Identification of Small Molecules Inhibiting Cardiomyocyte Necrosis and Apoptosis by Autophagy Induction and Metabolism Reprogramming
Abstract
1. Introduction
2. Material and Methods
2.1. Phenotypic High Throughput Screening
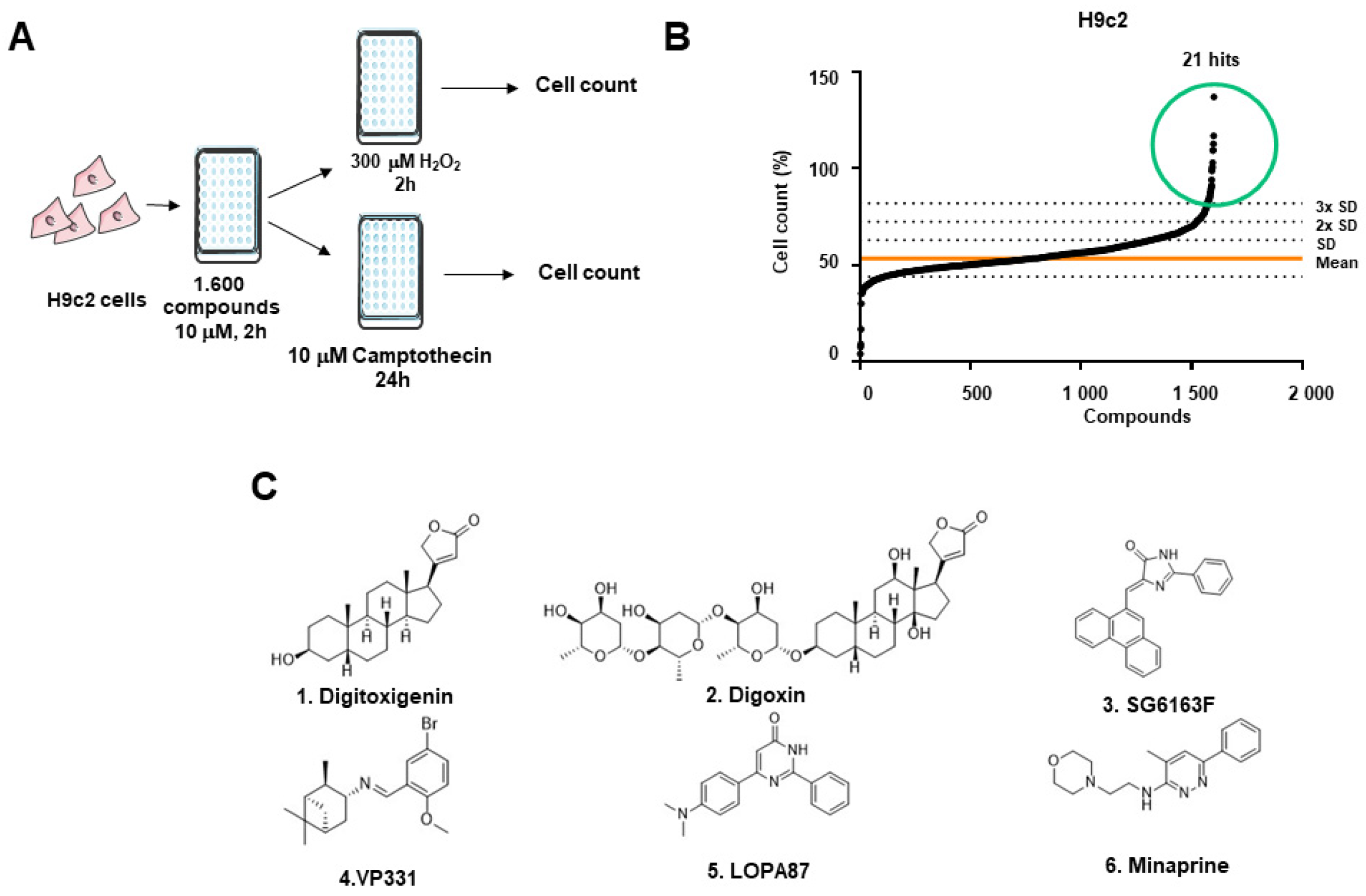
2.1.1. Chemical Libraries
2.1.2. Cellular Treatments
2.1.3. Viability Measurement and Hit Selection
2.2. Neonatal Cardiomyocyte Isolation
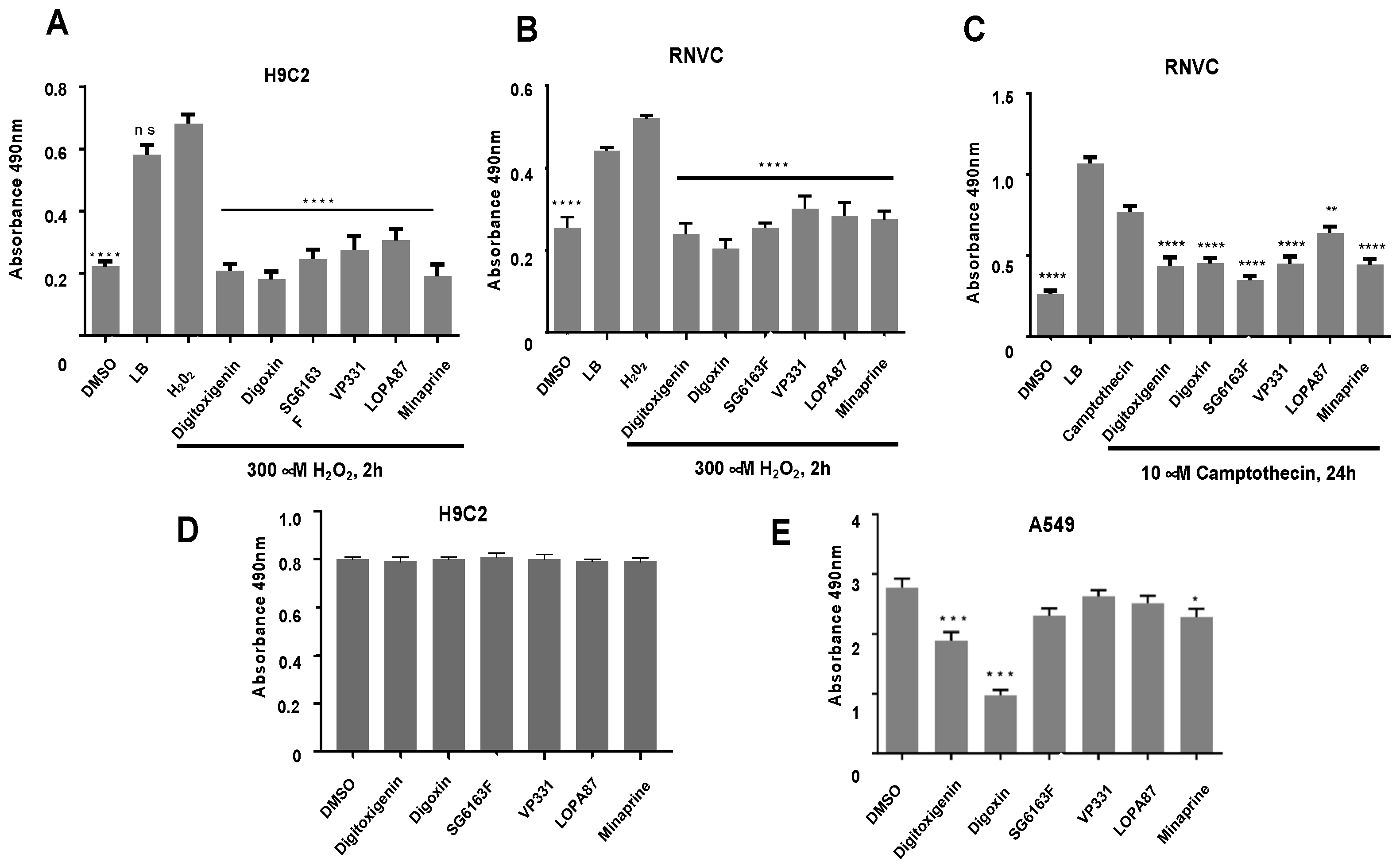
2.3. LDH Release Assay
2.4. Plasma Cell Membrane Permeabilization Assay
2.5. Plasmid Transfection
2.6. Mitochondrial Network Analysis by Confocal Microscopy and Transmission Electron Microscopy
2.6.1. Confocal Microscopy
2.6.2. Transmission Electron Microscopy
2.7. ROS Detection in RNVCs
2.8. Real-Time Bioenergetic Profile Analysis in H9c2 Cardiomyocytes
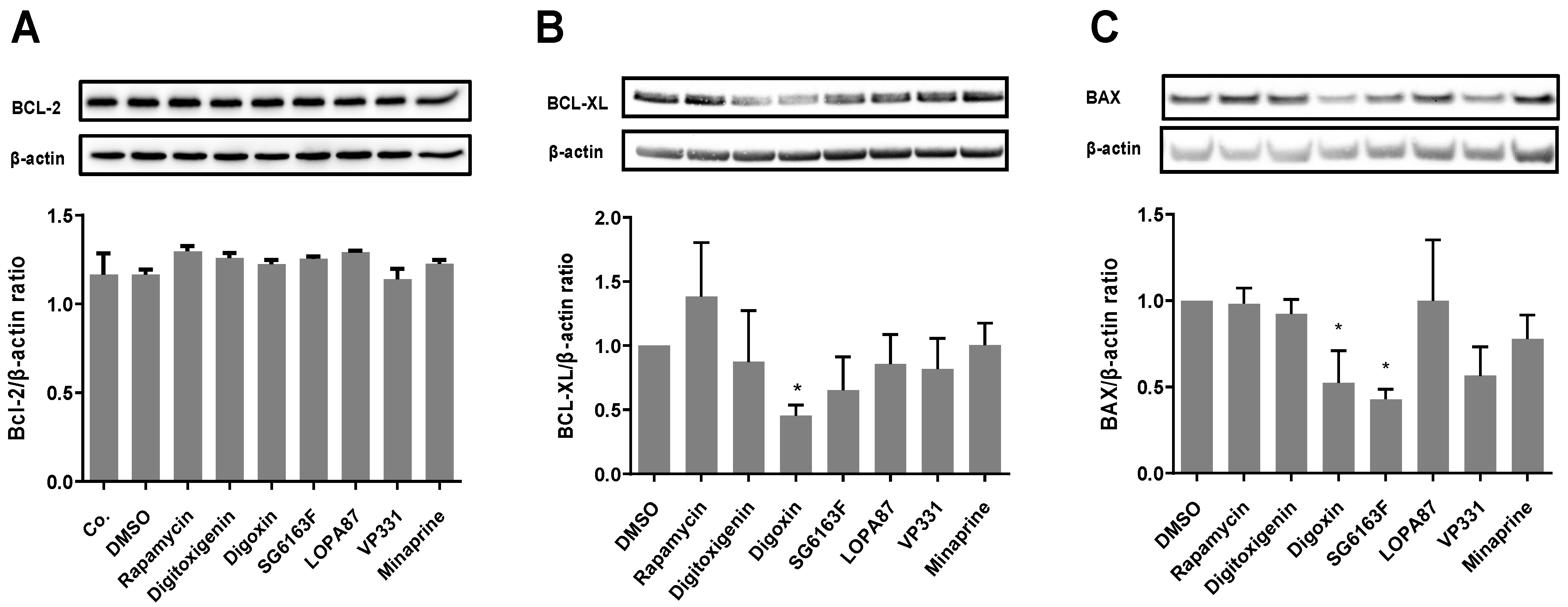
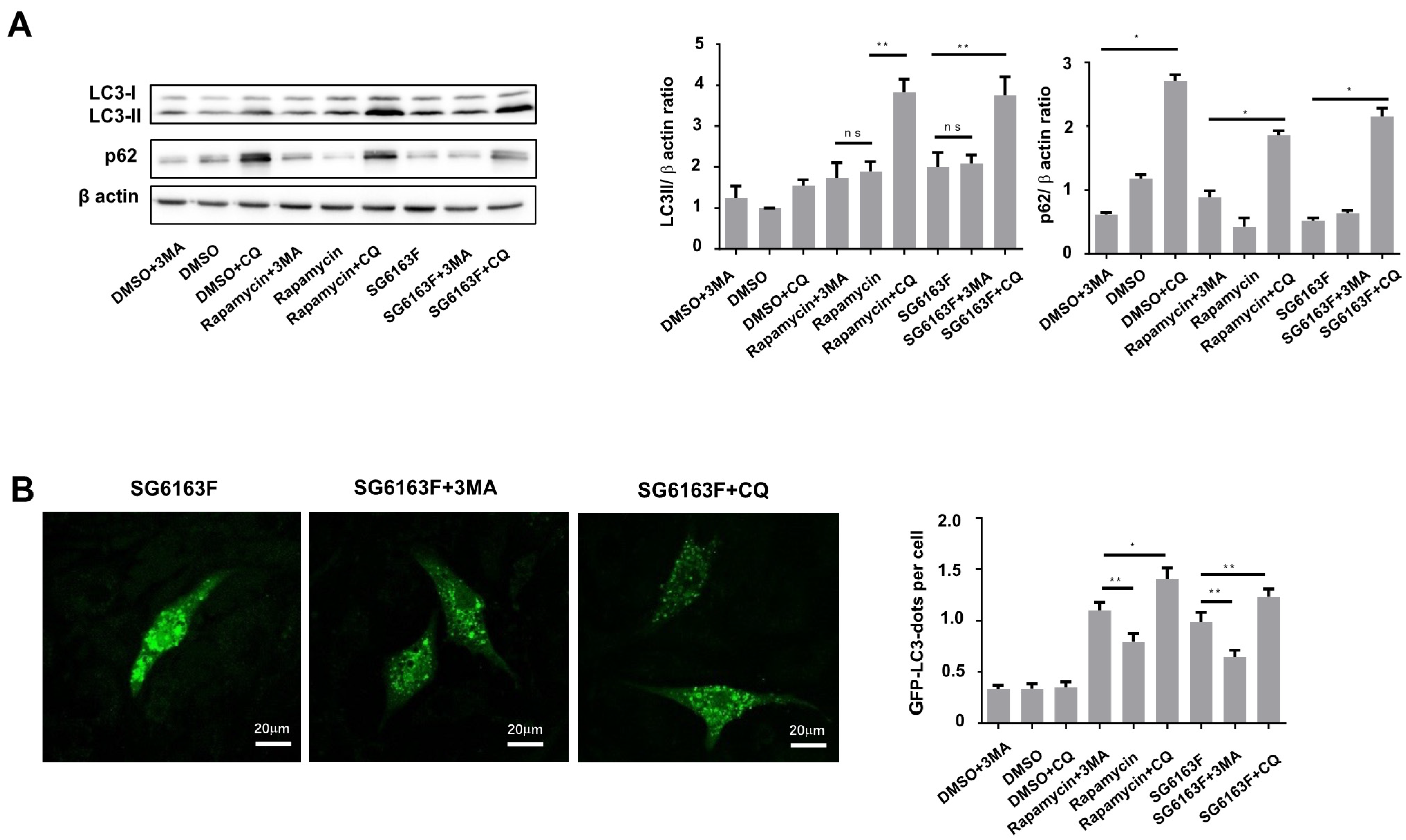
2.9. SDS-PAGE and Western Blot
2.10. Statistical Analysis
3. Results
3.1. Identification of Cardiomyocyte Apoptosis and Necrosis Inhibitors by High Throughput Screening
3.2. SG6163F Influences Autophagy Induction via ATG5 and BECLIN-1
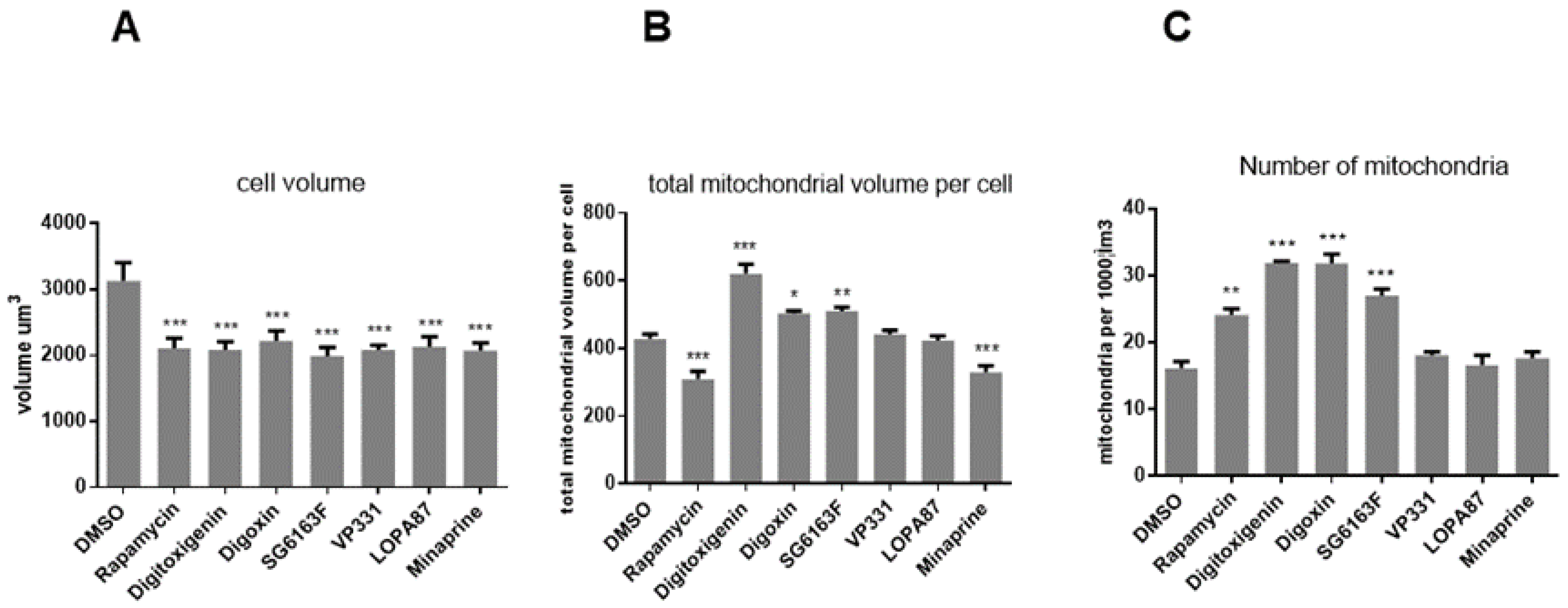
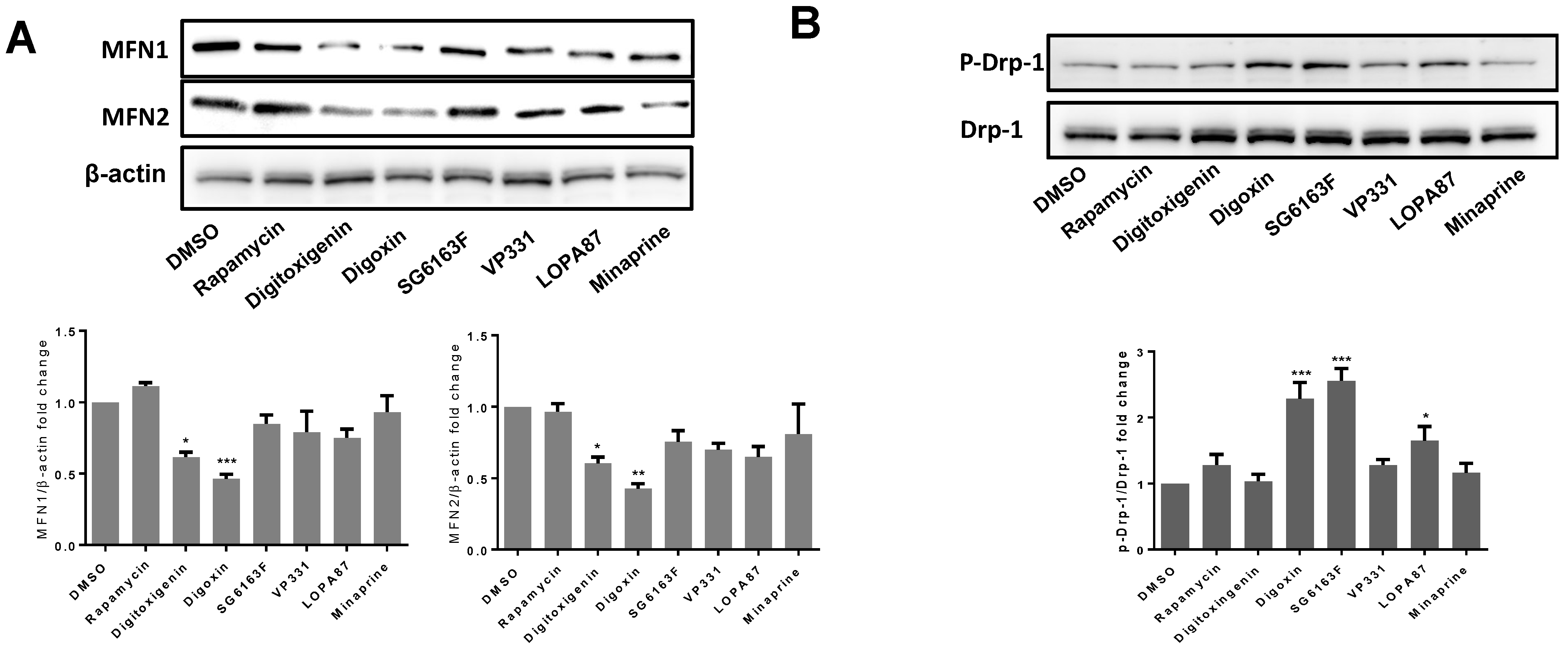
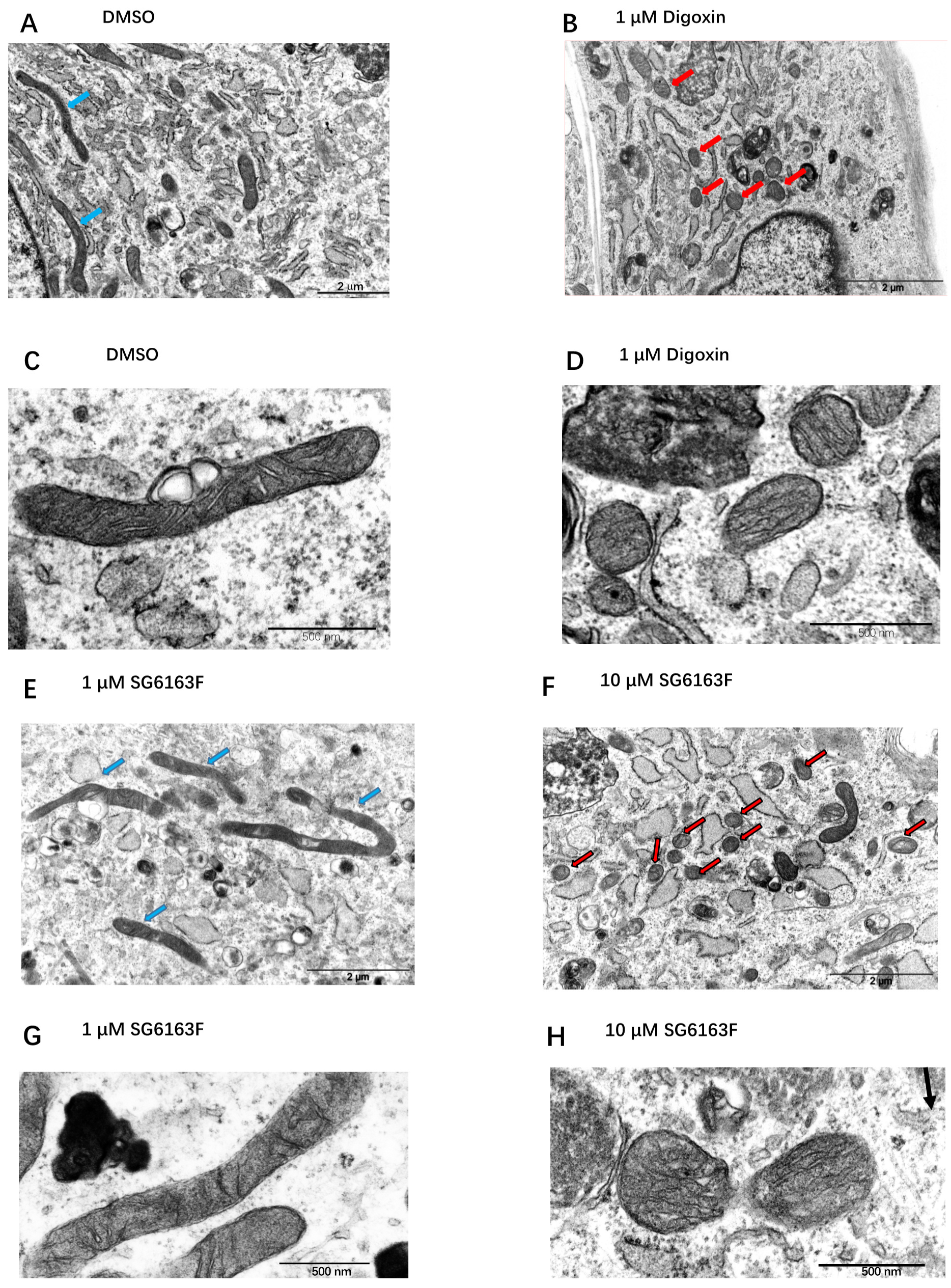
3.3. Compounds Impact on Mitochondrial Network Structure and Dynamics
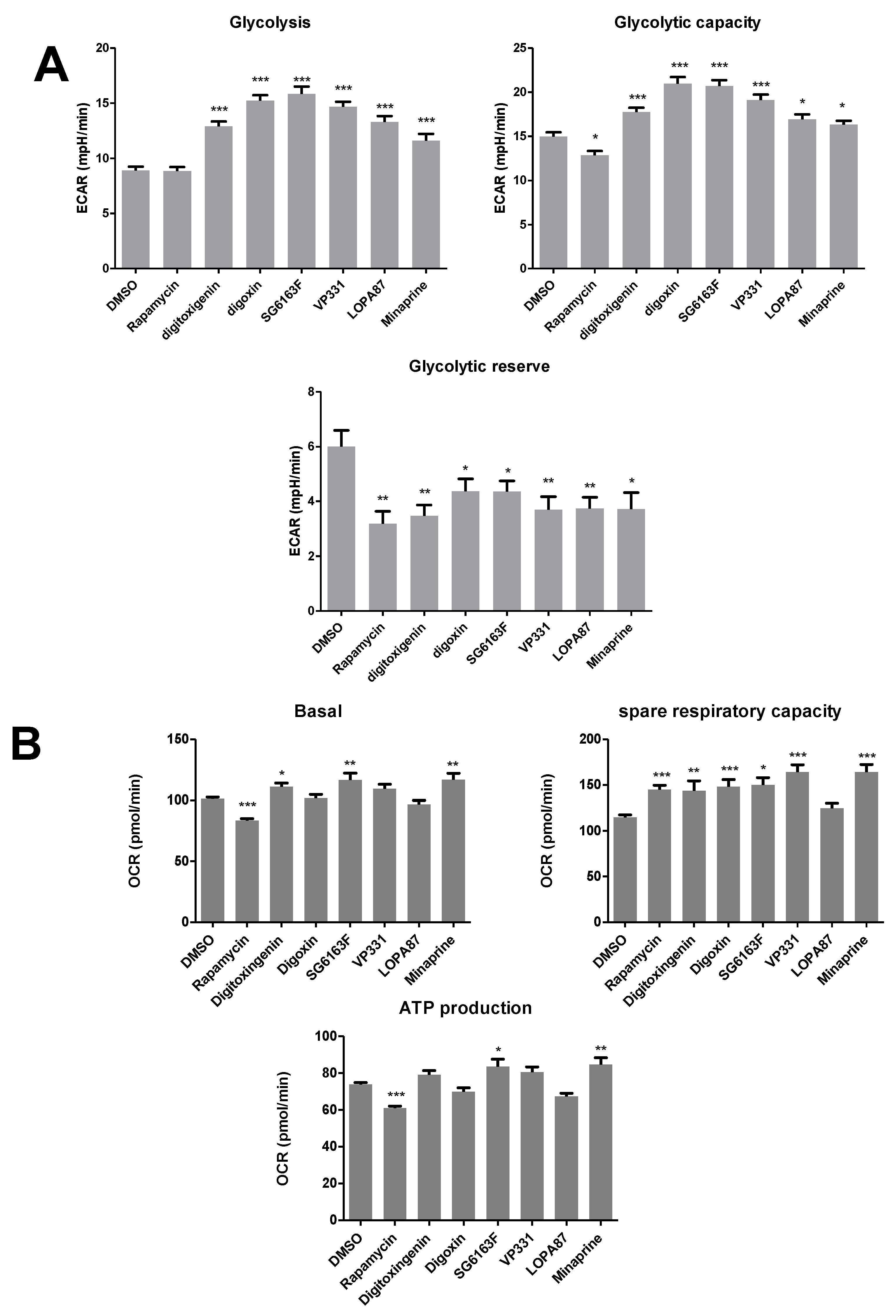
3.4. Metabolic Reprogramming in Cells Treated with Selected Compounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LDH | Lactate dehydrogenase |
| SD | Standard deviation |
| 3-MA | 3-methyladenine |
| CQ | Chloroquine |
References
- Mancilla, T.R.; Davis, L.R.; Aune, G.J. Doxorubicin-Induced P53 Interferes with Mitophagy in Cardiac Fibroblasts. PLoS ONE 2020, 15, e0238856. [Google Scholar] [CrossRef] [PubMed]
- Findlay, S.G.; Gill, J.H.; Plummer, R.; DeSantis, C.; Plummer, C. Chronic Cardiovascular Toxicity in the Older Oncology Patient Population. J. Geriatr. Oncol. 2019, 10, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Billingham, M.E.; Bristow, M.R.; Glatstein, E.; Mason, J.W.; Masek, M.A.; Daniels, J.R. Adriamycin Cardiotoxicity: Endomyocardial Biopsy Evidence of Enhancement by Irradiation. Am. J. Surg. Pathol. 1977, 1, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, M.; Nielsen, D.; Skovsgaard, T.; Hansen, J.; Jensen, B.V.; Dombernowsky, P. Epirubicin Cardiotoxicity: An Analysis of 469 Patients with Metastatic Breast Cancer. J. Clin. Oncol. 1998, 16, 3502–3508. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, R.; Grazette, L.; Yacobi, R.; Iliescu, C.; Patten, R.; Beahm, C.; Walters, B.; Shevtsov, S.; Pesant, S.; Clubb, F.J.; et al. Cardiotoxicity of the Cancer Therapeutic Agent Imatinib Mesylate. Nat. Med. 2006, 12, 908–916. [Google Scholar] [CrossRef]
- Chen, M.H.; Kerkelä, R.; Force, T. Mechanisms of Cardiac Dysfunction Associated with Tyrosine Kinase Inhibitor Cancer Therapeutics. Circulation 2008, 118, 84–95. [Google Scholar] [CrossRef]
- Carvalho, F.S.; Burgeiro, A.; Garcia, R.; Moreno, A.J.; Carvalho, R.A.; Oliveira, P.J. Doxorubicin-Induced Cardiotoxicity: From Bioenergetic Failure and Cell Death to Cardiomyopathy. Med. Res. Rev. 2014, 34, 106–135. [Google Scholar] [CrossRef]
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. Camptothecin Induces Protein-Linked DNA Breaks via Mammalian DNA Topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar] [CrossRef]
- Simůnek, T.; Stérba, M.; Popelová, O.; Adamcová, M.; Hrdina, R.; Gersl, V. Anthracycline-Induced Cardiotoxicity: Overview of Studies Examining the Roles of Oxidative Stress and Free Cellular Iron. Pharmacol. Rep. PR 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Micheau, O.; Solary, E.; Hammann, A.; Martin, F.; Dimanche-Boitrel, M.T. Sensitization of Cancer Cells Treated with Cytotoxic Drugs to Fas-Mediated Cytotoxicity. J. Natl. Cancer Inst. 1997, 89, 783–789. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.; Varin, A.; Nicolas, V.; Courilleau, D.; Mateo, P.; Caubere, C.; Rouet, P.; Gomez, A.-M.; Vandecasteele, G.; et al. A Cardiac Mitochondrial CAMP Signaling Pathway Regulates Calcium Accumulation, Permeability Transition and Cell Death. Cell Death Dis. 2016, 7, e2198. [Google Scholar] [CrossRef] [PubMed]
- Meyenberg Cunha-de Padua, M.; Fabbri, L.; Dufies, M.; Lacas-Gervais, S.; Contenti, J.; Voyton, C.; Fazio, S.; Irondelle, M.; Mograbi, B.; Rouleau, M.; et al. Evidences of a Direct Relationship between Cellular Fuel Supply and Ciliogenesis Regulated by Hypoxic VDAC1-ΔC. Cancers 2020, 12, 3484. [Google Scholar] [CrossRef] [PubMed]
- Gabillet, S.; Loreau, O.; Specklin, S.; Rasalofonjatovo, E.; Taran, F. A Phosphine-Catalyzed Preparation of 4-Arylidene-5-Imidazolones. J. Org. Chem. 2014, 79, 9894–9898. [Google Scholar] [CrossRef] [PubMed]
- Perfettini, J.L.; Deutsch, E.; Brenner, C.; Cintrat, J.-C.; Taran, F. Enhancers of Cellular Cannibalism for Use to Senzitize Tumors to Radiation Therapy. U.S. Patent Application No. 16/479,415, 28 November 2018. [Google Scholar]
- Yin, Z.; Pascual, C.; Klionsky, D. Autophagy: Machinery and Regulation. Microb. Cell 2016, 3, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.G.; Fingar, D.C. Mammalian Target of Rapamycin (MTOR): Conducting the Cellular Signaling Symphony. J. Biol. Chem. 2010, 285, 14071–14077. [Google Scholar] [CrossRef]
- Esteban-Martínez, L.; Boya, P. Autophagic Flux Determination in Vivo and Ex Vivo. Methods 2015, 75, 79–86. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Martel, C.; Huynh, L.H.; Garnier, A.; Ventura-Clapier, R.; Brenner, C. Inhibition of the Mitochondrial Permeability Transition for Cytoprotection: Direct versus Indirect Mechanisms. Biochem. Res. Int. 2012, 2012, 213403. [Google Scholar] [CrossRef]
- Schieke, S.M.; Phillips, D.; McCoy, J.P.; Aponte, A.M.; Shen, R.-F.; Balaban, R.S.; Finkel, T. The Mammalian Target of Rapamycin (MTOR) Pathway Regulates Mitochondrial Oxygen Consumption and Oxidative Capacity. J. Biol. Chem. 2006, 281, 27643–27652. [Google Scholar] [CrossRef]
- Prassas, I.; Diamandis, E.P. Novel Therapeutic Applications of Cardiac Glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- McConkey, D.J.; Lin, Y.; Nutt, L.K.; Ozel, H.Z.; Newman, R.A. Cardiac Glycosides Stimulate Ca2+ Increases and Apoptosis in Androgen-Independent, Metastatic Human Prostate Adenocarcinoma Cells. Cancer Res. 2000, 60, 3807–3812. [Google Scholar] [PubMed]
- Menger, L.; Vacchelli, E.; Adjemian, S.; Martins, I.; Ma, Y.; Shen, S.; Yamazaki, T.; Sukkurwala, A.Q.; Michaud, M.; Mignot, G.; et al. Cardiac Glycosides Exert Anticancer Effects by Inducing Immunogenic Cell Death. Sci. Transl. Med. 2012, 4, 143ra99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shoji-Kawata, S.; Sumpter, R.M.; Wei, Y.; Ginet, V.; Zhang, L.; Posner, B.; Tran, K.A.; Green, D.R.; Xavier, R.J.; et al. Autosis Is a Na+,K+-ATPase-Regulated Form of Cell Death Triggered by Autophagy-Inducing Peptides, Starvation, and Hypoxia-Ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, 20364–20371. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Le Toumelin, G.; Criollo, A.; Rain, J.-C.; Gautier, F.; Juin, P.; Tasdemir, E.; Pierron, G.; Troulinaki, K.; Tavernarakis, N.; et al. Functional and Physical Interaction between Bcl-X(L) and a BH3-like Domain in Beclin-1. EMBO J. 2007, 26, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Isobe, M.; Sadoshima, J. Regulation of Autophagy by Beclin 1 in the Heart. J. Mol. Cell. Cardiol. 2016, 95, 19–25. [Google Scholar] [CrossRef]
- Susin, S.A.; Zamzami, N.; Larochette, N.; Dallaporta, B.; Marzo, I.; Brenner, C.; Hirsch, T.; Petit, P.X.; Geuskens, M.; Kroemer, G. A Cytofluorometric Assay of Nuclear Apoptosis Induced in a Cell-Free System: Application to Ceramide-Induced Apoptosis. Exp. Cell Res. 1997, 236, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Belzacq-Casagrande, A.-S.; Martel, C.; Pertuiset, C.; Borgne-Sanchez, A.; Jacotot, E.; Brenner, C. Pharmacological Screening and Enzymatic Assays for Apoptosis. Front. Biosci. Landmark Ed. 2009, 14, 3550–3562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Z.; Nicolas, C.; Fischmeister, R.; Brenner, C. Enzymatic Assays for Probing Mitochondrial Apoptosis. In Mitochondrial Medicine; Weissig, V., Edeas, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1265, pp. 407–414. ISBN 978-1-4939-2287-1. [Google Scholar]
- Liu, D.; Lai, H.T.; Peyre, F.; Brenner, C. Multiple analysis of mitochondrial metabolism, autophagy and cell death. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Galluzzi, L.; Kepp, O.; Vander Heiden, M.G.; Kroemer, G. Metabolic Targets for Cancer Therapy. Nat. Rev. Drug Discov. 2013, 12, 829–846. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Obach, R.S. The Prediction of Human Clearance from Hepatic Microsomal Metabolism Data. Curr. Opin. Drug Discov. Devel. 2001, 4, 36–44. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Peyre, F.; Loissell-Baltazar, Y.A.; Courilleau, D.; Lacas-Gervais, S.; Nicolas, V.; Jacquet, E.; Dokudovskaya, S.; Taran, F.; Cintrat, J.-C.; et al. Identification of Small Molecules Inhibiting Cardiomyocyte Necrosis and Apoptosis by Autophagy Induction and Metabolism Reprogramming. Cells 2022, 11, 474. https://doi.org/10.3390/cells11030474
Liu D, Peyre F, Loissell-Baltazar YA, Courilleau D, Lacas-Gervais S, Nicolas V, Jacquet E, Dokudovskaya S, Taran F, Cintrat J-C, et al. Identification of Small Molecules Inhibiting Cardiomyocyte Necrosis and Apoptosis by Autophagy Induction and Metabolism Reprogramming. Cells. 2022; 11(3):474. https://doi.org/10.3390/cells11030474
Chicago/Turabian StyleLiu, Dawei, Félix Peyre, Yahir Alberto Loissell-Baltazar, Delphine Courilleau, Sandra Lacas-Gervais, Valérie Nicolas, Eric Jacquet, Svetlana Dokudovskaya, Frédéric Taran, Jean-Christophe Cintrat, and et al. 2022. "Identification of Small Molecules Inhibiting Cardiomyocyte Necrosis and Apoptosis by Autophagy Induction and Metabolism Reprogramming" Cells 11, no. 3: 474. https://doi.org/10.3390/cells11030474
APA StyleLiu, D., Peyre, F., Loissell-Baltazar, Y. A., Courilleau, D., Lacas-Gervais, S., Nicolas, V., Jacquet, E., Dokudovskaya, S., Taran, F., Cintrat, J.-C., & Brenner, C. (2022). Identification of Small Molecules Inhibiting Cardiomyocyte Necrosis and Apoptosis by Autophagy Induction and Metabolism Reprogramming. Cells, 11(3), 474. https://doi.org/10.3390/cells11030474






