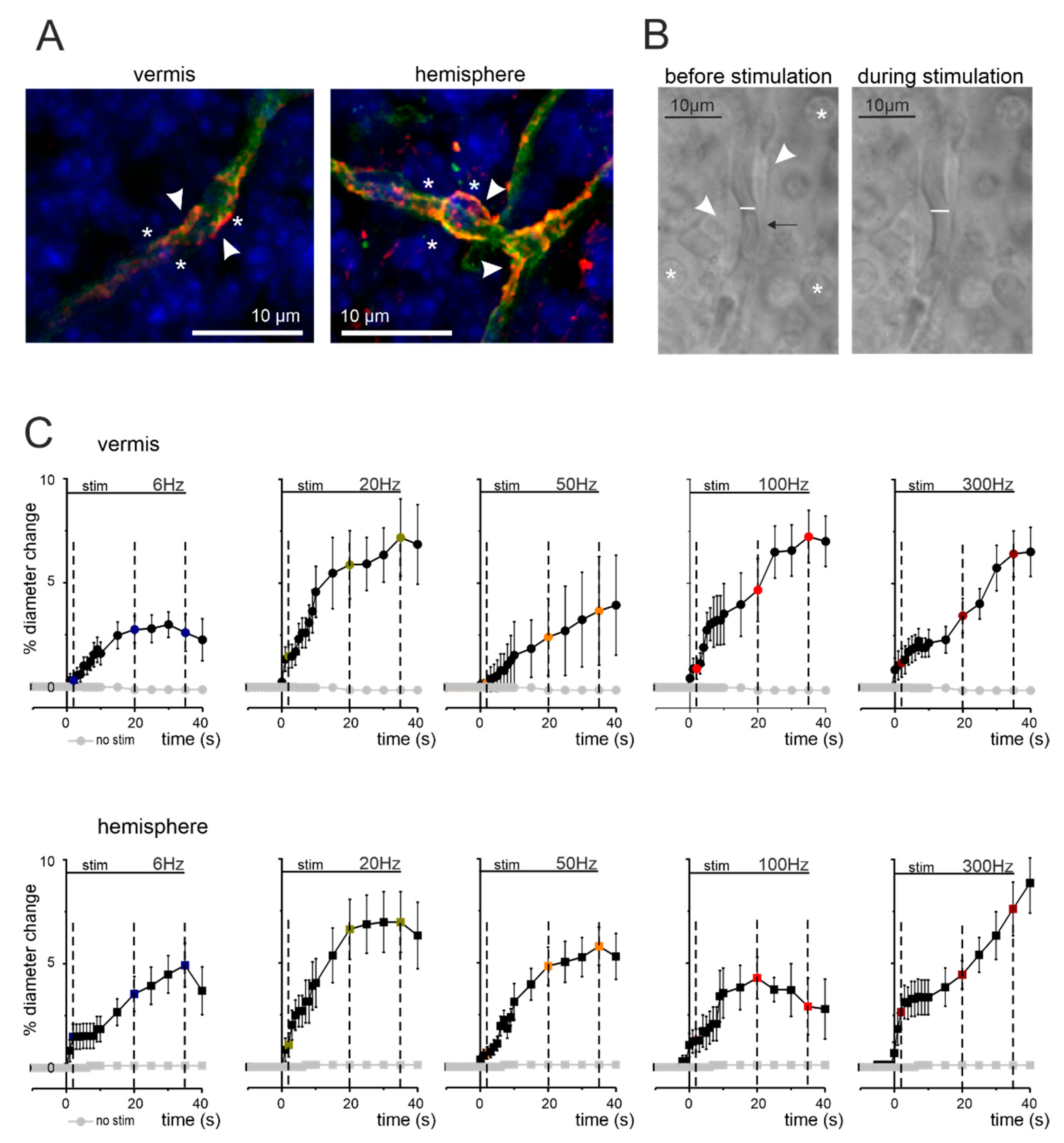
2.3. Time-Lapse Acquisition and Analysis of Capillary Diameter Changes
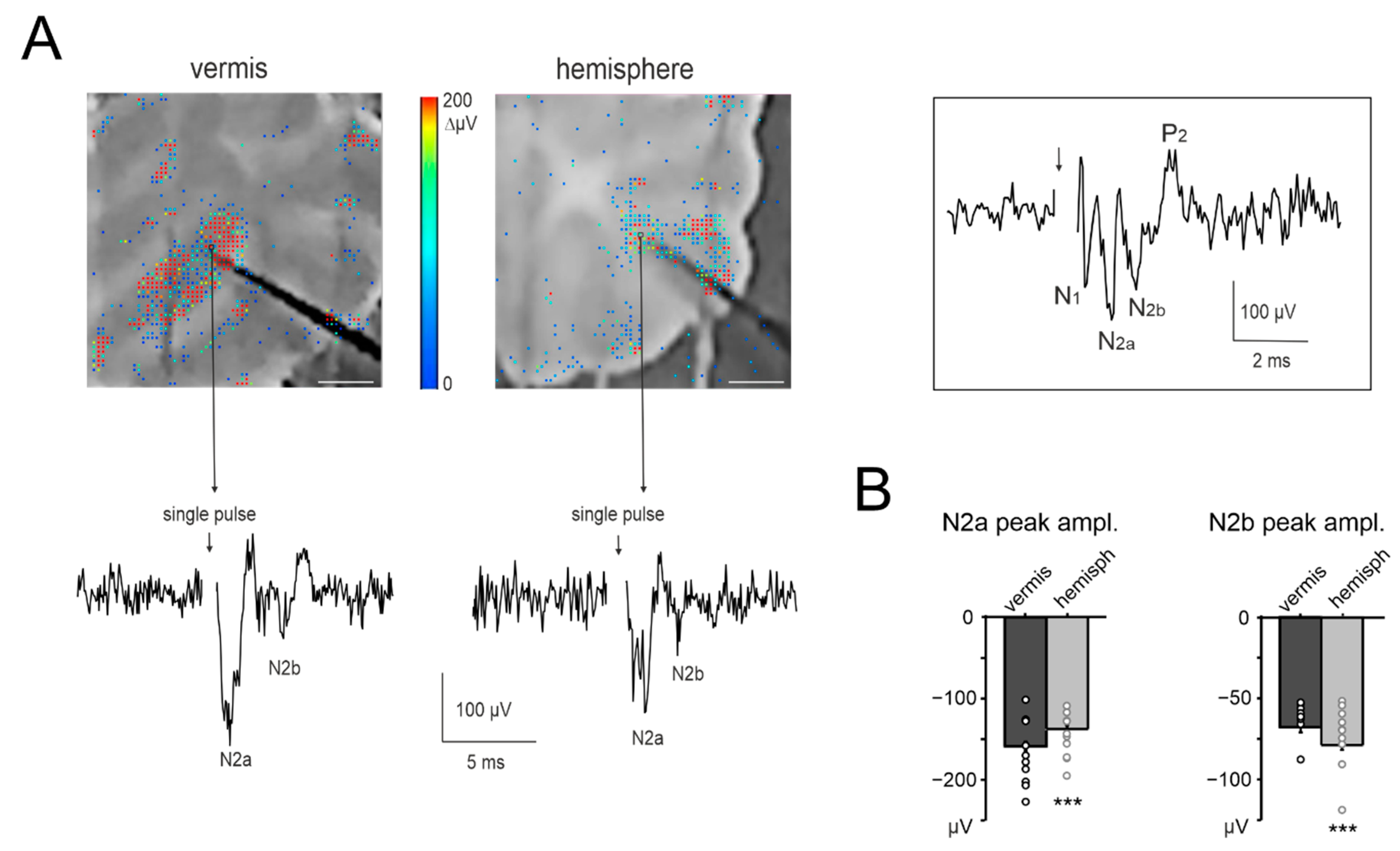
2.4. Electrophysiological Recordings of Neuronal Activity in Cerebellar Slices
2.5. Computational Model of the Granular Layer
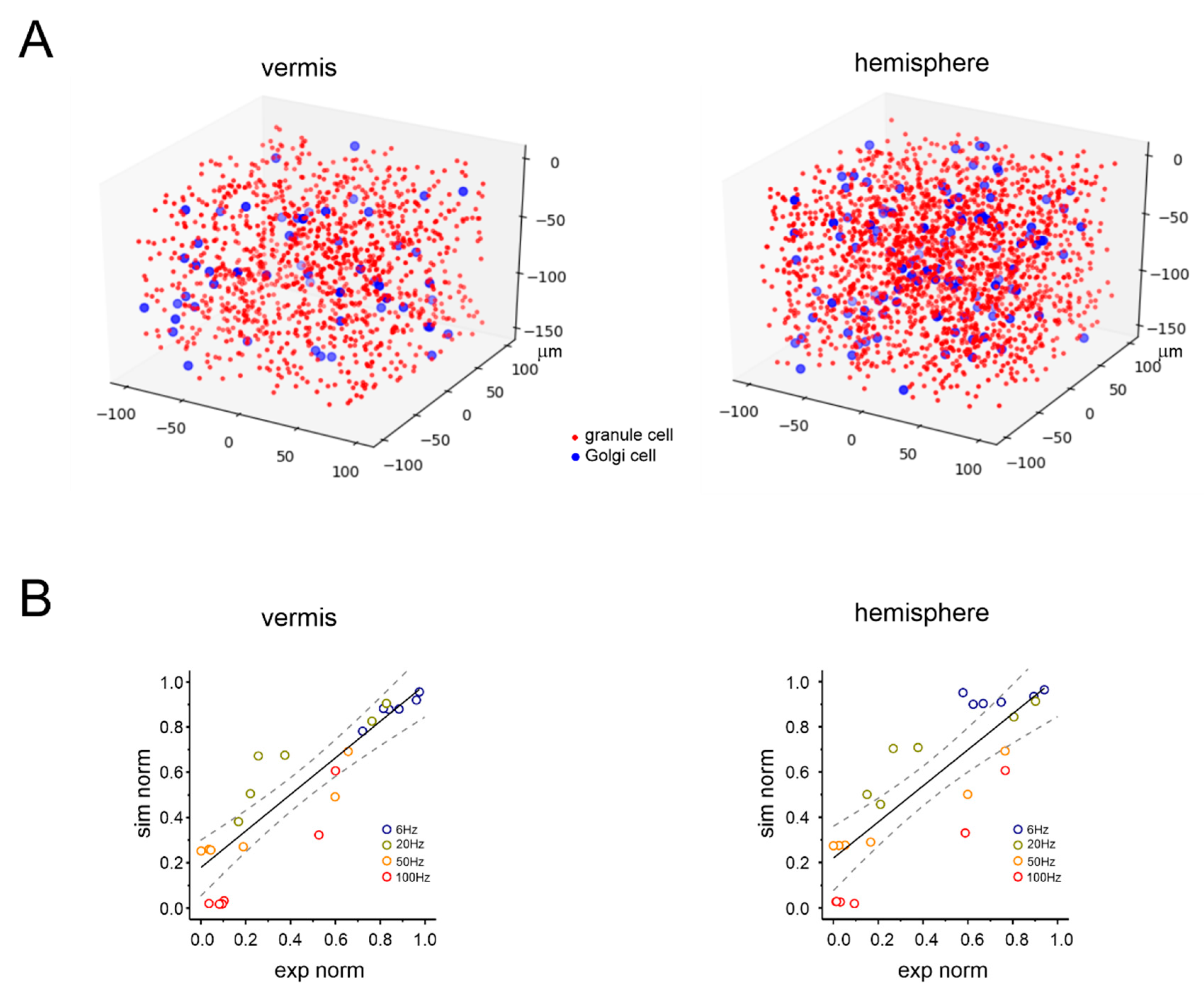
2.6. Model Validation
2.7. Data Analysis
3. Results
3.1. Anatomical Organization of Neurovascular Components in the Granular Layer of Vermis and Hemisphere
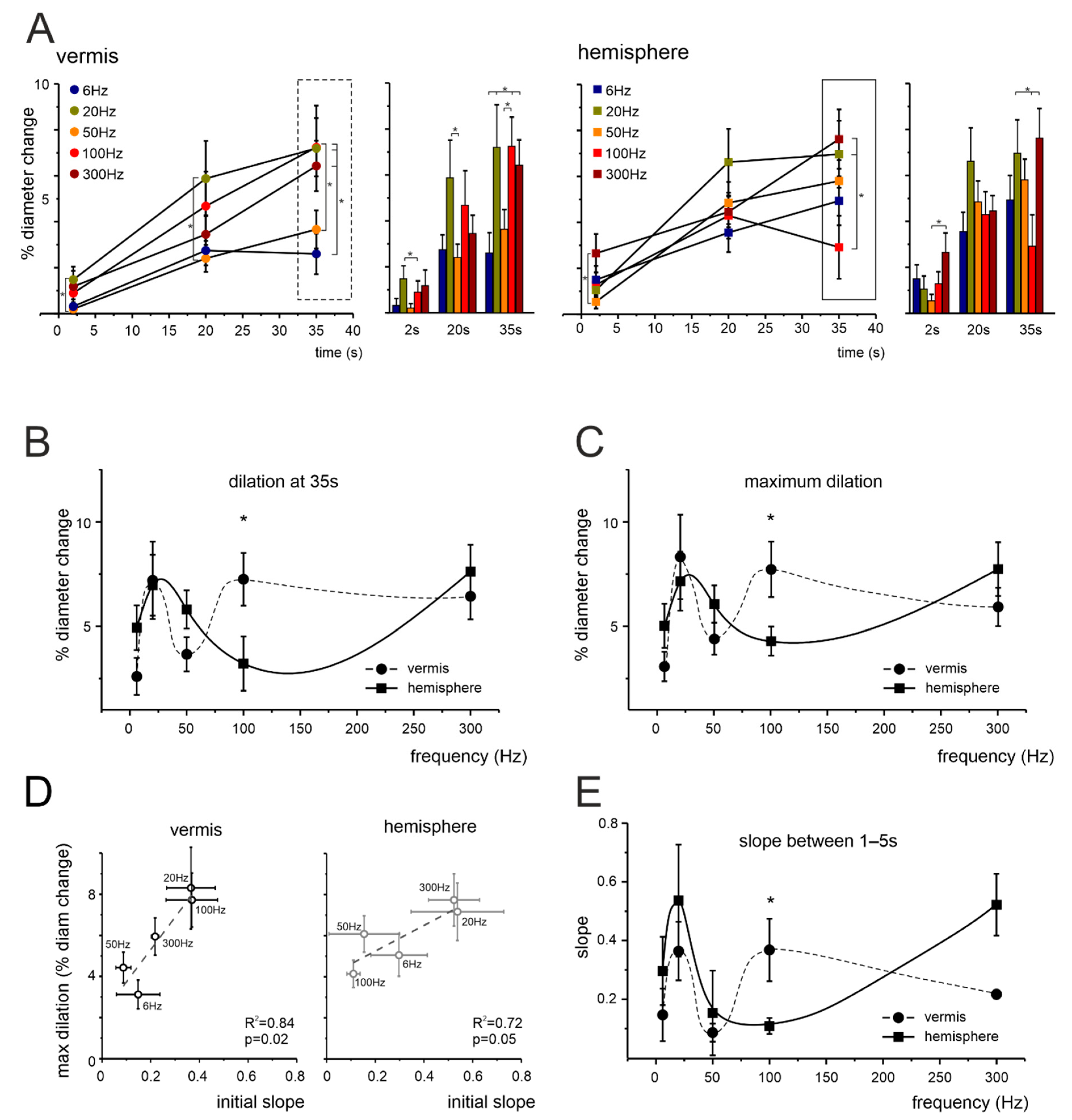
3.2. Non-Linear Frequency-Dependent Dilation of Granular Layer Capillaries (NVC)
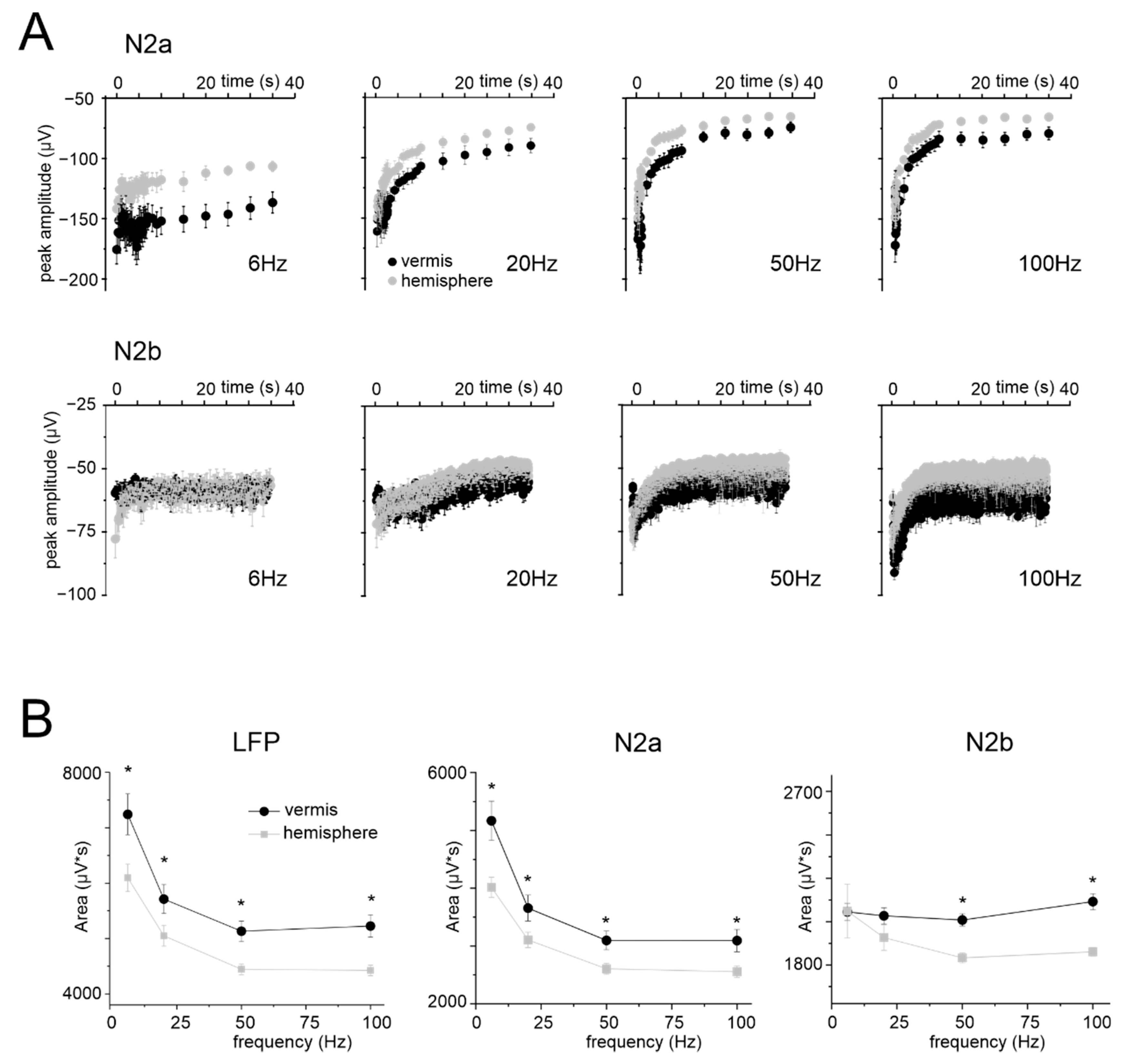
3.3. Granular Layer Responses to Mossy Fibers Stimulation (NA)
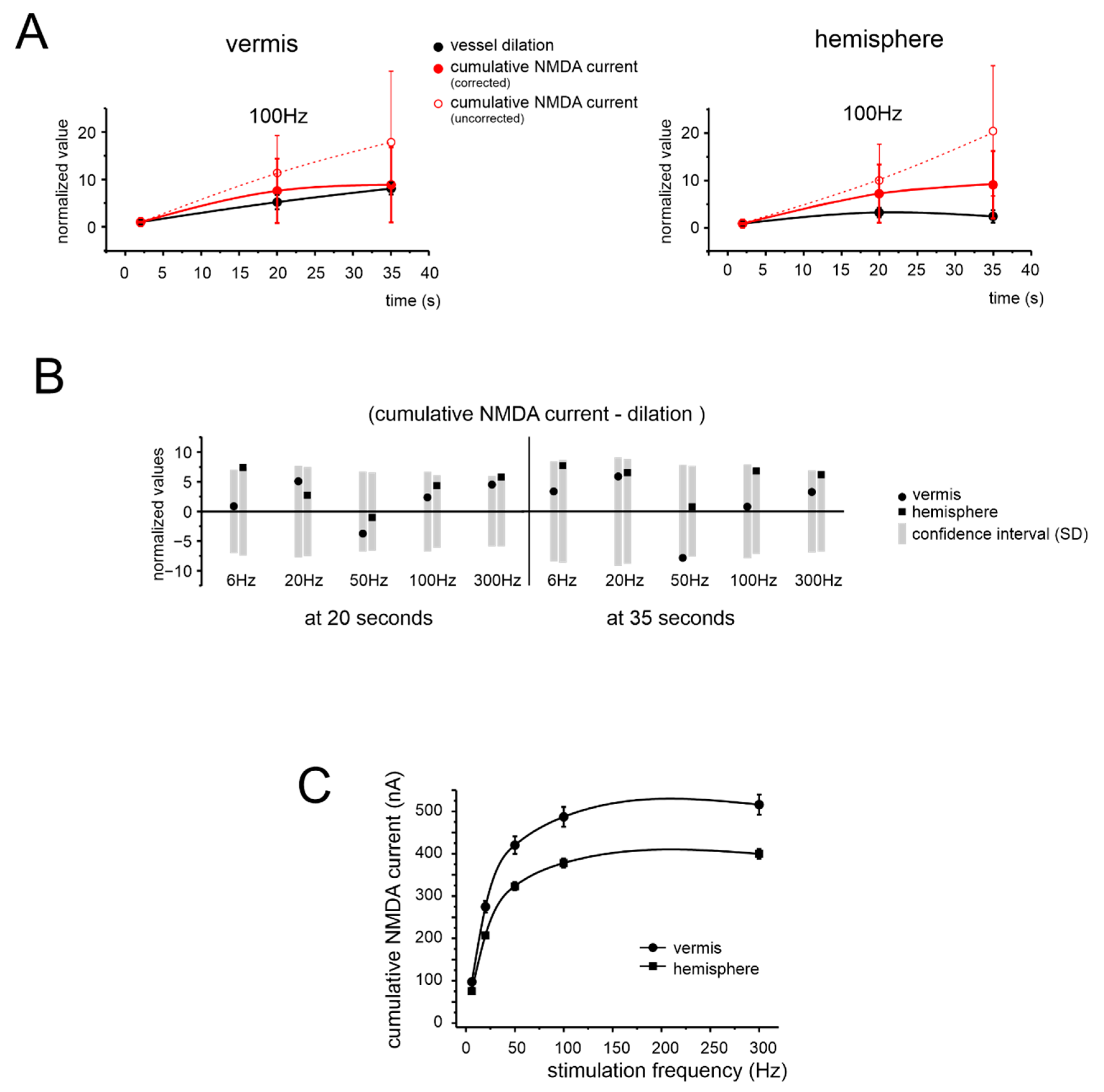
3.4. Simulated NMDA Currents Correlate with NVC Time-Course
4. Discussion
4.1. Non-Linearity and Region Specificity of Cerebellar NVC
4.2. NA and NVC in the Cerebellar Vermis and Hemisphere
4.3. NMDAR Currents Drive the Time Course of Dilation but Do Not Determine Its Frequency Dependence
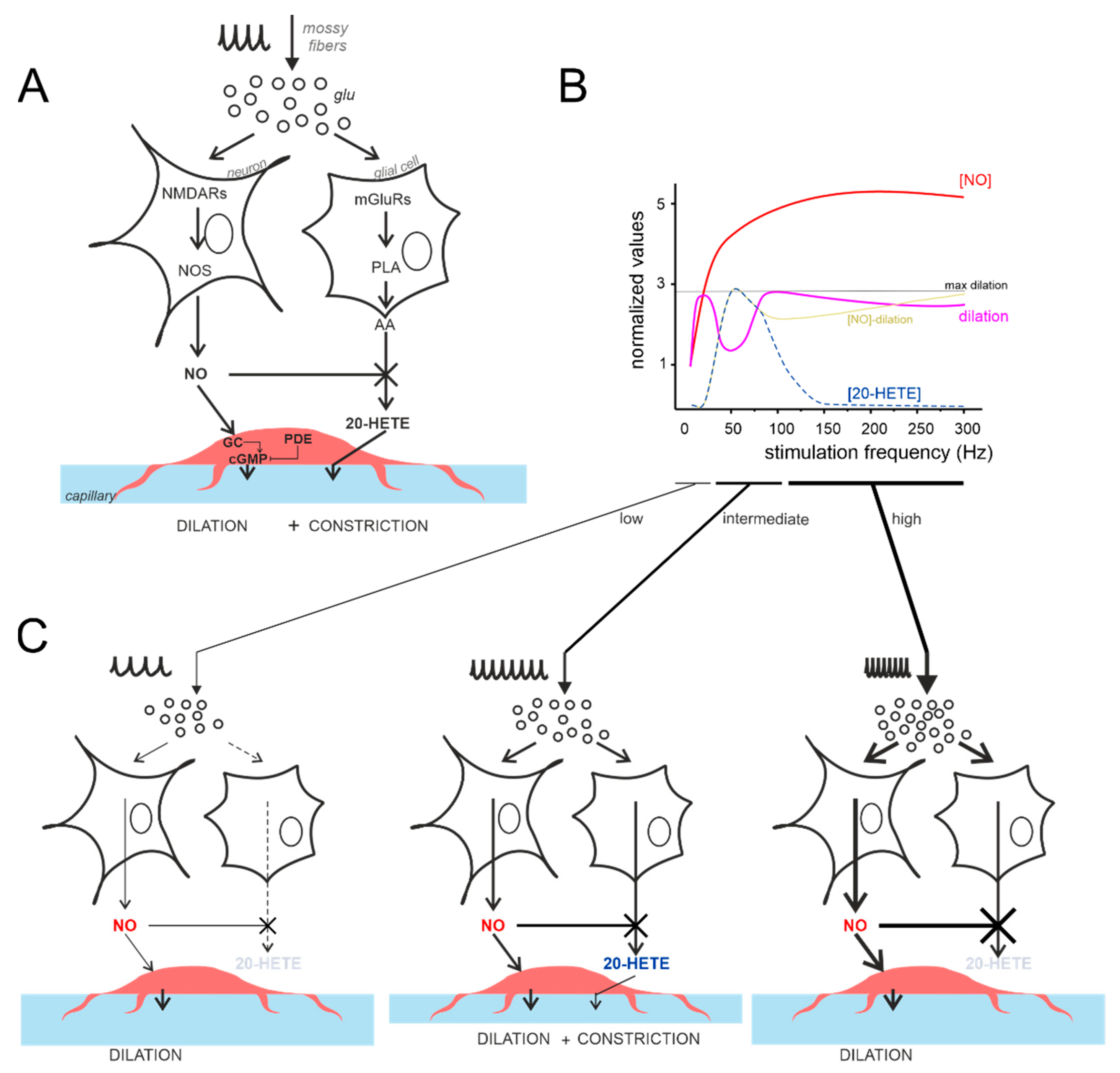
4.4. The Vasodilation–Vasoconstriction Competition Hypothesis
4.5. Comparing Different NVC Hypotheses and the Case for Cerebellar Region Specificity
4.6. Considerations on the Development of Cerebellar NVC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Hillman, E.M. Coupling mechanism and significance of the BOLD signal: A status report. Annu. Rev. Neurosci. 2014, 37, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Howarth, C.; Kurth-Nelson, Z.; Mishra, A. Interpreting BOLD: Towards a dialogue between cognitive and cellular neuroscience. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2016, 371, 20150348. [Google Scholar] [CrossRef] [PubMed]
- Lindauer, U.; Leithner, C.; Kaasch, H.; Rohrer, B.; Foddis, M.; Füchtemeier, M.; Offenhauser, N.; Steinbrink, J.; Royl, G.; Kohl-Bareis, M.; et al. Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Devor, A.; Hillman, E.M.; Tian, P.; Waeber, C.; Teng, I.C.; Ruvinskaya, L.; Shalinsky, M.H.; Zhu, H.; Haslinger, R.H.; Narayanan, S.N.; et al. Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 14347–14357. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.A. Cortical specificity in neurovascular coupling. J. Neurophysiol. 2015, 114, 3031–3032. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Bonev, A.D.; Straub, S.V.; Meredith, A.L.; Wilkerson, M.K.; Aldrich, R.W.; Nelson, M.T. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006, 9, 1397–1403. [Google Scholar] [CrossRef]
- Nippert, A.R.; Biesecker, K.R.; Newman, E.A. Mechanisms mediating functional hyperemia in the brain. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2018, 24, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Blanco, V.M.; Stern, J.E.; Filosa, J.A. Tone-dependent vascular responses to astrocyte-derived signals. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2855–H2863. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, A.; Anderson, G.K.; Sprick, J.D. Cerebrovascular control: What’s so base-ic about it? J. Physiol. 2021, 599, 2787–2788. [Google Scholar] [CrossRef] [PubMed]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Hosford, P.S.; Gourine, A.V. What is the key mediator of the neurovascular coupling response? Neurosci. Biobehav. Rev. 2019, 96, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Preller, K.H.; Mathys, C.; Cagnan, H.; Heinzle, J.; Razi, A.; Zeidman, P. Dynamic causal modelling revisited. Neuroimage 2019, 199, 730–744. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E. The organization of plasticity in the cerebellar cortex: From synapses to control. Prog. Brain Res. 2014, 210, 31–58. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, G.; Higgins, D.; Spolidoro, M.; Carrel, D.; Mathieu, B.; Léna, C.; Dieudonné, S.; Barbour, B.; Brunel, N.; Casado, M. Burst-dependent bidirectional plasticity in the cerebellum is driven by presynaptic NMDA receptors. Cell Rep. 2016, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.; Peppiatt-Wildman, C.M.; Attwell, D. The energy use associated with neural computation in the cerebellum. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 403–414. [Google Scholar] [CrossRef]
- Howarth, C.; Gleeson, P.; Attwell, D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2012, 32, 1222–1232. [Google Scholar] [CrossRef]
- Diedrichsen, J.; Verstynen, T.; Schlerf, J.; Wiestler, T. Advances in functional imaging of the human cerebellum. Curr. Opin. Neurol. 2010, 23, 382–387. [Google Scholar] [CrossRef]
- Mapelli, L.; Gagliano, G.; Soda, T.; Laforenza, U.; Moccia, F.; D’Angelo, E.U. Granular layer neurons control cerebellar neurovascular coupling through an NMDA receptor/no-dependent system. J. Neurosci. 2017, 37, 1340–1351. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.B.; Attwell, D.; Hall, C.N. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front. Neuroenergetics 2010, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.A.; Pardini, M.; Samson, R.S.; D’Angelo, E.; Friston, K.J.; Toosy, A.T.; Gandini Wheeler-Kingshott, C.A. Differential involvement of cortical and cerebellar areas using dominant and nondominant hands: An FMRI study. Hum. Brain Mapp. 2015, 36, 5079–5100. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.A.; Samson, R.S.; Gasston, D.; Pardini, M.; Friston, K.J.; D’Angelo, E.; Toosy, A.T.; Wheeler-Kingshott, C.A. Complex motor task associated with non-linear BOLD responses in cerebro-cortical areas and cerebellum. Brain Struct. Funct. 2016, 221, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.A.; Pardini, M.; Samson, R.S.; Friston, K.J.; Toosy, A.T.; D’Angelo, E.; Gandini Wheeler-Kingshott, C.A. Cerebellar lobules and dentate nuclei mirror cortical force-related-BOLD responses: Beyond all (linear) expectations. Hum. Brain Mapp. 2017, 38, 2566–2579. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.A.; Pardini, M.; Samson, R.S.; D’Angelo, E.; Friston, K.J.; Toosy, A.T.; Wheeler-Kingshott, C.A.G. Blood oxygenation level-dependent response to multiple grip forces in multiple sclerosis: Going beyond the main effect of movement in brodmann area 4a and 4p. Front. Cell. Neurosci. 2021, 15, 616028. [Google Scholar] [CrossRef] [PubMed]
- Iordanova, B.; Vazquez, A.L.; Poplawsky, A.J.; Fukuda, M.; Kim, S.G. Neural and hemodynamic responses to optogenetic and sensory stimulation in the rat somatosensory cortex. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2015, 35, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, H.W.; Enager, P.; Thomsen, K.J.; Lauritzen, M.J. Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007, 27, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, C.; Caesar, K.; Akgören, N.; Lauritzen, M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J. Physiol. 1998, 512 Pt 2, 555–566. [Google Scholar] [CrossRef]
- Mathiesen, C.; Caesar, K.; Lauritzen, M. Temporal coupling between neuronal activity and blood flow in rat cerebellar cortex as indicated by field potential analysis. J. Physiol. 2000, 523 Pt 1, 235–246. [Google Scholar] [CrossRef]
- Nemoto, M.; Sheth, S.; Guiou, M.; Pouratian, N.; Chen, J.W.; Toga, A.W. Functional signal- and paradigm-dependent linear relationships between synaptic activity and hemodynamic responses in rat somatosensory cortex. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 3850–3861. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Nemoto, M.; Guiou, M.; Walker, M.; Pouratian, N.; Toga, A.W. Evaluation of coupling between optical intrinsic signals and neuronal activity in rat somatosensory cortex. Neuroimage 2003, 19, 884–894. [Google Scholar] [CrossRef]
- Sheth, S.A.; Nemoto, M.; Guiou, M.; Walker, M.; Pouratian, N.; Toga, A.W. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron 2004, 42, 347–355. [Google Scholar] [CrossRef]
- Devonshire, I.M.; Papadakis, N.G.; Port, M.; Berwick, J.; Kennerley, A.J.; Mayhew, J.E.; Overton, P.G. Neurovascular coupling is brain region-dependent. Neuroimage 2012, 59, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Hewson-Stoate, N.; Jones, M.; Martindale, J.; Berwick, J.; Mayhew, J. Further nonlinearities in neurovascular coupling in rodent barrel cortex. Neuroimage 2005, 24, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.S.; Yu, L.; Murari, K.; Kiss, Z.H. Neurovascular coupling during deep brain stimulation. Brain Stimul. 2020, 13, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Akgören, N.; Dalgaard, P.; Lauritzen, M. Cerebral blood flow increases evoked by electrical stimulation of rat cerebellar cortex: Relation to excitatory synaptic activity and nitric oxide synthesis. Brain Res. 1996, 710, 204–214. [Google Scholar] [CrossRef]
- D’Angelo, E.; De Filippi, G.; Rossi, P.; Taglietti, V. Synaptic excitation of individual rat cerebellar granule cells in situ: Evidence for the role of NMDA receptors. J. Physiol. 1995, 484 Pt 2, 397–413. [Google Scholar] [CrossRef]
- Armano, S.; Rossi, P.; Taglietti, V.; D’Angelo, E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J. Neurosci. 2000, 20, 5208–5216. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, L.; Rossi, P.; Nieus, T.; D’Angelo, E. Tonic activation of GABAB receptors reduces release probability at inhibitory connections in the cerebellar glomerulus. J. Neurophysiol. 2009, 101, 3089–3099. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; O’farrell, F.M.; Reynell, C.; Hamilton, N.B.; Hall, C.N.; Attwell, D. Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nat. Protoc. 2014, 9, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Peppiatt, C.M.; Howarth, C.; Mobbs, P.; Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006, 443, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, J.; D’Angelo, E. The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J. Neurosci. 2007, 27, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Casali, S.; Tognolina, M.; Gandolfi, D.; Mapelli, J.; D’Angelo, E. Cellular-resolution mapping uncovers spatial adaptive filtering at the rat cerebellum input stage. Commun. Biol. 2020, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, R.; Geminiani, A.; Masoli, S.; Rizza, M.F.; Antonietti, A.; Casellato, C.; D’Angelo, E. Scaffold modelling captures the structure-function-dynamics relationship in brain microcircuits. boRxiv 2021. [Google Scholar] [CrossRef]
- Solinas, S.; Nieus, T.; D’Angelo, E. A realistic large-scale model of the cerebellum granular layer predicts circuit spatio-temporal filtering properties. Front. Cell. Neurosci. 2010, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Dalkara, T.; Alarcon-Martinez, L. Cerebral microvascular pericytes and neurogliovascular signaling in health and disease. Brain Res. 2015, 1623, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Maffei, A.; Prestori, F.; Rossi, P.; Taglietti, V.; D’Angelo, E. Presynaptic current changes at the mossy fiber-granule cell synapse of cerebellum during LTP. J. Neurophysiol. 2002, 88, 627–638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diwakar, S.; Lombardo, P.; Solinas, S.; Naldi, G.; D’Angelo, E. Local field potential modeling predicts dense activation in cerebellar granule cells clusters under LTP and LTD control. PLoS ONE 2011, 6, e21928. [Google Scholar] [CrossRef] [PubMed]
- Nieus, T.; Sola, E.; Mapelli, J.; Saftenku, E.; Rossi, P.; D’angelo, E. LTP regulates burst initiation and frequency at mossy fiber-granule cell synapses of rat cerebellum: Experimental observations and theoretical predictions. J. Neurophysiol. 2006, 95, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, A.M.; Bartus, K.; Reynell, C.; Constantinou, S.; Halvey, E.J.; Held, K.F.; Dostmann, W.R.; Vernon, J.; Garthwaite, J. Exquisite sensitivity to subsecond, picomolar nitric oxide transients conferred on cells by guanylyl cyclase-coupled receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 22060–22065. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Ishikawa, T.; Tsunoda, Y.; Lee, J.; Hoffman, D.S.; Kakei, S. Information processing in the hemisphere of the cerebellar cortex for control of wrist movement. J. Neurophysiol. 2016, 115, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Rancz, E.A.; Ishikawa, T.; Duguid, I.; Chadderton, P.; Mahon, S.; Häusser, M. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature 2007, 450, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Delvendahl, I.; Hallermann, S. The cerebellar mossy fiber synapse as a model for high-frequency transmission in the mammalian CNS. Trends Neurosci. 2016, 39, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Van Beugen, B.J.; Gao, Z.; Boele, H.J.; Hoebeek, F.; De Zeeuw, C.I. High frequency burst firing of granule cells ensures transmission at the parallel fiber to purkinje cell synapse at the cost of temporal coding. Front. Neural Circuits 2013, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, M.I.; DeStefano, J.G.; Linville, R.M.; Wong, A.D.; Searson, P.C. Cerebrovascular plasticity: Processes that lead to changes in the architecture of brain microvessels. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2019, 39, 1413–1432. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.R.; Poser, B.A.; Kaas, A.L.; Fear, E.J.; Dresbach, S.; Berwick, J.; Goebel, R.; Turner, R.; Kennerley, A.J. Validating layer-specific VASO across species. Neuroimage 2021, 237, 118195. [Google Scholar] [CrossRef]
- Benito, E.; Valor, L.M.; Jimenez-Minchan, M.; Huber, W.; Barco, A. cAMP response element-binding protein is a primary hub of activity-driven neuronal gene expression. J. Neurosci. 2011, 31, 18237–18250. [Google Scholar] [CrossRef] [PubMed]
- Huo, B.X.; Smith, J.B.; Drew, P.J. Neurovascular coupling and decoupling in the cortex during voluntary locomotion. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 10975–10981. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Stoica, B.A.; Faden, A.I. Metabotropic glutamate receptor-mediated signaling in neuroglia. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 136–150. [Google Scholar] [CrossRef] [PubMed]
- D’Antoni, S.; Berretta, A.; Bonaccorso, C.M.; Bruno, V.; Aronica, E.; Nicoletti, F.; Catania, M.V. Metabotropic glutamate receptors in glial cells. Neurochem. Res. 2008, 33, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.J.; Challiss, R.A. G protein-coupled receptor signalling in astrocytes in health and disease: A focus on metabotropic glutamate receptors. Biochem. Pharmacol. 2012, 84, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.S.; Schell, M.J.; Atkinson, P.J.; Johnston, N.R.; Nahorski, S.R.; Challiss, R.J. Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J. Biol. Chem. 2002, 277, 35947–35960. [Google Scholar] [CrossRef] [PubMed]
- MacVicar, B.; Newman, E. Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Ge, Y.; Lv, W.; Elliott, M.R.; Muroya, Y.; Hirata, T.; Booz, G.W.; Roman, R.J. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front. Biosci. 2016, 21, 1427. [Google Scholar] [CrossRef]
- D’Angelo, E.; Gandini Wheeler-Kingshott, C. Modelling the brain: Elementary components to explain ensemble functions. La Riv. Del Nuovo Cim. 2017, 40, 297–333. [Google Scholar] [CrossRef]
- Stackhouse, T.; Mishra, A. Neurovascular coupling in development and disease: Focus on astrocytes. Front. Cell Dev. Biol. 2021, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Kozberg, M.G.; Chen, B.R.; DeLeo, S.E.; Bouchard, M.B.; Hillman, E.M. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc. Natl. Acad. Sci. USA 2013, 110, 4380–4385. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Rossi, P.; Taglietti, V. Different proportions of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptor currents at the mossy fibre-granule cell synapse of developing rat cerebellum. Neuroscience 1993, 53, 121–130. [Google Scholar] [CrossRef]
- D’Angelo, E.; Rossi, P.; De Filippi, G.; Magistretti, J.; Taglietti, V. The relationship between synaptogenesis and expression of voltage-dependent currents in cerebellar granule cells in situ. J. Physiol.-Paris 1994, 88, 197–207. [Google Scholar] [CrossRef]
- Rossi, P.; D’Angelo, E.; Magistretti, J.; Toselli, M.; Taglietti, V. Age-dependent expression of high-voltage activated calcium currents during cerebellar granule cell development in situ. Pflug. Arch. Eur. J. Physiol. 1994, 429, 107–116. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Rossi, P.; Armano, S.; Taglietti, V. Evidence for NMDA and mGlu receptor-dependent long-term potentiation of mossy fiber-granule cell transmission in rat cerebellum. J. Neurophysiol. 1999, 81, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Sola, E.; Prestori, F.; Rossi, P.; Taglietti, V.; D’Angelo, E. Increased neurotransmitter release during long-term potentiation at mossy fibre-granule cell synapses in rat cerebellum. J. Physiol. 2004, 557, 843–861. [Google Scholar] [CrossRef] [PubMed]
- Chan-Palay, V.; Palay, S. The form of velate astrocytes in the cerebellar cortex of monkey and rat: High voltage electron microscopy of rapid Golgi preparations. Z. Anat. Entwickl. 1972, 138, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Buffo, A.; Rossi, F. Origin, lineage and function of cerebellar glia. Prog. Neurobiol. 2013, 109, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, O.A.; Fuentealba, L.C.; Alvarez-Buylla, A.; Rowitch, D.H. Astrocyte development and heterogeneity. Cold Spring Harb. Perspect. Biol. 2014, 7, a020362. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, V.; Parmigiani, E.; Figueres-Oñate, M.; Betizeau, M.; Aprato, J.; Nanavaty, I.; Berchialla, P.; Luzzati, F.; de’Sperati, C.; Lopez-Mascaraque, L.; et al. Multiple origins and modularity in the spatiotemporal emergence of cerebellar astrocyte heterogeneity. PLoS Biol. 2018, 16, e2005513. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliano, G.; Monteverdi, A.; Casali, S.; Laforenza, U.; Gandini Wheeler-Kingshott, C.A.M.; D’Angelo, E.; Mapelli, L. Non-Linear Frequency Dependence of Neurovascular Coupling in the Cerebellar Cortex Implies Vasodilation–Vasoconstriction Competition. Cells 2022, 11, 1047. https://doi.org/10.3390/cells11061047
Gagliano G, Monteverdi A, Casali S, Laforenza U, Gandini Wheeler-Kingshott CAM, D’Angelo E, Mapelli L. Non-Linear Frequency Dependence of Neurovascular Coupling in the Cerebellar Cortex Implies Vasodilation–Vasoconstriction Competition. Cells. 2022; 11(6):1047. https://doi.org/10.3390/cells11061047
Chicago/Turabian StyleGagliano, Giuseppe, Anita Monteverdi, Stefano Casali, Umberto Laforenza, Claudia A. M. Gandini Wheeler-Kingshott, Egidio D’Angelo, and Lisa Mapelli. 2022. "Non-Linear Frequency Dependence of Neurovascular Coupling in the Cerebellar Cortex Implies Vasodilation–Vasoconstriction Competition" Cells 11, no. 6: 1047. https://doi.org/10.3390/cells11061047
APA StyleGagliano, G., Monteverdi, A., Casali, S., Laforenza, U., Gandini Wheeler-Kingshott, C. A. M., D’Angelo, E., & Mapelli, L. (2022). Non-Linear Frequency Dependence of Neurovascular Coupling in the Cerebellar Cortex Implies Vasodilation–Vasoconstriction Competition. Cells, 11(6), 1047. https://doi.org/10.3390/cells11061047