Cathelicidin-Related Antimicrobial Peptide Negatively Regulates Bacterial Endotoxin-Induced Glial Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Model of Neuroinflammation
2.3. Glial Cell Culture
2.4. Immunofluorescence Staining
2.5. Quantification of Immunostaining
2.6. Measurement of Nitric Oxide Production
2.7. Assessment of Cell Viability
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Quantitative Real-Time Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results
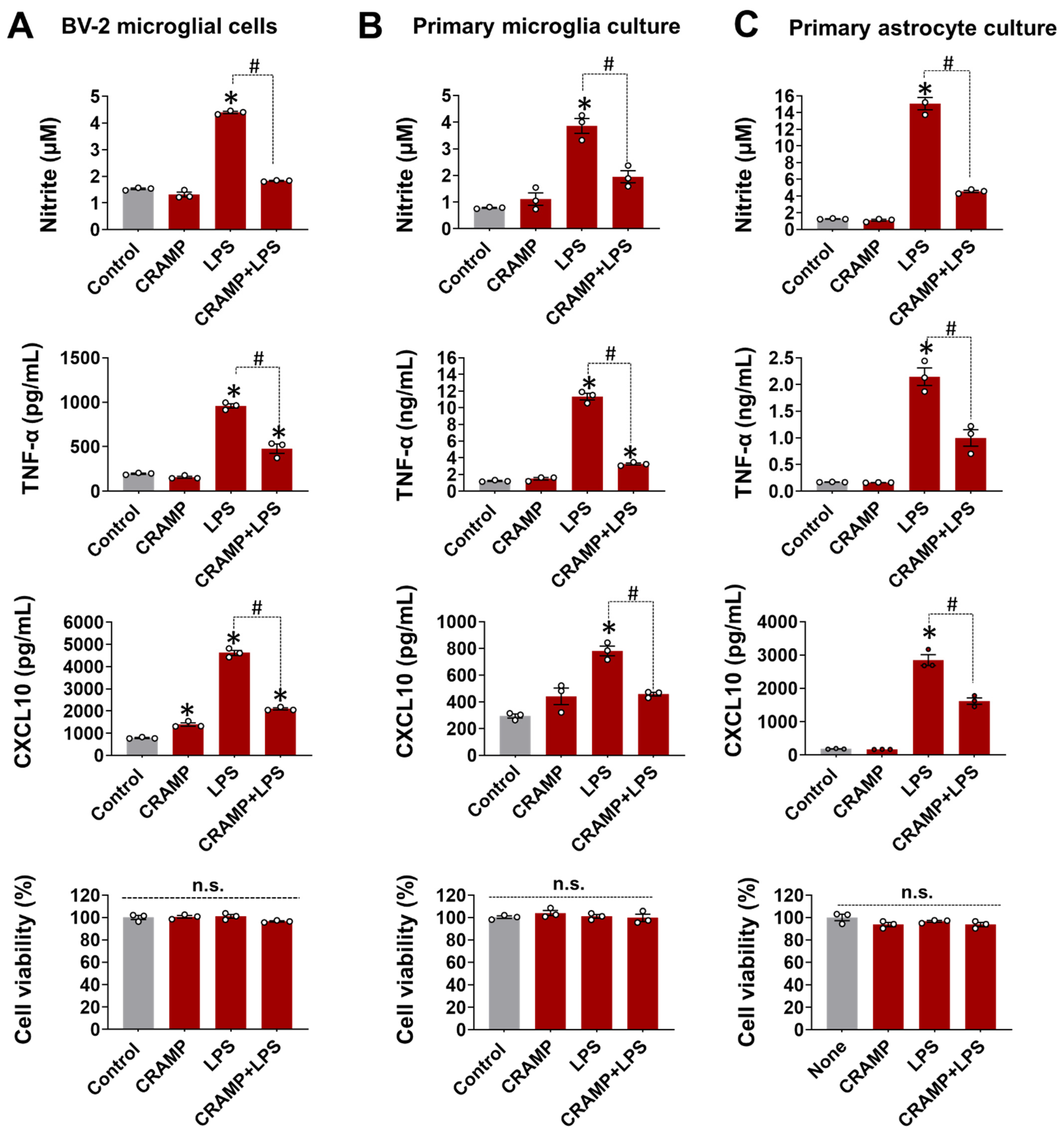
3.1. CRAMP Peptide Treatment Attenuates LPS-Induced Production of Inflammatory Mediators in Cultured Glial Cells
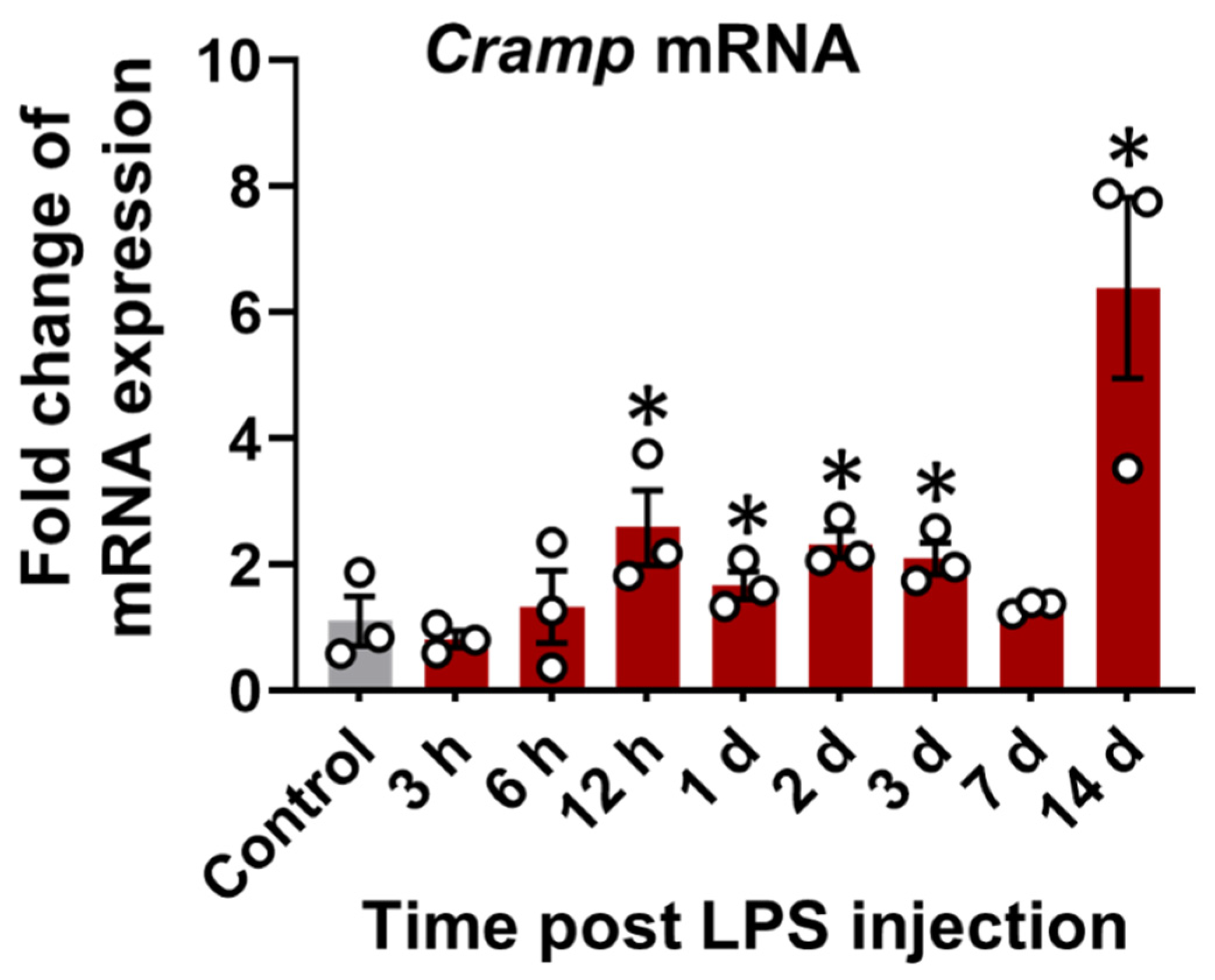
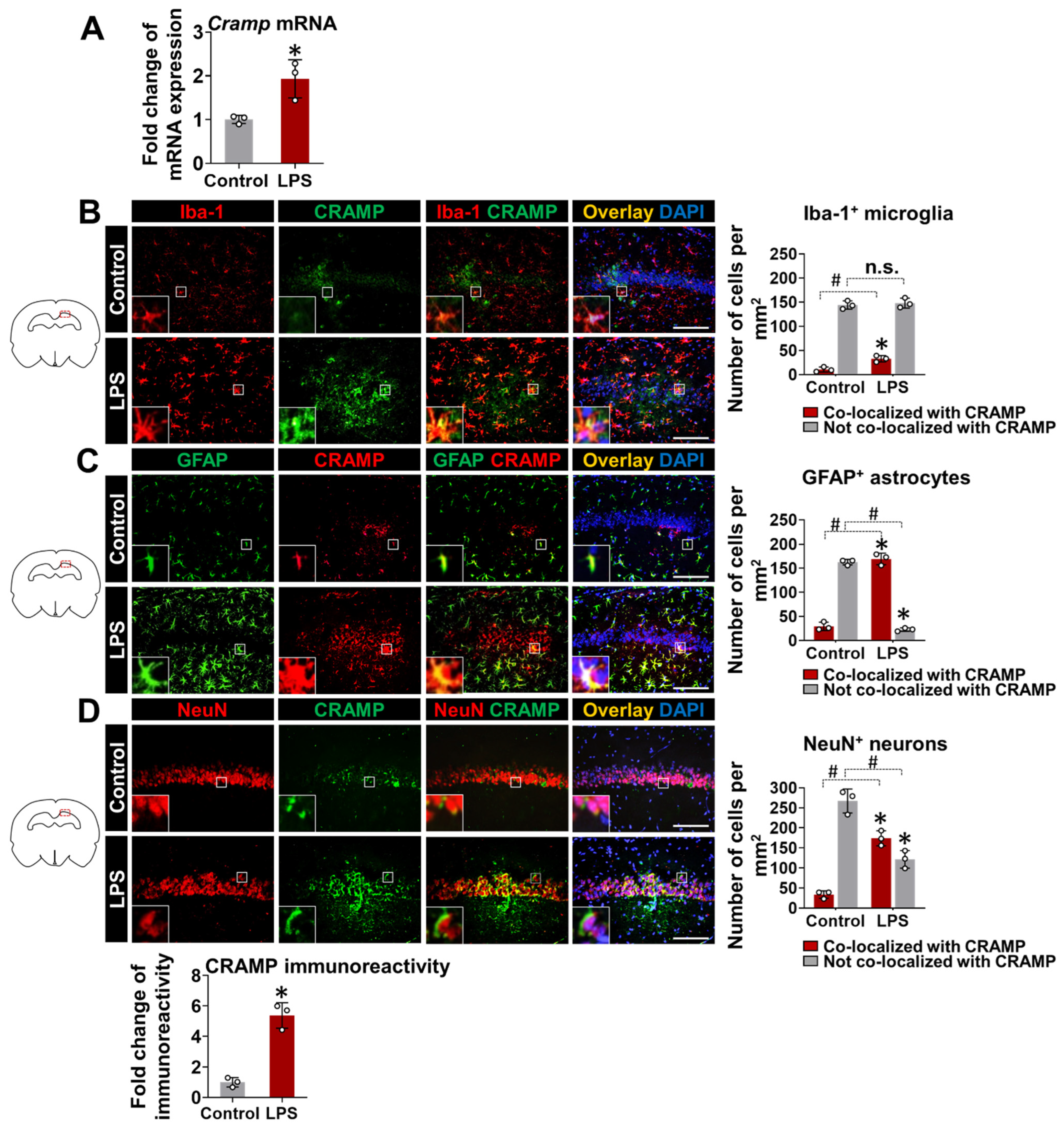
3.2. Cramp mRNA and CRAMP Protein Are Expressed in the Brain of LPS-Injected Mice
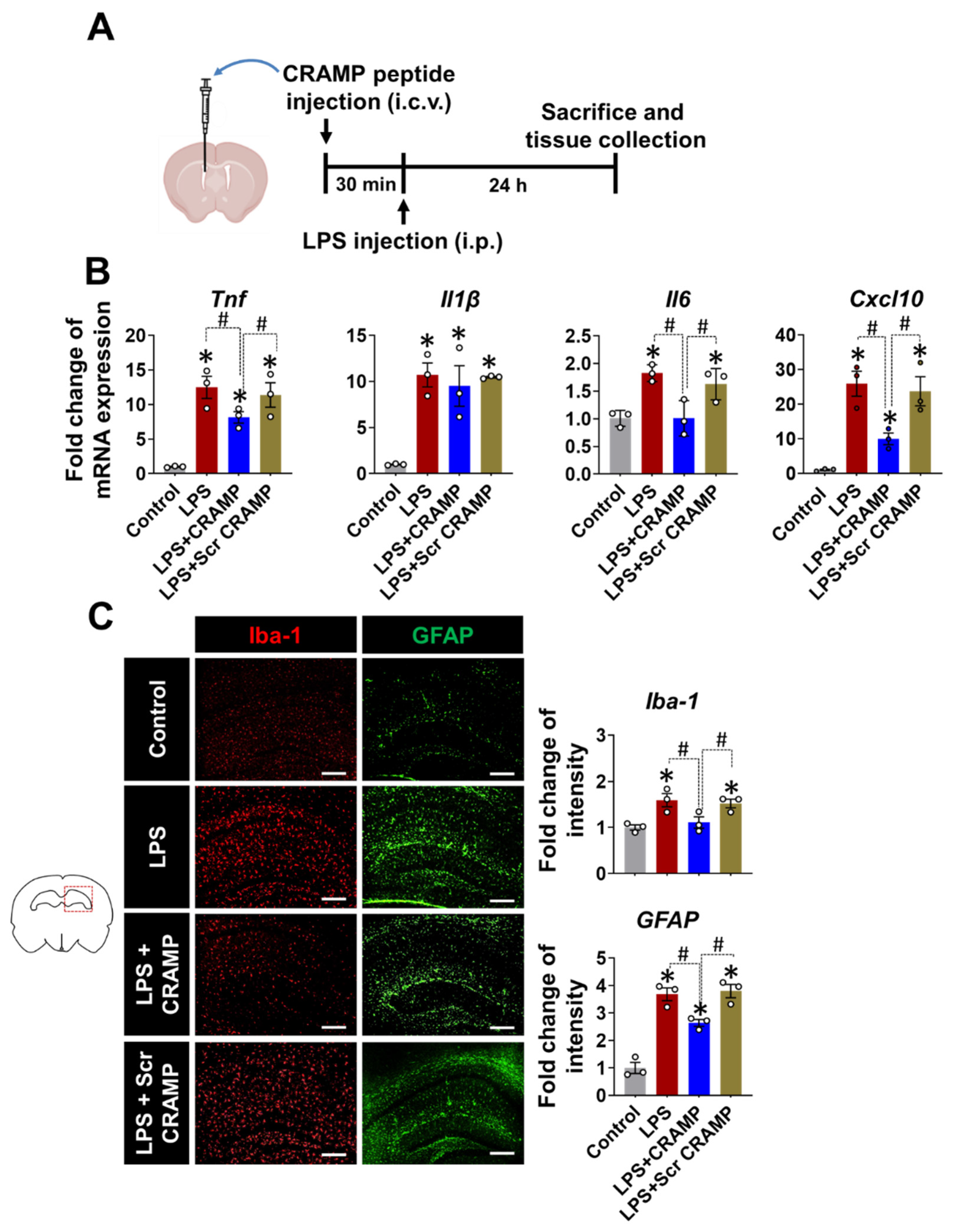
3.3. CRAMP Peptide Administration Attenuates Neuroinflammation in LPS-Injected Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bucki, R.; Leszczynska, K.; Namiot, A.; Sokolowski, W. Cathelicidin LL-37: A multitask antimicrobial peptide. Arch. Immunol. Ther. Exp. 2010, 58, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Henzler Wildman, K.A.; Lee, D.K.; Ramamoorthy, A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 2003, 42, 6545–6558. [Google Scholar] [CrossRef] [PubMed]
- Schromm, A.B.; Paulowski, L.; Kaconis, Y.; Kopp, F.; Koistinen, M.; Donoghue, A.; Keese, S.; Nehls, C.; Wernecke, J.; Garidel, P.; et al. Cathelicidin and PMB neutralize endotoxins by multifactorial mechanisms including LPS interaction and targeting of host cell membranes. Proc. Natl. Acad. Sci. USA 2021, 118, e2101721118. [Google Scholar] [CrossRef] [PubMed]
- Golec, M. Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide. Ann. Agric. Environ. Med. 2007, 14, 1–4. [Google Scholar]
- Suzuki, K.; Murakami, T.; Kuwahara-Arai, K.; Tamura, H.; Hiramatsu, K.; Nagaoka, I. Human anti-microbial cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of endothelial cells. Int. Immunol. 2011, 23, 185–193. [Google Scholar] [CrossRef]
- Nagaoka, I.; Hirota, S.; Niyonsaba, F.; Hirata, M.; Adachi, Y.; Tamura, H.; Tanaka, S.; Heumann, D. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin. Diagn. Lab. Immunol. 2002, 9, 972–982. [Google Scholar] [CrossRef]
- Nagaoka, I.; Hirota, S.; Niyonsaba, F.; Hirata, M.; Adachi, Y.; Tamura, H.; Heumann, D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14+ cells. J. Immunol. 2001, 167, 3329–3338. [Google Scholar] [CrossRef]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef]
- Mookherjee, N.; Lippert, D.N.; Hamill, P.; Falsafi, R.; Nijnik, A.; Kindrachuk, J.; Pistolic, J.; Gardy, J.; Miri, P.; Naseer, M.; et al. Intracellular receptor for human host defense peptide LL-37 in monocytes. J. Immunol. 2009, 183, 2688–2696. [Google Scholar] [CrossRef]
- Bhusal, A.; Nam, Y.; Seo, D.; Rahman, M.H.; Hwang, E.M.; Kim, S.C.; Lee, W.H.; Suk, K. Cathelicidin-related antimicrobial peptide promotes neuroinflammation through astrocyte-microglia communication in experimental autoimmune encephalomyelitis. Glia 2022, 70, 1902–1926. [Google Scholar] [CrossRef]
- Smith, K.J.; Minns, D.; McHugh, B.J.; Holloway, R.K.; O’Connor, R.; Williams, A.; Melrose, L.; McPherson, R.; Miron, V.E.; Davidson, D.J.; et al. The antimicrobial peptide cathelicidin drives development of experimental autoimmune encephalomyelitis in mice by affecting Th17 differentiation. PLoS Biol. 2022, 20, e3001554. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Kim, J.H.; Song, G.J.; Seo, M.; Hwang, E.M.; Suk, K. Astrocytic Orosomucoid-2 Modulates Microglial Activation and Neuroinflammation. J. Neurosci. 2017, 37, 2878–2894. [Google Scholar] [CrossRef]
- Saura, J.; Tusell, J.M.; Serratosa, J. High-yield isolation of murine microglia by mild trypsinization. Glia 2003, 44, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Chang, C.Y.; Jeon, S.B.; Yoon, H.J.; Ahn, Y.H.; Kim, H.S.; Kim, I.H.; Jeon, S.H.; Johnson, R.S.; Park, E.J. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat. Commun. 2015, 6, 6340. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kwon, O.; Bhusal, A.; Lee, J.; Hwang, E.M.; Ryu, H.; Park, J.Y.; Suk, K. Neuroinflammation Induced by Transgenic Expression of Lipocalin-2 in Astrocytes. Front. Cell. Neurosci. 2022, 16, 839118. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. Little peptide, big effects: The role of LL-37 in inflammation and autoimmune disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef]
- Elssner, A.; Duncan, M.; Gavrilin, M.; Wewers, M.D. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J. Immunol. 2004, 172, 4987–4994. [Google Scholar] [CrossRef]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Nizet, V.; Gallo, R.L. Cathelicidins and innate defense against invasive bacterial infection. Scand. J. Infect. Dis. 2003, 35, 670–676. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhusal, A.; Nam, Y.; Seo, D.; Lee, W.-H.; Suk, K. Cathelicidin-Related Antimicrobial Peptide Negatively Regulates Bacterial Endotoxin-Induced Glial Activation. Cells 2022, 11, 3886. https://doi.org/10.3390/cells11233886
Bhusal A, Nam Y, Seo D, Lee W-H, Suk K. Cathelicidin-Related Antimicrobial Peptide Negatively Regulates Bacterial Endotoxin-Induced Glial Activation. Cells. 2022; 11(23):3886. https://doi.org/10.3390/cells11233886
Chicago/Turabian StyleBhusal, Anup, Youngpyo Nam, Donggun Seo, Won-Ha Lee, and Kyoungho Suk. 2022. "Cathelicidin-Related Antimicrobial Peptide Negatively Regulates Bacterial Endotoxin-Induced Glial Activation" Cells 11, no. 23: 3886. https://doi.org/10.3390/cells11233886
APA StyleBhusal, A., Nam, Y., Seo, D., Lee, W.-H., & Suk, K. (2022). Cathelicidin-Related Antimicrobial Peptide Negatively Regulates Bacterial Endotoxin-Induced Glial Activation. Cells, 11(23), 3886. https://doi.org/10.3390/cells11233886






