Self- and Cross-Pollination in Argane Tree and their Implications on Breeding Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Site Study
2.2. Breeding System and Pollination Methods
2.3. In Vitro Germination of Pollen
2.4. Flowering Observations and Fruit Maturity Cycle
2.5. Fruit Set
2.6. Statistical Analysis
3. Results
3.1. In-Vitro Germination of Pollen
3.2. Blooming Periods, Floral Morphology and Fruit Maturity Cycle
3.3. Cross/Self-(in)Compatibility Study and Pollination Strategies to Improve Fruit Set in Argane Orchards
3.3.1. Cross-Compatibility
3.3.2. Self-Incompatibility
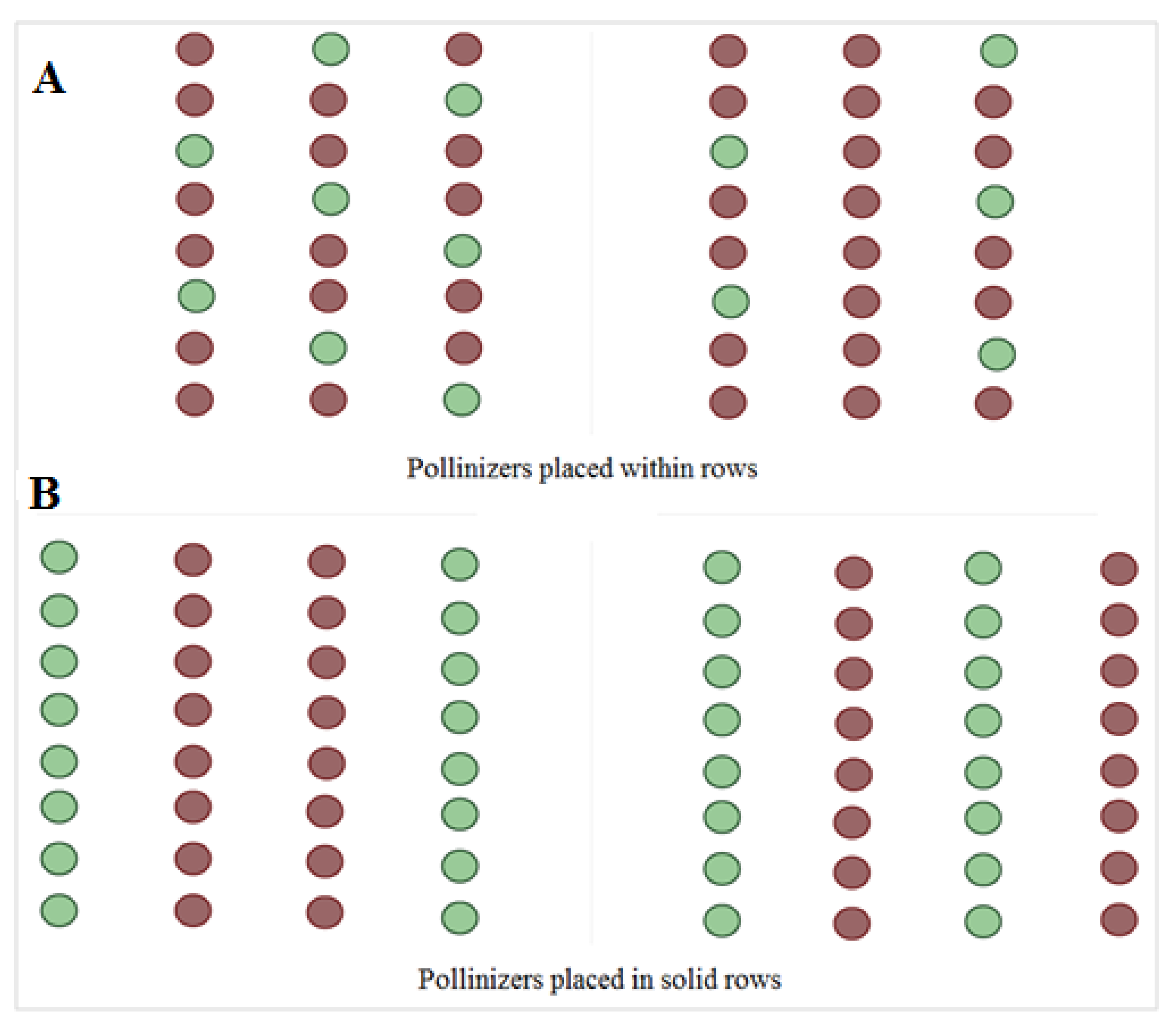
3.3.3. Pollination Strategies to Improve Fruit Set in Orchards of Argane
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benzyane, M. Estimation de la Biomasse et Etudes de la Croissance de L’arganier (ArganiaspinosaL.Skeels) dans le Plateau de Haha (Essaouira); Mémoire 3th Cycle; Institut Agronomique et Vétérinaire Hassan II: Rabat, Morocco, 1989; 224p. [Google Scholar]
- Bani-Aameur, F. Argania spinosa(L.) Skeels flowering phenology. Genet. Resour. Crop Evol. 2002, 49, 11–19. [Google Scholar] [CrossRef]
- AitAabd, N.; Msanda, F.; El Mousadik, A. Univariate and Multivariate Analysis of Agronomical Traits of Preselected Argan Trees. Not. Bot. HortiAgrobot. Cluj-Napoca 2012, 40, 308–316. [Google Scholar] [CrossRef] [Green Version]
- AitAabd, N.; Bouharroud, R.; Tahiri, A.; Wifaya, A.; Mimouni, A.; El Mousadik, A. Genetic Diversity, Conservation and Breeding of Argan Tree (Argania spinosa L. Skeels). In Advances in Plant Breeding Strategies: Nut and Beverage Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 31–56. [Google Scholar] [CrossRef]
- Mouhaddab, J.; Msanda, F.; Filali-Maltouf, A.; Belkadi, B.; Ferradouss, A.; El Modafar, C.; IbnSouda-Koraichi, S.; El Mousadik, A. Using microsatellite markers to map genetic diversity and population structure of an endangered Moroccan endemic tree (Argania spinosa L. Skeels) and development of a core collection. Plant Genet. 2017, 10, 51–59. [Google Scholar] [CrossRef]
- Pakhrou, O.; Medraoui, L.; Yatrib, C.; Alami, M.; Ibn Souda-Kouraichi, S.; Ferradous, A.; Msanda, F.; Belkadi, B.; El Modafar, C.; Filali-Maltouf, A.; et al. Study of genetic diversity and differentiation of argan tree population (Argania spinosa L.) using AFLP markers. Aust. J. Crop Sci. 2016, 10, 990–999. [Google Scholar] [CrossRef]
- Darwin, C. The Effects of Cross and Self-Fertilization in the Vegetable Kingdom; John Murray: London, UK, 1876. [Google Scholar]
- Benlahbil, S.; Bani-Aameur, F. At what phenological phase is the stigma of argan (Argania spinosa (L) Skeels) flower receptive to pollen adhesion and germination? For. Genet. 2003, 9, 257–262. [Google Scholar]
- Lush, W.M.; Opat, A.S.; Nie, I.; Clarke, A.E. An in vitro assay for assessing the effect of growth factors on Nicotianaalata pollen tube. Sex. PlantReprod. 1997, 10, 351–357. [Google Scholar] [CrossRef]
- Cerović, R.; Ružić, Đ. Pollen tube growth in sour cherry (Prunuscerasus L.) at different temperatures. J. Hortic. Sci. 1992, 67, 333–340. [Google Scholar] [CrossRef]
- Herrero, M.; Hormaza, J. Pistil strategies controlling pollen tube growth. Sex. Plant Reprod. 1996, 9, 343–347. [Google Scholar] [CrossRef]
- Selak, G.V.; Cuevas, J.; Pinillos, V.; Ban, S.G.; Perica, S. Pollen tube performance in assessment of compatibility in olive (Oleaeuropaea L.) cultivars. Sci. Hortic. 2014, 165, 36–43. [Google Scholar] [CrossRef]
- Radunić, M.; Jazbec, A.; Ercisli, S.; Čmelik, Z.; Ban, S.G. Pollen-pistil interaction influence on the fruit set of sweet cherry Mira. Sci. Hortic. 2017, 224, 358–366. [Google Scholar] [CrossRef]
- Matsumoto, D. Gametophytic Self-Incompatability. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2017; Volume 2, pp. 275–280. [Google Scholar]
- Ramíreza, F.; Davenport, T.L. Mango (Mangifera indica L.) pollination: A review. Sci. Hortic. 2016, 203, 158–168. [Google Scholar] [CrossRef]
- Chapman, L.A.; Goring, D.R. Pollen–pistil interactions regulating successful fertilization in the Brassicaceae. J. Exp. Bot. 2010, 61, 1987–1999. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Srivastav, M.; Rymbai, H.; Chaudhary, R.; Singh, A.; Dubey, A.; Lal, K. Pollen–pistil interaction studies in mango (Mangiferaindica L.) cultivars. Sci. Hort. 2013, 160, 213–221. [Google Scholar] [CrossRef]
- Devi, C.P.; Munshi, A.D.; Behera, T.K.; Choudhary, H.; Gurung, B.V.; Saha, P. Cross compatibility in interspecific hybridization of eggplant, Solanum melongena, with its wild relatives. Sci. Hortic. 2015, 193, 353–358. [Google Scholar] [CrossRef]
- Péreza, V.M.; Herrerob, J.I.; Hormaza, N. Self-fertility and preferential cross-fertilization in mango (Mangifera indica). Sci. Hortic. 2016, 26, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Kuroki, K.; Takemura, Y.; Mingfeng, J.; Marumori, H.; Teratani, N.; Matsumotob, K.; Matsumoto, T.; Tamura, F. Pear pollen selection using higher germination properties at low temperatures and the effect on the fruit set and quality of Japanese pear cultivars. Sci. Hortic. 2017, 216, 200–204. [Google Scholar] [CrossRef]
- Nerd, A.; Irijimovich, V.; Mizrahi, Y. Phenology, breeding system and fruit development of Argan (Argania spinosa, Sapotaceae) cultivated in Israel. Econ. Bot. 1998, 52, 161–167. [Google Scholar] [CrossRef]
- Ajerrar, A.; Akroud, H.; AitAabd, N.; Qessaoui, R.; Amarraque, A.; Lahmyd, H.; Zaafrani, M.; Chebli, B.; Mayad, H.; Bouharroud, R. Pollination system and effective pollinators of Arganiaspinosa (L. Skeels). J. Saudi Soc. Agric. Sci. 2020, 19, 375–382. [Google Scholar] [CrossRef]
- Ramirez, N.; Brito, Y. Reproductive biology of a tropical plant swamp community in the Venezuelan Lla- nos. Am. J. Bot. 1990, 77, 1260–1271. [Google Scholar] [CrossRef]
- Dickinson, G.; Lee, D.J.; Wallace, H.M. Influence of pre- and post-zygotic barriers on hybridisation between Corymbia sections. Ann. Bot. 2012, 109, 1215–1226. [Google Scholar] [CrossRef] [Green Version]
- Gehrke-Vélez, M.; Castillo-Vera, A.; Ruiz-Bello, C.; Moreno-Martinez, J.L.; Moreno-Basurto, G. Delayed self-incompatibility causes morphological alterations and crop reduction in ‘Ataúlfo’ mango (Mangiferaindica L.). N. Zeal. J. Crop Hortic. 2012, 40, 215–227. [Google Scholar] [CrossRef] [Green Version]
- De Nettancourt, D. Incompatibility in Angiosperms; Springer Science & Business Media: New York, NY, USA, 2013; Volume 3. [Google Scholar]
- Gibbs, P.E. Late-acting self-incompatibility-the pariah breeding system in flowering plants. New Phytol. 2014, 203, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, M.; Zhao, L. Dissecting stylar responses to self-pollination in wild tomato selfcompatible and self-incompatible species using comparative proteomics. Plant Physiol. Biochem. 2016, 106, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Miranda, A.S.; Lima, H.A. Agaristarevoluta (Ericaceae): A generalist plant with self-compatible and self-incompatible individuals. Flora 2017, 234, 7–14. [Google Scholar] [CrossRef]



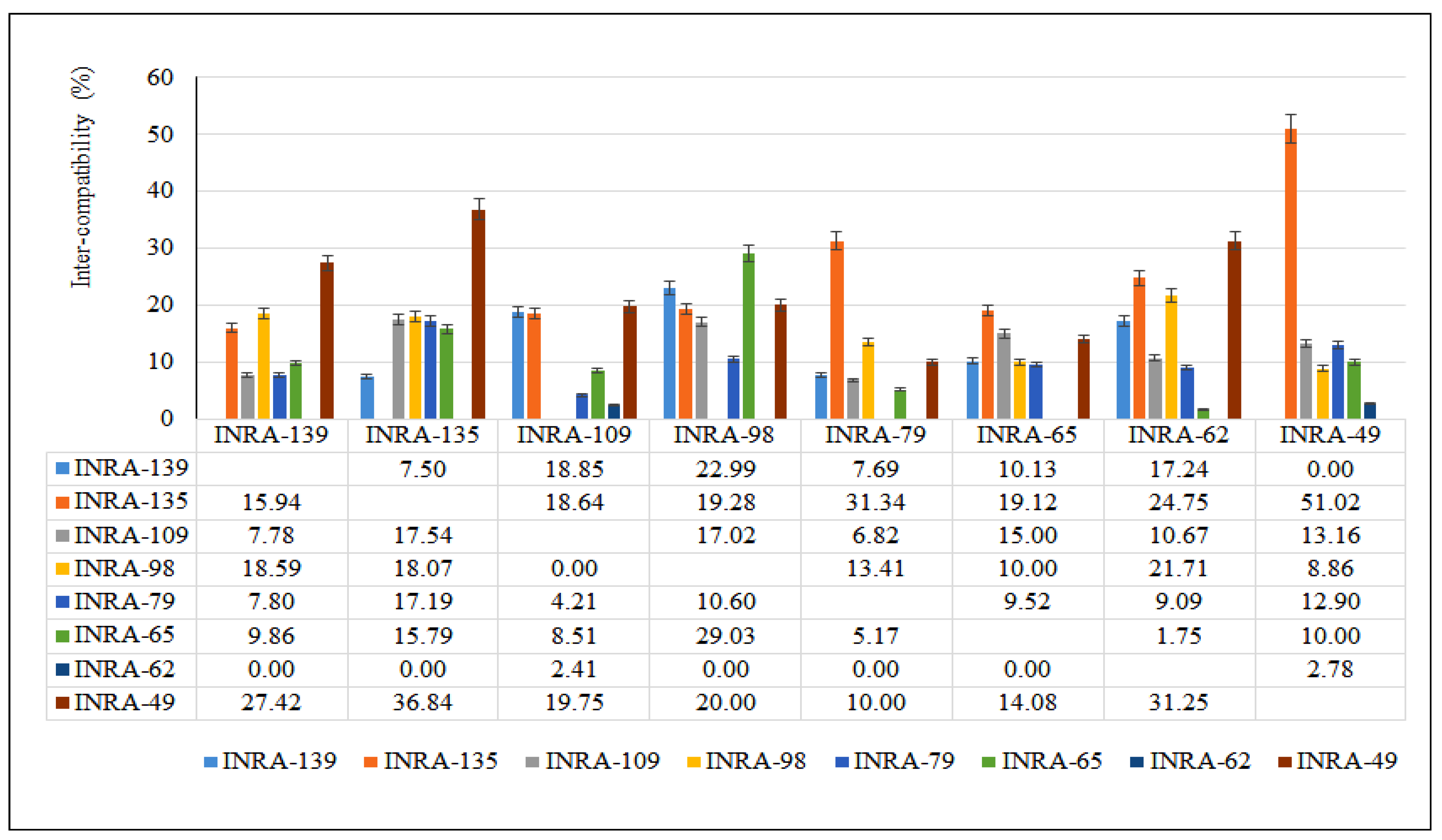



| INRA-139 | INRA-135 | INRA-109 | INRA-98 | INRA-79 | INRA-65 | INRA-62 | INRA-49 | ||
|---|---|---|---|---|---|---|---|---|---|
| ♀♂ | |||||||||
| INRA-139 | √ | √ | √ | √ | √ | √ | √ | ||
| INRA-135 | √ | √ | √ | √ | √ | √ | √ | ||
| INRA-109 | √ | √ | √ | √ | √ | √ | √ | ||
| INRA-98 | √ | √ | √ | √ | √ | √ | √ | ||
| INRA-79 | √ | √ | √ | √ | √ | √ | √ | ||
| INRA-65 | √ | √ | √ | √ | √ | √ | √ | ||
| INRA-62 | √ | √ | √ | √ | √ | √ | √ | ||
| INRA-49 | √ | √ | √ | √ | √ | √ | √ | ||
| INRA-132 | INRA-94 | INRA-67 | INRA-54 | INRA-39 | INRA-32 | ||
|---|---|---|---|---|---|---|---|
| ♀♂ | |||||||
| INRA-132 | √ | √ | √ | √ | √ | ||
| INRA-94 | √ | √ | √ | √ | √ | ||
| INRA-67 | √ | √ | √ | √ | √ | ||
| INRA-54 | √ | √ | √ | √ | √ | ||
| INRA-39 | √ | √ | √ | √ | √ | ||
| INRA-32 | √ | √ | √ | √ | √ | ||
| Genotypes | Fruit Maturity Cycle | Fruit Shape | Floral Morphs | Number Of Flowering Periods/Year |
|---|---|---|---|---|
| INRA-139 | 12 months | Round | Mst | 1 time/year (March–April) |
| INRA-135 | 9 months | Round | Bst | 2 times/year (March–April) and (June–July) |
| INRA-132 | 12 months | Drop | Bst | 2 times/year (March–April) and (June–July) |
| INRA-109 | 12 months | Oval | Bst | 1 time/year (March–April) |
| INRA-98 | 12 months | Oval | Bst | 2 times/year (March–April) and (June–July) |
| INRA-94 | 12 months | Oval | Mst | 1 time/year (March–April) |
| INRA-79 | 12 months | Oval | Mst | 1 time/year (March–April) |
| INRA-67 | 12 months | Oval | Lst | 1 time/year (March–April) |
| INRA-65 | 15 months | Oval | Mst | 1 time/year (March–April) |
| INRA-62 | 12 months | Oval | Mst | 1 time/year (March–April) |
| INRA-54 | 12 months | Oval | Bst | 2 times/year (March–April) and (June–July) |
| INRA-49 | 12 months | Oval | Mst | 1 time/year (March–April) |
| INRA-39 | 12 months | Oval | Mst | 1 time/year (March–April) |
| INRA-32 | 12 months | Oval | Mst | 1 time/year (March–April) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Aabd, N.; Tahiri, A.; Qessaoui, R.; Mimouni, A.; Bouharroud, R. Self- and Cross-Pollination in Argane Tree and their Implications on Breeding Programs. Cells 2022, 11, 828. https://doi.org/10.3390/cells11050828
Ait Aabd N, Tahiri A, Qessaoui R, Mimouni A, Bouharroud R. Self- and Cross-Pollination in Argane Tree and their Implications on Breeding Programs. Cells. 2022; 11(5):828. https://doi.org/10.3390/cells11050828
Chicago/Turabian StyleAit Aabd, Naima, Abdelghani Tahiri, Redouan Qessaoui, Abdelaziz Mimouni, and Rachid Bouharroud. 2022. "Self- and Cross-Pollination in Argane Tree and their Implications on Breeding Programs" Cells 11, no. 5: 828. https://doi.org/10.3390/cells11050828
APA StyleAit Aabd, N., Tahiri, A., Qessaoui, R., Mimouni, A., & Bouharroud, R. (2022). Self- and Cross-Pollination in Argane Tree and their Implications on Breeding Programs. Cells, 11(5), 828. https://doi.org/10.3390/cells11050828






