MRCK-Alpha and Its Effector Myosin II Regulatory Light Chain Bind ABCB4 and Regulate Its Membrane Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Yeast Two-Hybrid Screen
2.3. DNA Constructs
2.4. Reverse Transcription Quantitative PCR (RT-qPCR)
2.5. CRISPR Cas9 Experiments
2.6. Cell Culture, Transfection and Immunofluorescence
2.7. Coimmunoprecipitation and Western Blotting
2.8. siRNA Knockdown
2.9. Cell Surface Staining
2.10. Measurement of PC Secretion
2.11. Statistical Analysis
3. Results
3.1. MRCKα Binds the N-Terminal Domain of ABCB4
3.2. MRCKα Silencing Increases ABCB4 Protein Expression
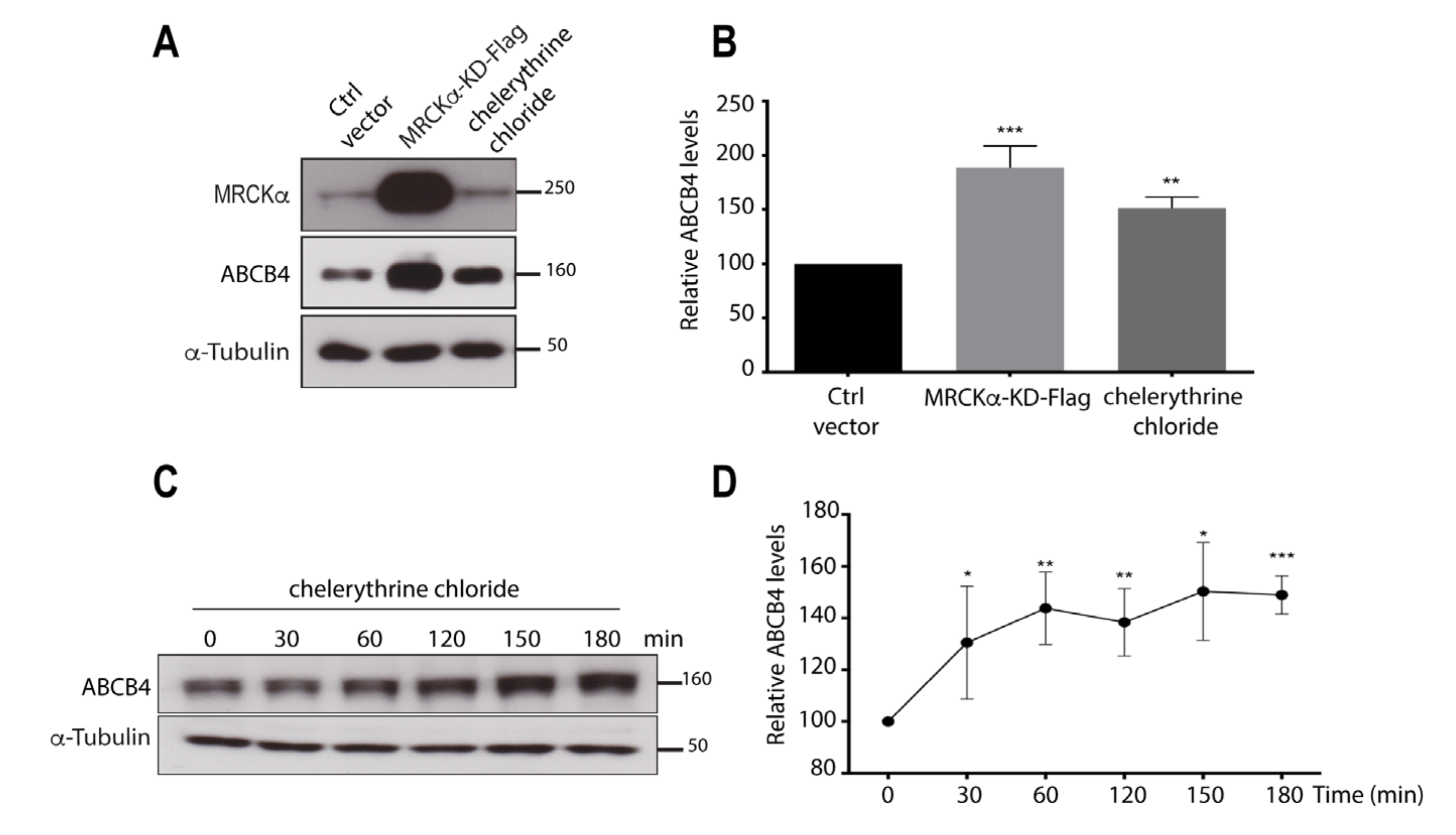
3.3. Inhibition of the Kinase Activity of MRCKα Increases ABCB4 Protein Expression
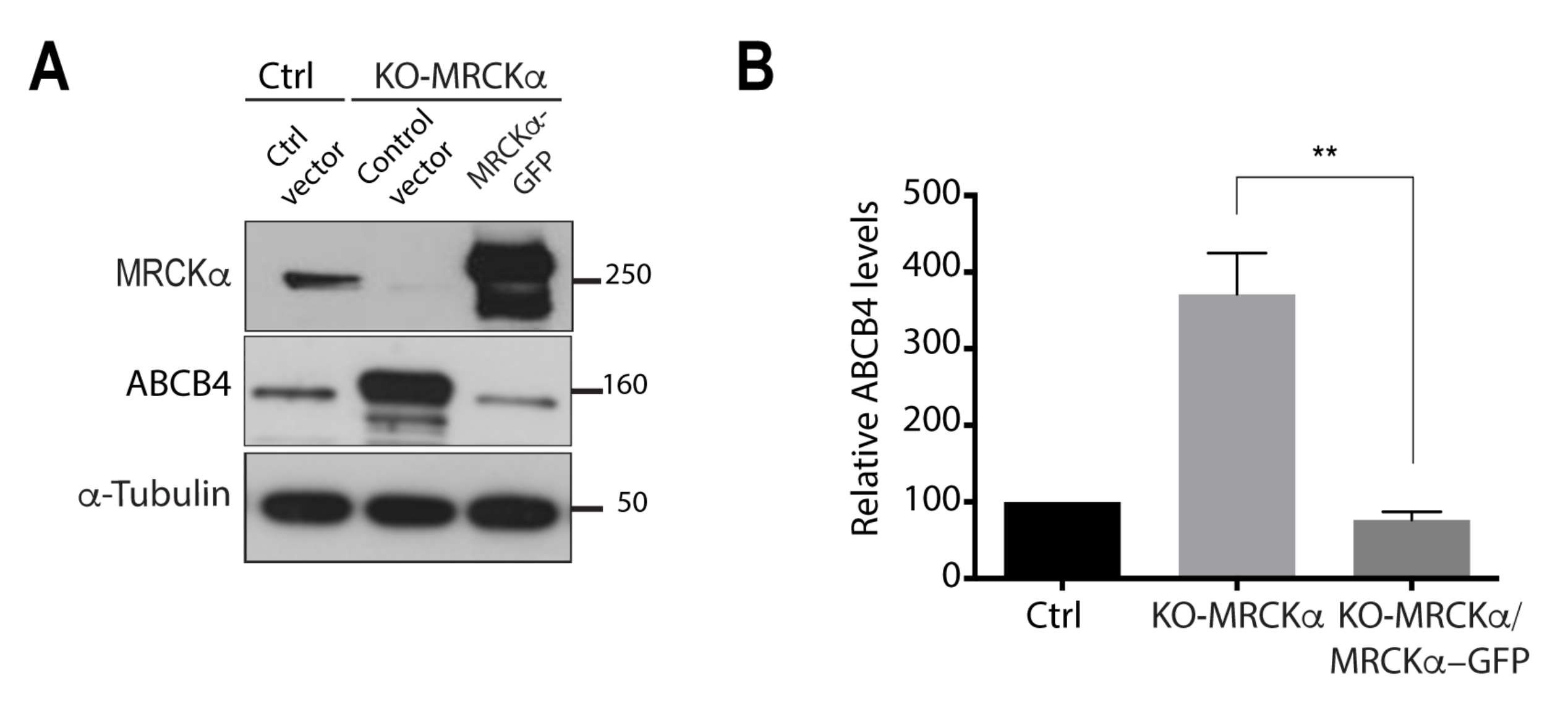
3.4. MRCKα Knockout Increases ABCB4 Protein Expression
3.5. The Effect of MRCKα on ABCB4 Depends on Its Effector MRLC
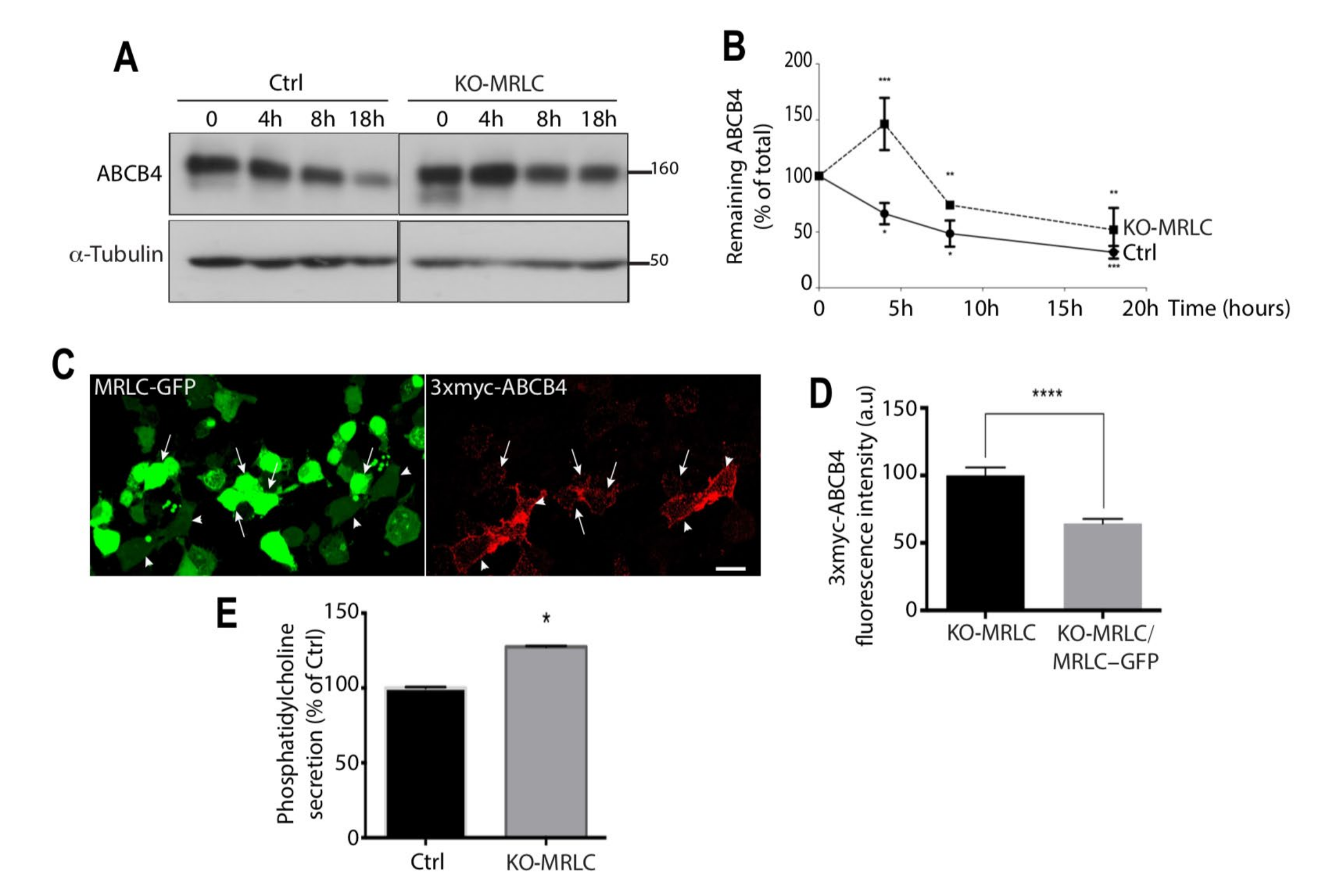
3.6. MRLC Knockout Increases ABCB4 Protein Stability and Increases Its Cell Surface Expression
3.7. MRLC Knockout Increases ABCB4 Function
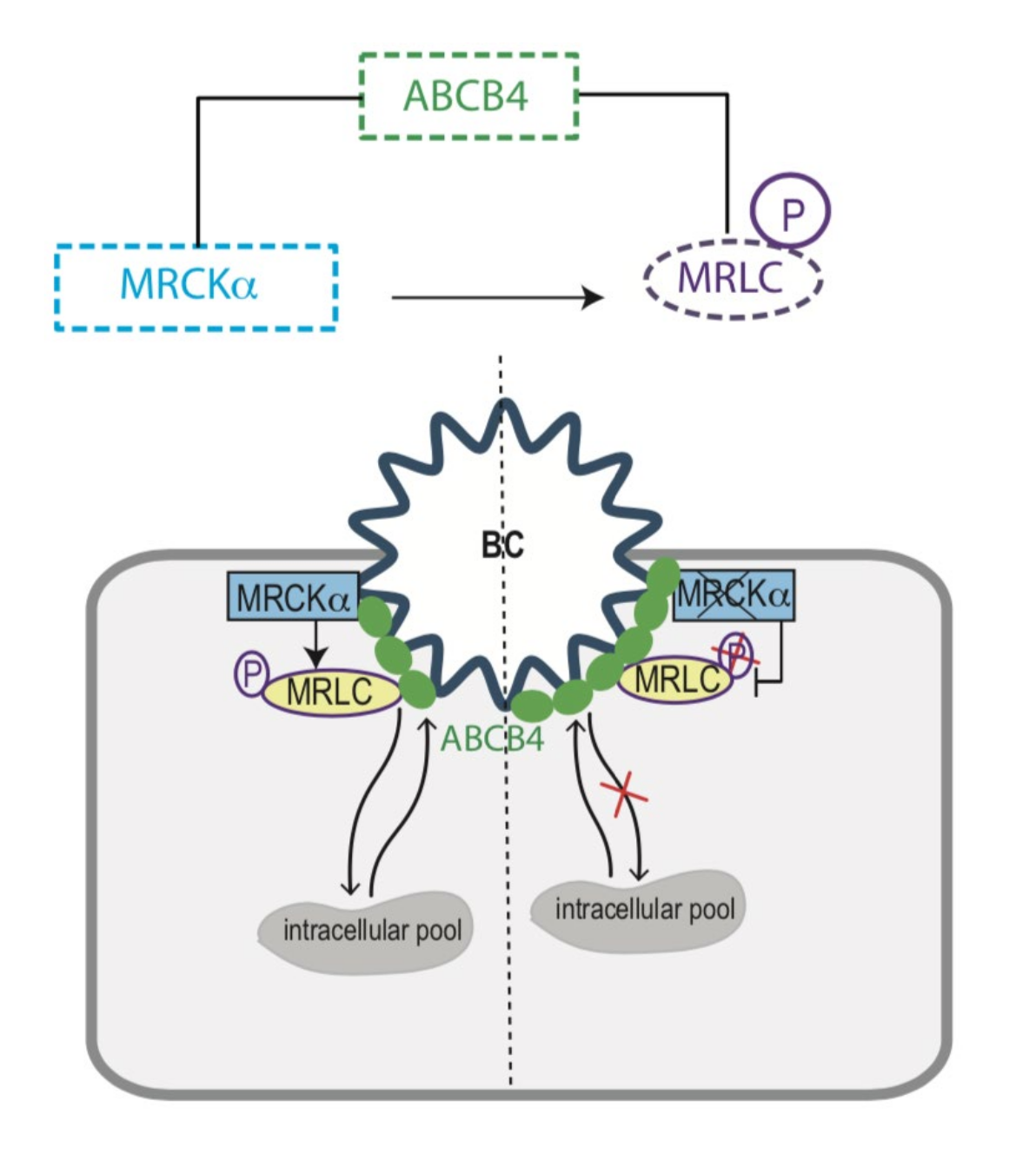
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-Binding Cassette |
| HEK | Human Embryonic Kidney |
| HS1 | Hematopoietic cell specific protein 1 |
| KO | KnockOut |
| MRCK | Myotonic dystrophy kinase-Related Cdc42-binding Kinase |
| MRLC | MyosinII Regulatory Light Chain |
| PC | PhosphatidylCholine |
| siRNA | small interfering RNA |
| WT | Wild Type |
References
- Prescher, M.; Kroll, T.; Schmitt, L. ABCB4/MDR3 in health and disease-at the crossroads of biochemistry and medicine. Biol. Chem. 2019, 400, 1245–1259. [Google Scholar] [CrossRef]
- Borst, P.; Zelcer, N.; van Helvoort, A. ABC transporters in lipid transport. Biochim. Biophys. Acta 2000, 1486, 128–144. [Google Scholar] [CrossRef]
- Wang, D.Q.; Cohen, D.E.; Carey, M.C. Biliary lipids and cholesterol gallstone disease. J. Lipid. Res. 2009, 50, S406–S411. [Google Scholar] [CrossRef] [Green Version]
- Jacquemin, E.; De Vree, J.M.; Cresteil, D.; Sokal, E.M.; Sturm, E.; Dumont, M.; Scheffer, G.L.; Paul, M.; Burdelski, M.; Bosma, P.J.; et al. The wide spectrum of multidrug resistance 3 deficiency: From neonatal cholestasis to cirrhosis of adulthood. Gastroenterology 2001, 120, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Rosmorduc, O.; Hermelin, B.; Poupon, R. MDR3 gene defect in adults with symptomatic intrahepatic and gallbladder cholesterol cholelithiasis. Gastroenterology 2001, 120, 1459–1467. [Google Scholar] [CrossRef]
- Davit-Spraul, A.; Gonzales, E.; Baussan, C.; Jacquemin, E. The spectrum of liver diseases related to ABCB4 gene mutations: Pathophysiology and clinical aspects. Semin. Liver Dis. 2010, 30, 134–146. [Google Scholar] [CrossRef]
- Geenes, V.; Williamson, C. Intrahepatic cholestasis of pregnancy. World J. Gastroenterol. 2009, 15, 2049–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaunay, J.L.; Durand-Schneider, A.M.; Delautier, D.; Rada, A.; Gautherot, J.; Jacquemin, E.; Ait-Slimane, T.; Maurice, M. A missense mutation in ABCB4 gene involved in progressive familial intrahepatic cholestasis type 3 leads to a folding defect that can be rescued by low temperature. Hepatology 2009, 49, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Gautherot, J.; Delautier, D.; Maubert, M.A.; Ait-Slimane, T.; Bolbach, G.; Delaunay, J.L.; Durand-Schneider, A.M.; Firrincieli, D.; Barbu, V.; Chignard, N.; et al. Phosphorylation of ABCB4 impacts its function: Insights from disease-causing mutations. Hepatology 2014, 60, 610–621. [Google Scholar] [CrossRef]
- Delaunay, J.L.; Durand-Schneider, A.M.; Dossier, C.; Falguieres, T.; Gautherot, J.; Davit-Spraul, A.; Ait-Slimane, T.; Housset, C.; Jacquemin, E.; Maurice, M. A functional classification of ABCB4 variations causing progressive familial intrahepatic cholestasis type 3. Hepatology 2016, 63, 1620–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Saad, A.; Bruneau, A.; Mareux, E.; Lapalus, M.; Delaunay, J.L.; Gonzales, E.; Jacquemin, E.; Ait-Slimane, T.; Falguieres, T. Molecular Regulation of Canalicular ABC Transporters. Int. J. Mol. Sci. 2021, 22, 2113. [Google Scholar] [CrossRef]
- Olsen, J.A.; Alam, A.; Kowal, J.; Stieger, B.; Locher, K.P. Structure of the human lipid exporter ABCB4 in a lipid environment. Nat. Struct. Mol. Biol. 2020, 27, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.F.; Moseley, J.; Calderon, G.; Swift, A.L.; Li, S.; Arias, I.M. Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin-Darby canine kidney cells. J. Biol. Chem. 2004, 279, 32761–32770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.; Calderon, G.; Swift, A.L.; Moseley, J.; Li, S.; Hosoya, H.; Arias, I.M.; Ortiz, D.F. Myosin II regulatory light chain is required for trafficking of bile salt export protein to the apical membrane in Madin-Darby canine kidney cells. J. Biol. Chem. 2005, 280, 23741–23747. [Google Scholar] [PubMed] [Green Version]
- Venot, Q.; Delaunay, J.L.; Fouassier, L.; Delautier, D.; Falguieres, T.; Housset, C.; Maurice, M.; Ait-Slimane, T. A PDZ-Like Motif in the Biliary Transporter ABCB4 Interacts with the Scaffold Protein EBP50 and Regulates ABCB4 Cell Surface Expression. PLoS ONE 2016, 11, e0146962. [Google Scholar] [CrossRef] [Green Version]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Unbekandt, M.; Olson, M.F. The actin-myosin regulatory MRCK kinases: Regulation, biological functions and associations with human cancer. J. Mol. Med. 2014, 92, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, T.; Chen, X.Q.; Tan, I.; Manser, E.; Lim, L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol. Cell. Biol. 1998, 18, 130–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, I.; Ng, C.H.; Lim, L.; Leung, T. Phosphorylation of a novel myosin binding subunit of protein phosphatase 1 reveals a conserved mechanism in the regulation of actin cytoskeleton. J. Biol. Chem. 2001, 276, 21209–21216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, S.; Paterson, H.F.; Marshall, C.J. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat. Cell Biol. 2005, 7, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, P.A.; di Blasio, L.; Puliafito, A.; Seano, G.; Sessa, R.; Chianale, F.; Leung, T.; Bussolino, F.; Primo, L. PDK1-mediated activation of MRCKalpha regulates directional cell migration and lamellipodia retraction. J. Cell Biol. 2014, 206, 415–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumoto, K.; Uchimura, T.; Iwasaki, T.; Ueda, K.; Hosoya, H. Phosphorylation of myosin II regulatory light chain is necessary for migration of HeLa cells but not for localization of myosin II at the leading edge. Biochem. J. 2003, 370, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [Green Version]
- Aoudjehane, L.; Gautheron, J.; Le Goff, W.; Goumard, C.; Gilaizeau, J.; Nget, C.S.; Savier, E.; Atif, M.; Lesnik, P.; Morichon, R.; et al. Novel defatting strategies reduce lipid accumulation in primary human culture models of liver steatosis. Dis. Model. Mech. 2020, 13, dmm042663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, I.; Lai, J.; Yong, J.; Li, S.F.; Leung, T. Chelerythrine perturbs lamellar actomyosin filaments by selective inhibition of myotonic dystrophy kinase-related Cdc42-binding kinase. FEBS Lett. 2011, 585, 1260–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresnick, A.R. Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 1999, 11, 26–33. [Google Scholar] [CrossRef]
- Bajaj, G.; Rodriguez-Proteau, R.; Venkataraman, A.; Fan, Y.; Kioussi, C.; Ishmael, J.E. MDR1 function is sensitive to the phosphorylation state of myosin regulatory light chain. Biochem. Biophys. Res. Commun. 2010, 398, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Lam, P.; Xu, S.; Soroka, C.J.; Boyer, J.L. A C-terminal tyrosine-based motif in the bile salt export pump directs clathrin-dependent endocytosis. Hepatology 2012, 55, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, H.; Inamura, K.; Aida, K.; Naoi, S.; Horikawa, R.; Nagasaka, H.; Takatani, T.; Fukushima, T.; Hattori, A.; Yabuki, T.; et al. AP2 adaptor complex mediates bile salt export pump internalization and modulates its hepatocanalicular expression and transport function. Hepatology 2012, 55, 1889–1900. [Google Scholar] [CrossRef]
- Gonzales, E.; Taylor, S.A.; Davit-Spraul, A.; Thebaut, A.; Thomassin, N.; Guettier, C.; Whitington, P.F.; Jacquemin, E. MYO5B mutations cause cholestasis with normal serum gamma-glutamyl transferase activity in children without microvillous inclusion disease. Hepatology 2017, 65, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Overeem, A.W.; Li, Q.; Qiu, Y.L.; Carton-Garcia, F.; Leng, C.; Klappe, K.; Dronkers, J.; Hsiao, N.H.; Wang, J.S.; Arango, D.; et al. A Molecular Mechanism Underlying Genotype-Specific Intrahepatic Cholestasis Resulting From MYO5B Mutations. Hepatology 2020, 72, 213–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharanek, A.; Burban, A.; Burbank, M.; Le Guevel, R.; Li, R.; Guillouzo, A.; Guguen-Guillouzo, C. Rho-kinase/myosin light chain kinase pathway plays a key role in the impairment of bile canaliculi dynamics induced by cholestatic drugs. Sci. Rep. 2016, 6, 24709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burbank, M.G.; Burban, A.; Sharanek, A.; Weaver, R.J.; Guguen-Guillouzo, C.; Guillouzo, A. Early Alterations of Bile Canaliculi Dynamics and the Rho Kinase/Myosin Light Chain Kinase Pathway Are Characteristics of Drug-Induced Intrahepatic Cholestasis. Drug Metab. Dispos. 2016, 44, 1780–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantore, M.; Reinehr, R.; Sommerfeld, A.; Becker, M.; Haussinger, D. The Src family kinase Fyn mediates hyperosmolarity-induced Mrp2 and Bsep retrieval from canalicular membrane. J. Biol. Chem. 2011, 286, 45014–45029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonhoff, C.M.; Webster, C.R.; Anwer, M.S. Taurolithocholate-induced MRP2 retrieval involves MARCKS phosphorylation by protein kinase C in HUH-NTCP Cells. Hepatology 2013, 58, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, T.; Buch, T.; Urban, N.; Weirauch, U.; Schierle, K.; Aigner, A.; Schaefer, M.; Kalwa, H. Restoration of MARCKS enhances chemosensitivity in cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 843–858. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.; Cai, S.Y.; Liu, X.; Lian, W.; Chen, S.; Zhang, L.; Feng, X.; Cheng, Y.; He, X.; He, Y.; et al. Canalicular membrane MRP2/ABCC2 internalization is determined by Ezrin Thr567 phosphorylation in human obstructive cholestasis. J. Hepatol. 2015, 63, 1440–1448. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruneau, A.; Delaunay, J.-L.; Durand-Schneider, A.-M.; Vauthier, V.; Ben Saad, A.; Aoudjehane, L.; El Mourabit, H.; Morichon, R.; Falguières, T.; Gautheron, J.; et al. MRCK-Alpha and Its Effector Myosin II Regulatory Light Chain Bind ABCB4 and Regulate Its Membrane Expression. Cells 2022, 11, 617. https://doi.org/10.3390/cells11040617
Bruneau A, Delaunay J-L, Durand-Schneider A-M, Vauthier V, Ben Saad A, Aoudjehane L, El Mourabit H, Morichon R, Falguières T, Gautheron J, et al. MRCK-Alpha and Its Effector Myosin II Regulatory Light Chain Bind ABCB4 and Regulate Its Membrane Expression. Cells. 2022; 11(4):617. https://doi.org/10.3390/cells11040617
Chicago/Turabian StyleBruneau, Alix, Jean-Louis Delaunay, Anne-Marie Durand-Schneider, Virginie Vauthier, Amel Ben Saad, Lynda Aoudjehane, Haquima El Mourabit, Romain Morichon, Thomas Falguières, Jérémie Gautheron, and et al. 2022. "MRCK-Alpha and Its Effector Myosin II Regulatory Light Chain Bind ABCB4 and Regulate Its Membrane Expression" Cells 11, no. 4: 617. https://doi.org/10.3390/cells11040617
APA StyleBruneau, A., Delaunay, J.-L., Durand-Schneider, A.-M., Vauthier, V., Ben Saad, A., Aoudjehane, L., El Mourabit, H., Morichon, R., Falguières, T., Gautheron, J., Housset, C., & Aït-Slimane, T. (2022). MRCK-Alpha and Its Effector Myosin II Regulatory Light Chain Bind ABCB4 and Regulate Its Membrane Expression. Cells, 11(4), 617. https://doi.org/10.3390/cells11040617









