Aged Mouse Hippocampus Exhibits Signs of Chronic Hypoxia and an Impaired HIF-Controlled Response to Acute Hypoxic Exposures
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Acute Hypoxia
2.3. Tissue Processing
2.4. Slide Preparation
2.5. Fluorescent In Situ Hybridization (fISH)
2.6. Immunohistochemistry (IHC)
2.7. Statistical Analysis
3. Results
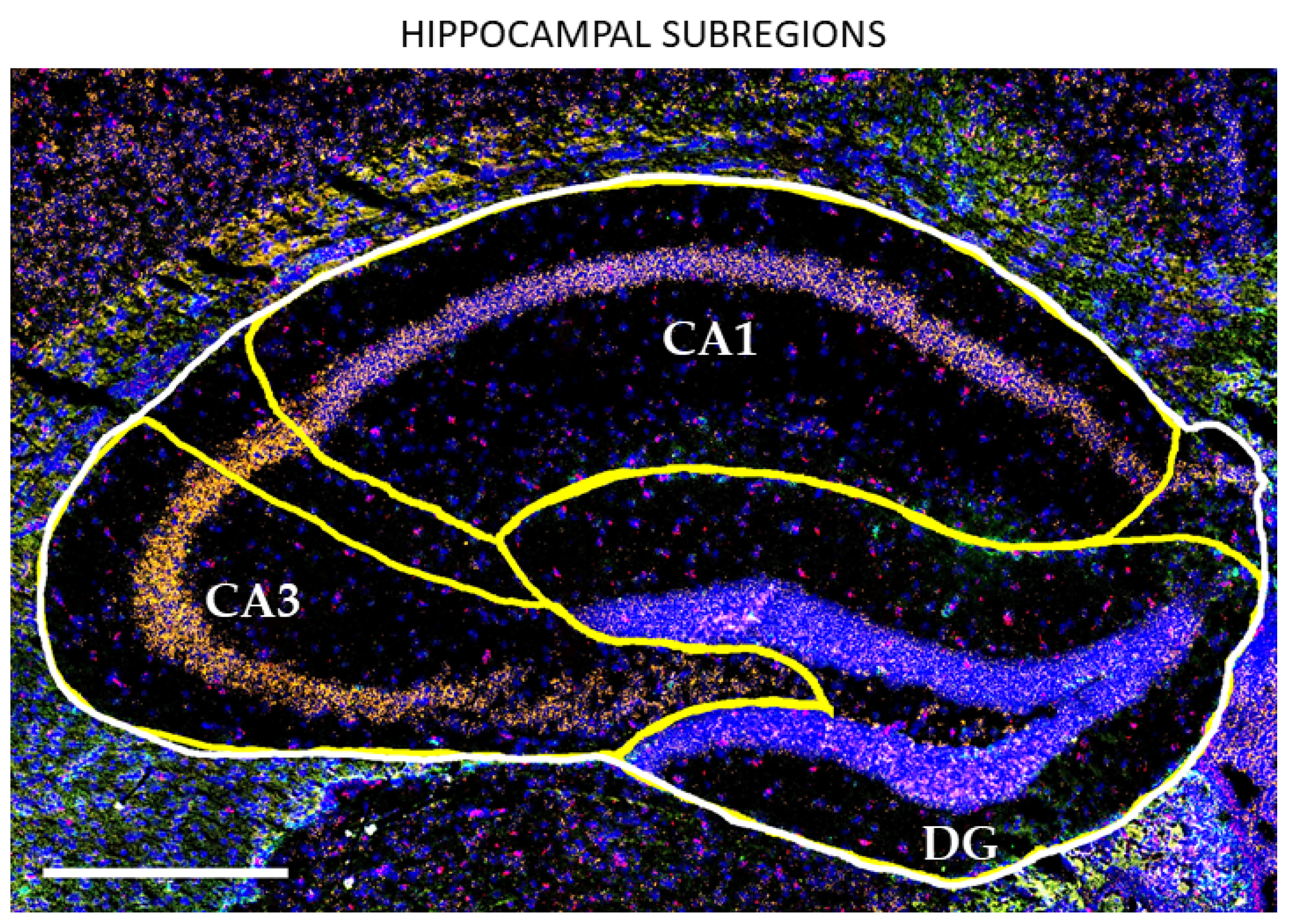
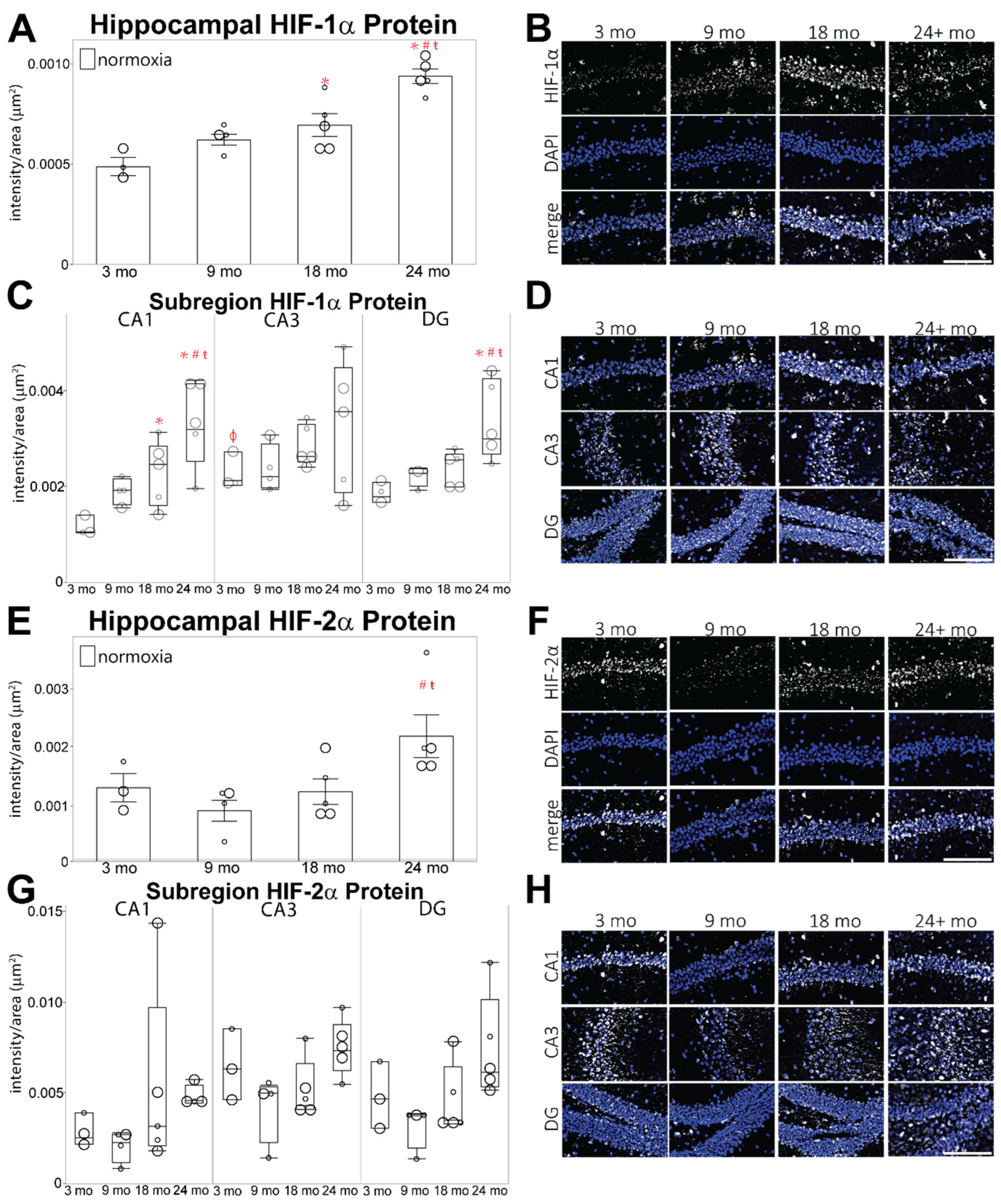
3.1. HIF-α Levels under Normoxia Are Increased in the Aged Hippocampus
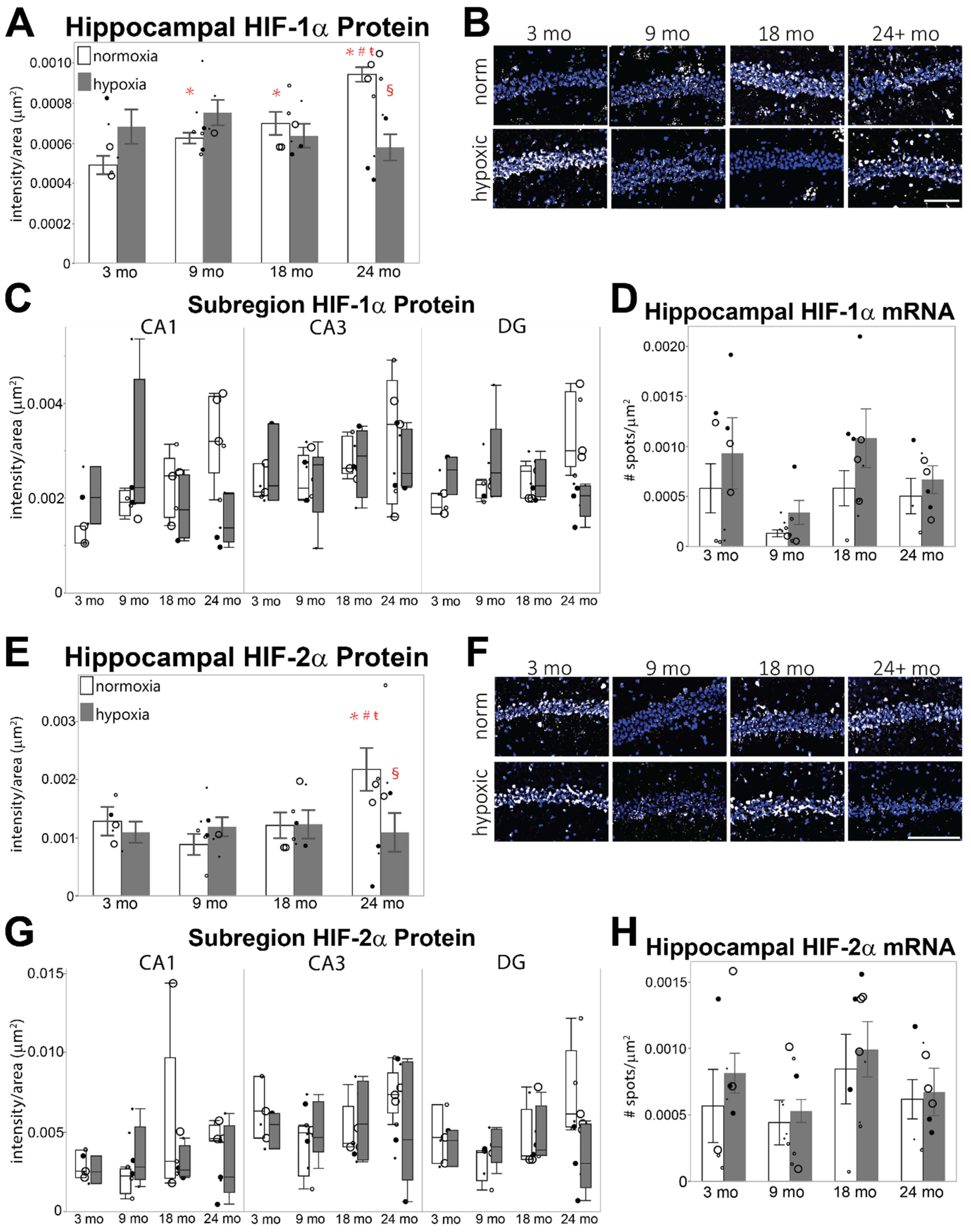
3.2. HIF-α Levels under Acute Hypoxia Are Diminished in Aged Hippocampus
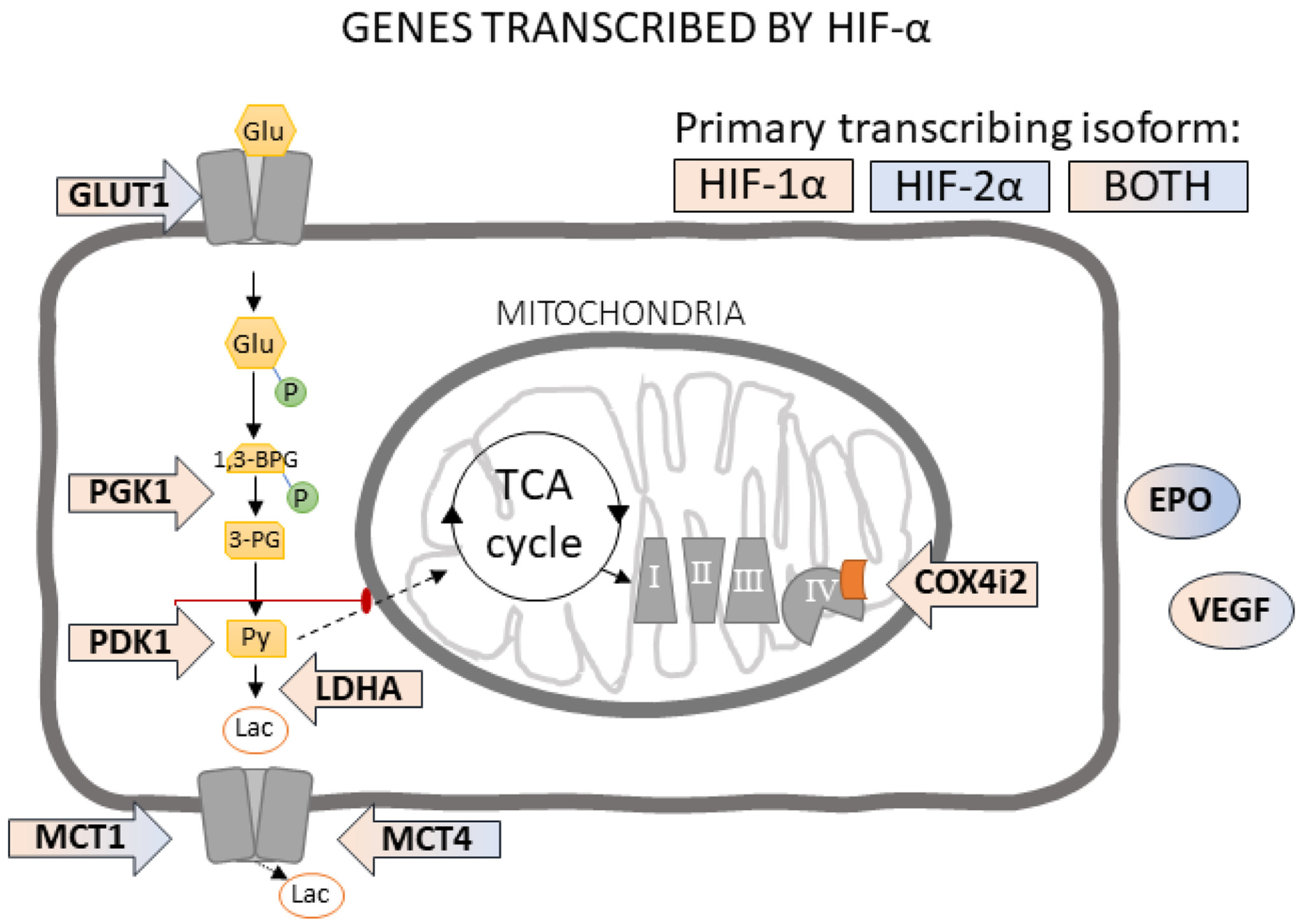
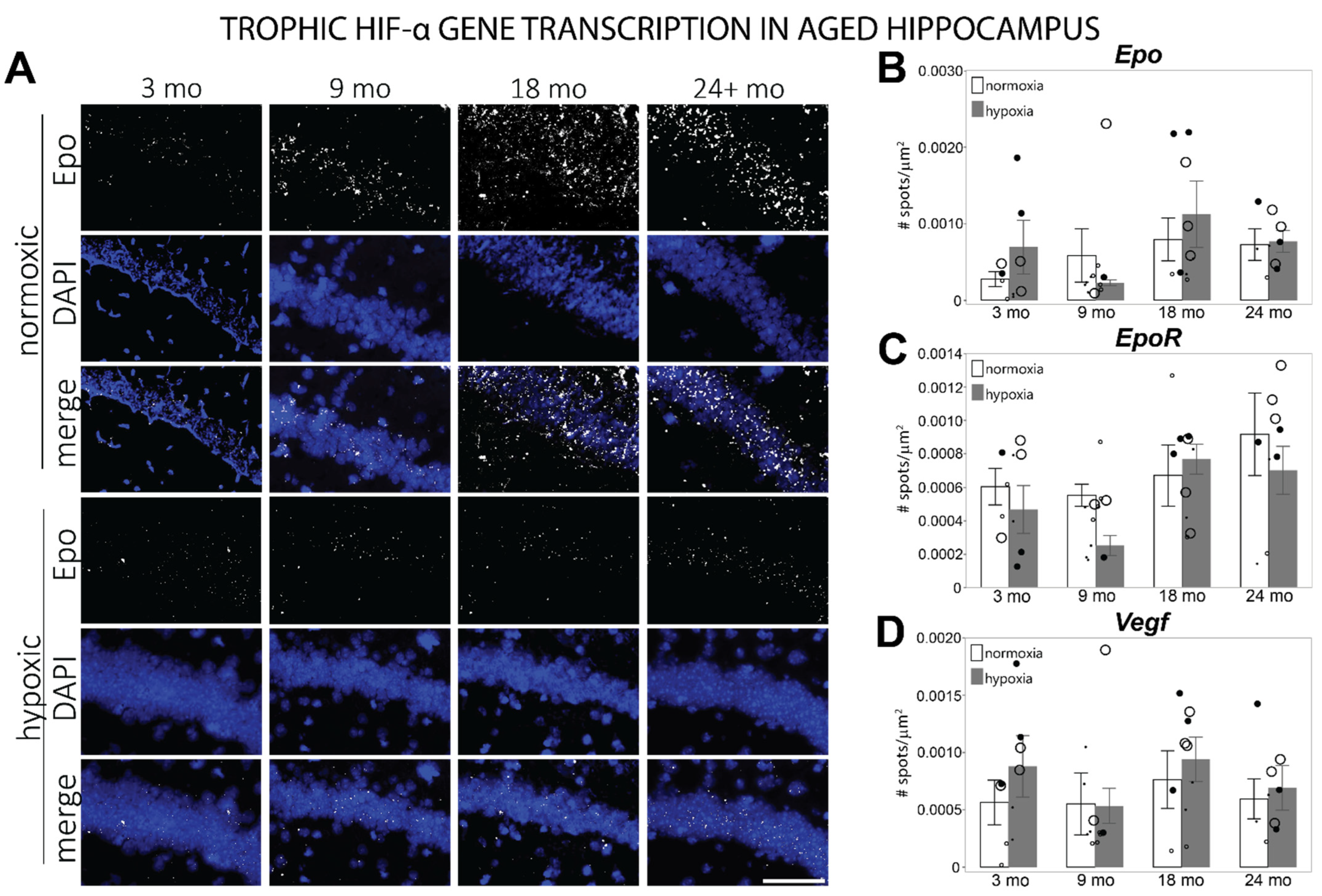
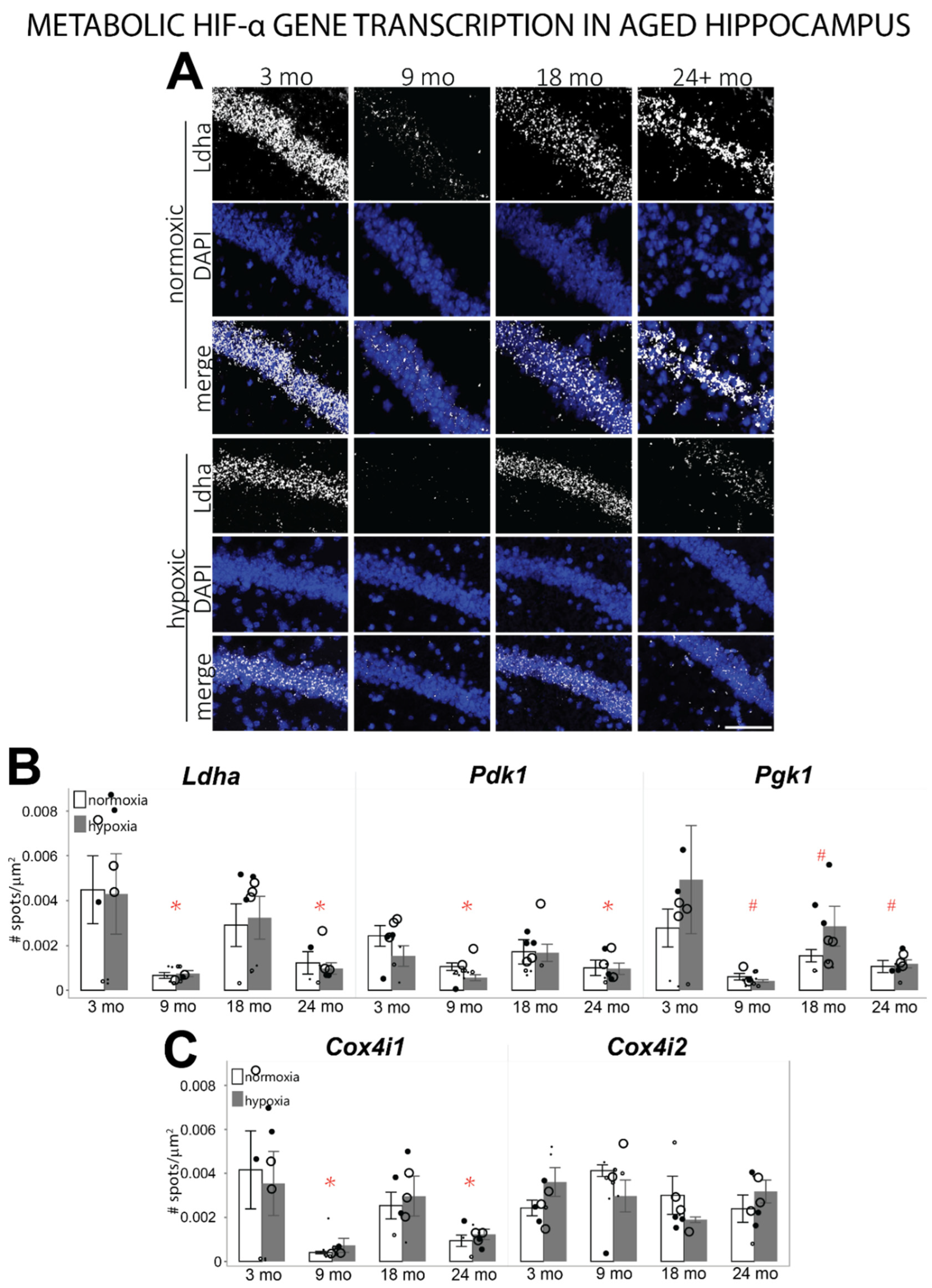
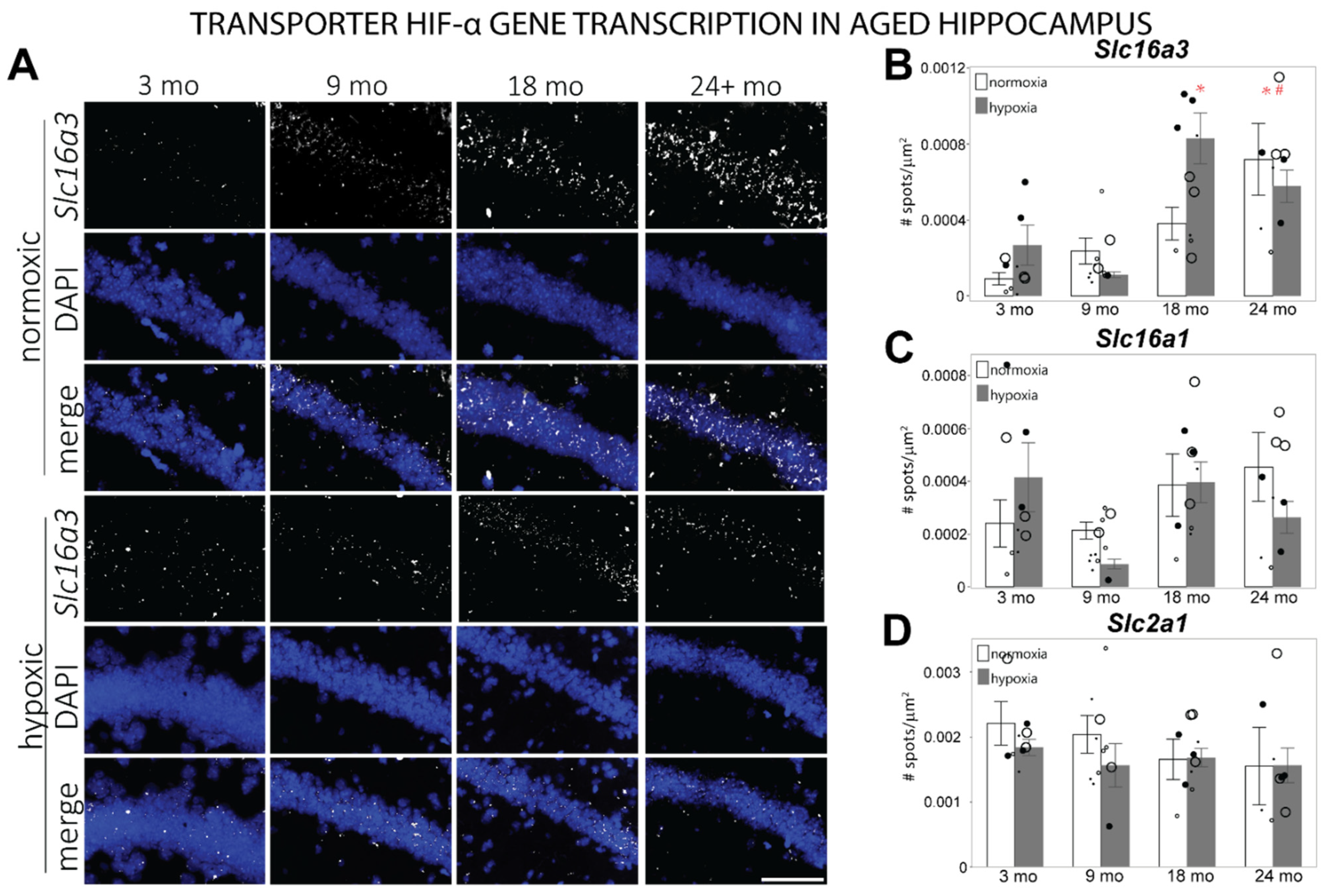
3.3. HIF-α Controlled Transcription Is Altered in Aged Hippocampus at Baseline
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Life Expectancy Data by Country. Available online: http://apps.who.int/gho/data/node.main.688?lang=en (accessed on 22 November 2021).
- Ruchała, M.; Bromińska, B.; Cyrańska-Chyrek, E.; Kuźnar-Kamińska, B.; Kostrzewska, M.; Batura-Gabryel, H. Obstructive sleep apnea and hormones—A novel insight. Arch. Med. Sci. AMS 2017, 13, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan 2014, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, P.G. Understanding anemia of chronic disease. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.; Simone, S.M.; Giovannetti, T.; Floyd, T.F. Cerebral Hypoxia: Its Role in Age-Related Chronic and Acute Cognitive Dysfunction. Anesth. Analg. 2021, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Giovannetti, T.; Price, C.C.; Fanning, M.; Messe, S.; Ratcliffe, S.J.; Lyon, A.; Kasner, S.E.; Seidel, G.; Bavaria, J.E.; Szeto, W.Y.; et al. Cognition and Cerebral Infarction in Older Adults After Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2019, 107, 787–794. [Google Scholar] [CrossRef]
- Li, M.; Bertout, J.A.; Ratcliffe, S.J.; Eckenhoff, M.F.; Simon, M.C.; Floyd, T.F. Acute anemia elicits cognitive dysfunction and evidence of cerebral cellular hypoxia in older rats with systemic hypertension. Anesthesiology 2010, 113, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Riba-Llena, I.; Alvarez-Sabin, J.; Romero, O.; Santamarina, E.; Sampol, G.; Maisterra, O.; Ferre, A.; Montaner, J.; Quintana, M.; Delgado, P. Nighttime hypoxia affects global cognition, memory, and executive function in community-dwelling individuals with hypertension. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2020, 16, 243–250. [Google Scholar] [CrossRef]
- Arjun, R.; Acharya, S.; Shender, B.S.; Rorres, C.; Hrebien, L.; Kam, M. Correlation of Cognitive Scores and the Onset of Hypoxia. Aerosp. Med. Hum. Perform. 2019, 90, 429–439. [Google Scholar] [CrossRef]
- Bouak, F.; Vartanian, O.; Hofer, K.; Cheung, B. Acute Mild Hypoxic Hypoxia Effects on Cognitive and Simulated Aircraft Pilot Performance. Aerosp. Med. Hum. Perform. 2018, 89, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Daniel, J.M.; Dohanich, G.P. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J. Neurosci. 2001, 21, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.N.; Anderson, M.; Snyder, B.; Duong, P.; Trieu, J.; Schreihofer, D.A.; Cunningham, R.L. Chronic Intermittent Hypoxia Induces Hormonal and Male Sexual Behavioral Changes: Hypoxia as an Advancer of Aging Physiology & Behavior. Physiol. Behav. 2018, 189, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, L.C.D.; Davies, K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017, 595, 7275–7309. [Google Scholar] [CrossRef] [PubMed]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional Mitochondria in Health and Disease. Front. Endocrinol. 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef]
- Collins, J.A.; Munoz, J.V.; Patel, T.R.; Loukas, M.; Tubbs, R.S. The anatomy of the aging aorta. Clin. Anat. 2014, 27, 463–466. [Google Scholar] [CrossRef]
- Shaw, K.; Bell, L.; Boyd, K.; Grijseels, D.M.; Clarke, D.; Bonnar, O.; Crombag, H.S.; Hall, C.N. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat. Commun. 2021, 12, 3190. [Google Scholar] [CrossRef]
- Catchlove, S.J.; Parrish, T.B.; Chen, Y.; Macpherson, H.; Hughes, M.E.; Pipingas, A. Regional Cerebrovascular Reactivity and Cognitive Performance in Healthy Aging. J. Exp. Neurosci. 2018, 12, 1179069518785151. [Google Scholar] [CrossRef]
- Lim, D.C.; Brady, D.C.; Soans, R.; Kim, E.Y.; Valverde, L.; Keenan, B.T.; Guo, X.; Kim, W.Y.; Park, M.J.; Galante, R.; et al. Different cyclical intermittent hypoxia severities have different effects on hippocampal microvasculature. J. Appl. Physiol. 2016, 121, 78–88. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsumoto, Y.; Ota, H.; Sugimura, K.; Takahashi, J.; Ito, K.; Miyata, S.; Furukawa, K.; Arai, H.; Fukumoto, Y.; et al. Hippocampal Blood Flow Abnormality Associated with Depressive Symptoms and Cognitive Impairment in Patients with Chronic Heart Failure. Circ. J. 2016, 80, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Tomita, S.; Nakagawa, T.; Ueki, M.; Iwanaga, Y.; Ono, J.; Onodera, M.; Huang, C.L.; Kanenishi, K.; Shimada, A.; et al. Effects of aging and HIF-1alpha deficiency on permeability of hippocampal vessels. Microsc. Res. Tech. 2006, 69, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Temido-Ferreira, M.; Coelho, J.E.; Pousinha, P.A.; Lopes, L.V. Novel Players in the Aging Synapse: Impact on Cognition. J. Caffeine Adenosine Res. 2019, 9, 104–127. [Google Scholar] [CrossRef]
- Lazarov, O.; Hollands, C. Hippocampal neurogenesis: Learning to remember. Prog. Neurobiol. 2016, 138–140, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Squire, L.R. Similarity in form and function of the hippocampus in rodents, monkeys, and humans. Proc. Natl. Acad. Sci. USA 2013, 110, 10365–10370. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.W.; Aggleton, J.P. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001, 2, 51–61. [Google Scholar] [CrossRef]
- Kushwah, N.; Jain, V.; Dheer, A.; Kumar, R.; Prasad, D.; Khan, N. Hypobaric Hypoxia-Induced Learning and Memory Impairment: Elucidating the Role of Small Conductance Ca(2+)-Activated K(+) Channels. Neuroscience 2018, 388, 418–429. [Google Scholar] [CrossRef]
- Feng, J.; Wu, Q.; Zhang, D.; Chen, B.Y. Hippocampal impairments are associated with intermittent hypoxia of obstructive sleep apnea. Chin. Med. J. 2012, 125, 696–701. [Google Scholar]
- Xu, H.; Lu, A.; Sharp, F.R. Regional genome transcriptional response of adult mouse brain to hypoxia. BMC Genom. 2011, 12, 499. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.C.; Baranova, O.; Lin, J.; Pichiule, P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J. Neurosci. 2006, 26, 9471–9481. [Google Scholar] [CrossRef] [PubMed]
- Leiton, C.V.; Chen, E.; Cutrone, A.; Conn, K.; Mellanson, K.; Malik, D.M.; Klingener, M.; Lamm, R.; Cutrone, M.; Petrie, J., IV; et al. Astrocyte HIF-2alpha supports learning in a passive avoidance paradigm under hypoxic stress. Hypoxia 2018, 6, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Iyer, S.; Sataur, A.; Covello, K.L.; Chodosh, L.A.; Simon, M.C. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol. Cell Biol. 2006, 26, 3514–3526. [Google Scholar] [CrossRef]
- Hu, C.J.; Wang, L.Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef]
- Warnecke, C.; Zaborowska, Z.; Kurreck, J.; Erdmann, V.A.; Frei, U.; Wiesener, M.; Eckardt, K.U. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: Erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 1462–1464. [Google Scholar] [CrossRef]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef]
- Semenza, G.L.; Prabhakar, N.R. Neural regulation of hypoxia-inducible factors and redox state drives the pathogenesis of hypertension in a rodent model of sleep apnea. J. Appl. Physiol. 2015, 119, 1152–1156. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Xie, C.; Jiang, C. The role of hypoxia-inducible factors in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 21–32. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y. Epigenetic regulators: Multifunctional proteins modulating hypoxia-inducible factor-α protein stability and activity. Cell. Mol. Life Sci. CMLS 2018, 75, 1043–1056. [Google Scholar] [CrossRef]
- Gruneberg, D.; Montellano, F.A.; Plaschke, K.; Li, L.; Marti, H.H.; Kunze, R. Neuronal prolyl-4-hydroxylase 2 deficiency improves cognitive abilities in a murine model of cerebral hypoperfusion. Exp. Neurol. 2016, 286, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili Tazangi, P.; Moosavi, S.M.; Shabani, M.; Haghani, M. Erythropoietin improves synaptic plasticity and memory deficits by decrease of the neurotransmitter release probability in the rat model of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2015, 130, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Melian, W.; Merceron-Martinez, D.; Pavon-Fuentes, N.; Alberti-Amador, E.; Leon-Martinez, R.; Ledon, N.; Delgado Ocana, S.; Bergado Rosado, J.A. Erythropoietin Promotes Neural Plasticity and Spatial Memory Recovery in Fimbria-Fornix-Lesioned Rats. Neurorehabilit. Neural Repair 2015, 29, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Dayyat, E.A.; Zhang, S.X.; Wang, Y.; Cheng, Z.J.; Gozal, D. Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci. 2012, 13, 77. [Google Scholar] [CrossRef]
- Harris, R.A.; Lone, A.; Lim, H.; Martinez, F.; Frame, A.K.; Scholl, T.J.; Cumming, R.C. Aerobic Glycolysis Is Required for Spatial Memory Acquisition But Not Memory Retrieval in Mice. eNeuro 2019, 6, ENEURO.0389-0318.2019. [Google Scholar] [CrossRef]
- Steinman, M.Q.; Gao, V.; Alberini, C.M. The Role of Lactate-Mediated Metabolic Coupling between Astrocytes and Neurons in Long-Term Memory Formation. Front. Integr. Neurosci. 2016, 10, 10. [Google Scholar] [CrossRef]
- Dalmases, M.; Torres, M.; Márquez-Kisinousky, L.; Almendros, I.; Planas, A.M.; Embid, C.; Martínez-Garcia, M.Á.; Navajas, D.; Farré, R.; Montserrat, J.M. Brain Tissue Hypoxia and Oxidative Stress Induced by Obstructive Apneas is Different in Young and Aged Rats. Sleep 2014, 37, 1249–1256. [Google Scholar] [CrossRef]
- Li, B.; Lu, X.; Moeini, M.; Sakadžić, S.; Thorin, E.; Lesage, F. Atherosclerosis is associated with a decrease in cerebral microvascular blood flow and tissue oxygenation. PLoS ONE 2019, 14, e0221547. [Google Scholar] [CrossRef]
- Iyalomhe, O.; Swierczek, S.; Enwerem, N.; Chen, Y.; Adedeji, M.O.; Allard, J.; Ntekim, O.; Johnson, S.; Hughes, K.; Kurian, P.; et al. The Role of Hypoxia-Inducible Factor 1 in Mild Cognitive Impairment. Cell Mol. Neurobiol. 2017, 37, 969–977. [Google Scholar] [CrossRef]
- Benderro, G.F.; Lamanna, J.C. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res. 2011, 1389, 50–60. [Google Scholar] [CrossRef]
- Ndubuizu, O.I.; Tsipis, C.P.; Li, A.; LaManna, J.C. Hypoxia-inducible factor-1 (HIF-1)-independent microvascular angiogenesis in the aged rat brain. Brain Res. 2010, 1366, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Sandhir, R.; Hamilton, E.S.; Berman, N.E. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J. Neurotrauma 2009, 26, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Ndubuizu, O.I.; Chavez, J.C.; LaManna, J.C. Increased prolyl 4-hydroxylase expression and differential regulation of hypoxia-inducible factors in the aged rat brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R158–R165. [Google Scholar] [CrossRef] [PubMed]
- Rapino, C.; Bianchi, G.; Di Giulio, C.; Centurione, L.; Cacchio, M.; Antonucci, A.; Cataldi, A. HIF-1alpha cytoplasmic accumulation is associated with cell death in old rat cerebral cortex exposed to intermittent hypoxia. Aging Cell 2005, 4, 177–185. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Sha, J.; Li, Y.; Zhang, R.; Bu, N. Expression of hypoxia inducible factor-1alpha and erythropoietin in the hippocampus of aging rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2009, 34, 856–860. [Google Scholar]
- Wu, H.Q.; Wang, H.Q.; Cheng, H.X.; Zhang, G.L.; Zhan, S.Q. Expression of hypoxia inducible factor-1alpha in different brain regions in aged rats. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 1897–1899. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Feng, G.; Mellor, R.H.; Bernstein, M.; Keller-Peck, C.; Nguyen, Q.T.; Wallace, M.; Nerbonne, J.M.; Lichtman, J.W.; Sanes, J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 2000, 28, 41–51. [Google Scholar] [CrossRef]
- Keller-Peck, C.R.; Walsh, M.K.; Gan, W.-B.; Feng, G.; Sanes, J.R.; Lichtman, J.W. Asynchronous Synapse Elimination in Neonatal Motor Units: Studies Using GFP Transgenic Mice. Neuron 2001, 31, 381–394. [Google Scholar] [CrossRef]
- Allen Mouse Brain Atlas. Available online: https://mouse.brain-map.org/experiment/thumbnails/100048576?image_type=atlas (accessed on 1 December 2021).
- Baxter, M.G.; Gallagher, M. Neurobiological substrates of behavioral decline: Models and data analytic strategies for individual differences in aging. Neurobiol. Aging 1996, 17, 491–495. [Google Scholar] [CrossRef]
- Sumien, N.; Sims, M.N.; Taylor, H.J.; Forster, M.J. Profiling psychomotor and cognitive aging in four-way cross mice. Age 2006, 28, 265–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogle, M.E.; Gu, X.; Espinera, A.R.; Wei, L. Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1α. Neurobiol. Dis. 2012, 45, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Rosenbaum, D.M.; Zhuang, J.; Poon, C.; Qin, P.; Rivera, K.; Lepore, J.; Willette, R.N.; Hu, E.; et al. The prolyl 4-hydroxylase inhibitor GSK360A decreases post-stroke brain injury and sensory, motor, and cognitive behavioral deficits. PLoS ONE 2017, 12, e0184049. [Google Scholar] [CrossRef]
- Kant, R.; Bali, A.; Singh, N.; Jaggi, A.S. Prolyl 4 hydroxylase: A critical target in the pathophysiology of diseases. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2013, 17, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, S.; Corcoran, A.E.; Wall, A.; Mukandala, G.; O’Connor, J.J. Acute hypoxic exposure and prolyl-hydroxylase inhibition improves synaptic transmission recovery time from a subsequent hypoxic insult in rat hippocampus. Brain Res. 2018, 1701, 212–218. [Google Scholar] [CrossRef]
- Carrica, L.; Li, L.; Newville, J.; Kenton, J.; Gustus, K.; Brigman, J.; Cunningham, L.A. Genetic inactivation of hypoxia inducible factor 1-alpha (HIF-1α) in adult hippocampal progenitors impairs neurogenesis and pattern discrimination learning. Neurobiol. Learn. Mem. 2019, 157, 79–85. [Google Scholar] [CrossRef]
- Gozal, D.; Nair, D.; Goldbart, A.D. Physical activity attenuates intermittent hypoxia-induced spatial learning deficits and oxidative stress. Am. J. Respir. Crit. Care Med. 2010, 182, 104–112. [Google Scholar] [CrossRef]
- Khuu, M.A.; Nallamothu, T.; Castro-Rivera, C.I.; Arias-Cavieres, A.; Szujewski, C.C.; Garcia Iii, A.J. Stage-dependent effects of intermittent hypoxia influence the outcome of hippocampal adult neurogenesis. Sci. Rep. 2021, 11, 6005. [Google Scholar] [CrossRef]
- Arias-Cavieres, A.; Khuu, M.A.; Nwakudu, C.U.; Barnard, J.E.; Dalgin, G.; Garcia, A.J., 3rd. A HIF1a-Dependent Pro-Oxidant State Disrupts Synaptic Plasticity and Impairs Spatial Memory in Response to Intermittent Hypoxia. eNeuro 2020, 7, ENEURO.0024-20.2020. [Google Scholar] [CrossRef]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef]
- Benitez, S.G.; Castro, A.E.; Patterson, S.I.; Munoz, E.M.; Seltzer, A.M. Hypoxic preconditioning differentially affects GABAergic and glutamatergic neuronal cells in the injured cerebellum of the neonatal rat. PLoS ONE 2014, 9, e102056. [Google Scholar] [CrossRef] [PubMed]
- Boroujerdi, A.; Milner, R. Defining the critical hypoxic threshold that promotes vascular remodeling in the brain. Exp. Neurol. 2015, 263, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Koizumi, S. Hypoxia-independent mechanisms of HIF-1alpha expression in astrocytes after ischemic preconditioning. Glia 2017, 65, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Smeyne, M.; Sladen, P.; Jiao, Y.; Dragatsis, I.; Smeyne, R.J. HIF1alpha is necessary for exercise-induced neuroprotection while HIF2alpha is needed for dopaminergic neuron survival in the substantia nigra pars compacta. Neuroscience 2015, 295, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Taie, S.; Ono, J.; Iwanaga, Y.; Tomita, S.; Asaga, T.; Chujo, K.; Ueki, M. Hypoxia-inducible factor-1 alpha has a key role in hypoxic preconditioning. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2009, 16, 1056–1060. [Google Scholar] [CrossRef]
- Ashok, B.S.; Ajith, T.A.; Sivanesan, S. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin. Exp. Pharmacol. Physiol. 2017, 44, 327–334. [Google Scholar] [CrossRef]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef]
- Yuan, L.B.; Dong, H.L.; Zhang, H.P.; Zhao, R.N.; Gong, G.; Chen, X.M.; Zhang, L.N.; Xiong, L. Neuroprotective effect of orexin-A is mediated by an increase of hypoxia-inducible factor-1 activity in rat. Anesthesiology 2011, 114, 340–354. [Google Scholar] [CrossRef]
- Lourhmati, A.; Buniatian, G.H.; Paul, C.; Verleysdonk, S.; Buecheler, R.; Buadze, M.; Proksch, B.; Schwab, M.; Gleiter, C.H.; Danielyan, L. Age-dependent astroglial vulnerability to hypoxia and glutamate: The role for erythropoietin. PLoS ONE 2013, 8, e77182. [Google Scholar] [CrossRef]
- Hernández, C.C.; Burgos, C.F.; Gajardo, A.H.; Silva-Grecchi, T.; Gavilan, J.; Toledo, J.R.; Fuentealba, J. Neuroprotective effects of erythropoietin on neurodegenerative and ischemic brain diseases: The role of erythropoietin receptor. Neural. Regen. Res. 2017, 12, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Onimaru, H.; Yamagata, A.; Itoh, S.; Matsubara, H.; Imakiire, T.; Nishida, Y.; Kumagai, H. Erythropoietin, a putative neurotransmitter during hypoxia, is produced in RVLM neurons and activates them in neonatal Wistar rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R700–R708. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, D.; Heinrich, R. Alternative Erythropoietin Receptors in the Nervous System. J. Clin. Med. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.-X. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008, 582, 359–364. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef]
- Yeo, E.-J. Hypoxia and aging. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Forster, M.J.; Popper, M.D.; Retz, K.C.; Lal, H. Age differences in acquisition and retention of one-way avoidance learning in C57BL/6NNia and autoimmune mice. Behav. Neural Biol. 1988, 49, 139–151. [Google Scholar] [CrossRef]
- Pereda, D.; Al-Osta, I.; Okorocha, A.E.; Easton, A.; Hartell, N.A. Changes in presynaptic calcium signalling accompany age-related deficits in hippocampal LTP and cognitive impairment. Aging Cell 2019, 18, e13008. [Google Scholar] [CrossRef]
- Morrison, J.H.; Baxter, M.G. The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012, 13, 240–250. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, J.; North, B.J.; Wei, W. Functional analyses of major cancer-related signaling pathways in Alzheimer’s disease etiology. Biochim. Et Biophys. Acta Rev. Cancer 2017, 1868, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.A.; Ogren, J.A.; Abouzeid, C.M.; Macey, P.M.; Sairafian, K.G.; Saharan, P.S.; Thompson, P.M.; Fonarow, G.C.; Hamilton, M.A.; Harper, R.M.; et al. Regional hippocampal damage in heart failure. Eur. J. Heart Fail. 2015, 17, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Cheng, H.M.; Mitchell, G.F.; Sung, S.H.; Chen, C.H.; Pan, W.H.; Hwang, A.C.; Chen, L.K.; Wang, P.N. Carotid Flow Velocities and Blood Pressures Are Independently Associated With Cognitive Function. Am. J. Hypertens 2019, 32, 289–297. [Google Scholar] [CrossRef] [PubMed]
- LaManna, J.C.; Vendel, L.M.; Farrell, R.M. Brain adaptation to chronic hypobaric hypoxia in rats. J. Appl. Physiol. 1992, 72, 2238–2243. [Google Scholar] [CrossRef]
- Moeini, M.; Lu, X.; Avti, P.K.; Damseh, R.; Bélanger, S.; Picard, F.; Boas, D.; Kakkar, A.; Lesage, F. Compromised microvascular oxygen delivery increases brain tissue vulnerability with age. Sci. Rep. 2018, 8, 8219. [Google Scholar] [CrossRef]
- Tsien, J.Z.; Huerta, P.T.; Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 1996, 87, 1327–1338. [Google Scholar] [CrossRef]
- Wall, A.M.; Corcoran, A.E.; O’Halloran, K.D.; O’Connor, J.J. Effects of prolyl-hydroxylase inhibition and chronic intermittent hypoxia on synaptic transmission and plasticity in the rat CA1 and dentate gyrus. Neurobiol. Dis. 2014, 62, 8–17. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Guo, H.; Zhang, G.; Zhang, R.; Zhan, S. Increased hypoxia-inducible factor 1alpha expression in rat brain tissues in response to aging. Neural Regen. Res. 2012, 7, 778–782. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Caetano, M.; Barbosa, R.M.; Laranjinha, J. Age-Dependent Impairment of Neurovascular and Neurometabolic Coupling in the Hippocampus. Front. Physiol. 2018, 9, 913. [Google Scholar] [CrossRef]
- Dodson, M.; Darley-Usmar, V.; Zhang, J. Cellular metabolic and autophagic pathways: Traffic control by redox signaling. Free Radic. Biol. Med. 2013, 63, 207–221. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Swerdlow, R.H. Mitochondrial links between brain aging and Alzheimer’s disease. Transl. Neurodegener. 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Bangen, K.J.; Clark, A.L.; Edmonds, E.C.; Evangelista, N.D.; Werhane, M.L.; Thomas, K.R.; Locano, L.E.; Tran, M.; Zlatar, Z.Z.; Nation, D.A.; et al. Cerebral Blood Flow and Amyloid-beta Interact to Affect Memory Performance in Cognitively Normal Older Adults. Front. Aging Neurosci. 2017, 9, 181. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snyder, B.; Wu, H.-K.; Tillman, B.; Floyd, T.F. Aged Mouse Hippocampus Exhibits Signs of Chronic Hypoxia and an Impaired HIF-Controlled Response to Acute Hypoxic Exposures. Cells 2022, 11, 423. https://doi.org/10.3390/cells11030423
Snyder B, Wu H-K, Tillman B, Floyd TF. Aged Mouse Hippocampus Exhibits Signs of Chronic Hypoxia and an Impaired HIF-Controlled Response to Acute Hypoxic Exposures. Cells. 2022; 11(3):423. https://doi.org/10.3390/cells11030423
Chicago/Turabian StyleSnyder, Brina, Hua-Kang Wu, Brianna Tillman, and Thomas F. Floyd. 2022. "Aged Mouse Hippocampus Exhibits Signs of Chronic Hypoxia and an Impaired HIF-Controlled Response to Acute Hypoxic Exposures" Cells 11, no. 3: 423. https://doi.org/10.3390/cells11030423
APA StyleSnyder, B., Wu, H.-K., Tillman, B., & Floyd, T. F. (2022). Aged Mouse Hippocampus Exhibits Signs of Chronic Hypoxia and an Impaired HIF-Controlled Response to Acute Hypoxic Exposures. Cells, 11(3), 423. https://doi.org/10.3390/cells11030423





