Comparison of the Immune Response in Vaccinated People Positive and Negative to SARS-CoV-2 Employing FTIR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Medical Data Collection
2.3. Saliva Sample Pre-Processing and Spectral Analysis
2.4. Immune Response Analysis through FTIR Spectroscopy
3. Results
3.1. Integration of the Population
3.2. Analysis of Medical Data
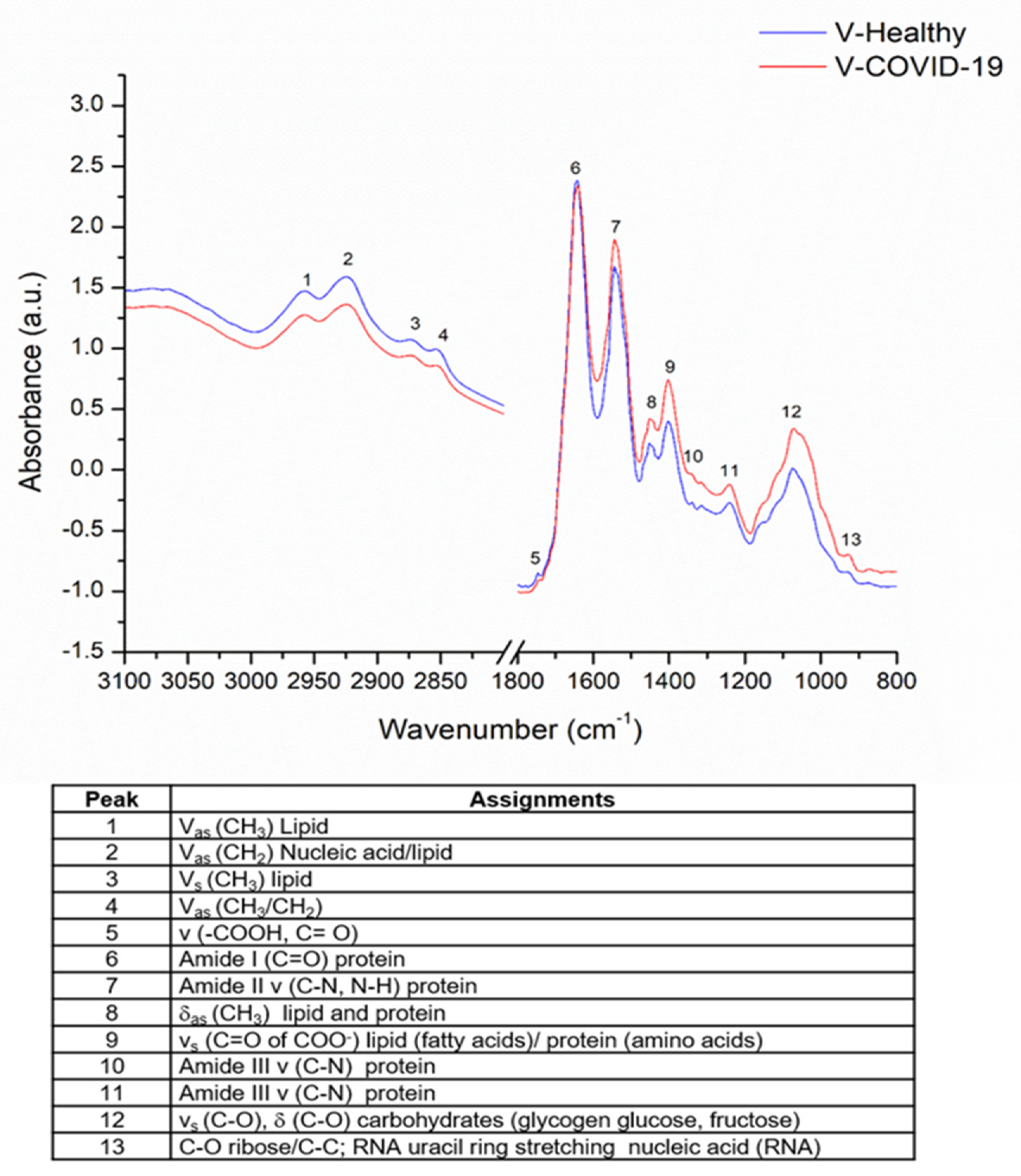
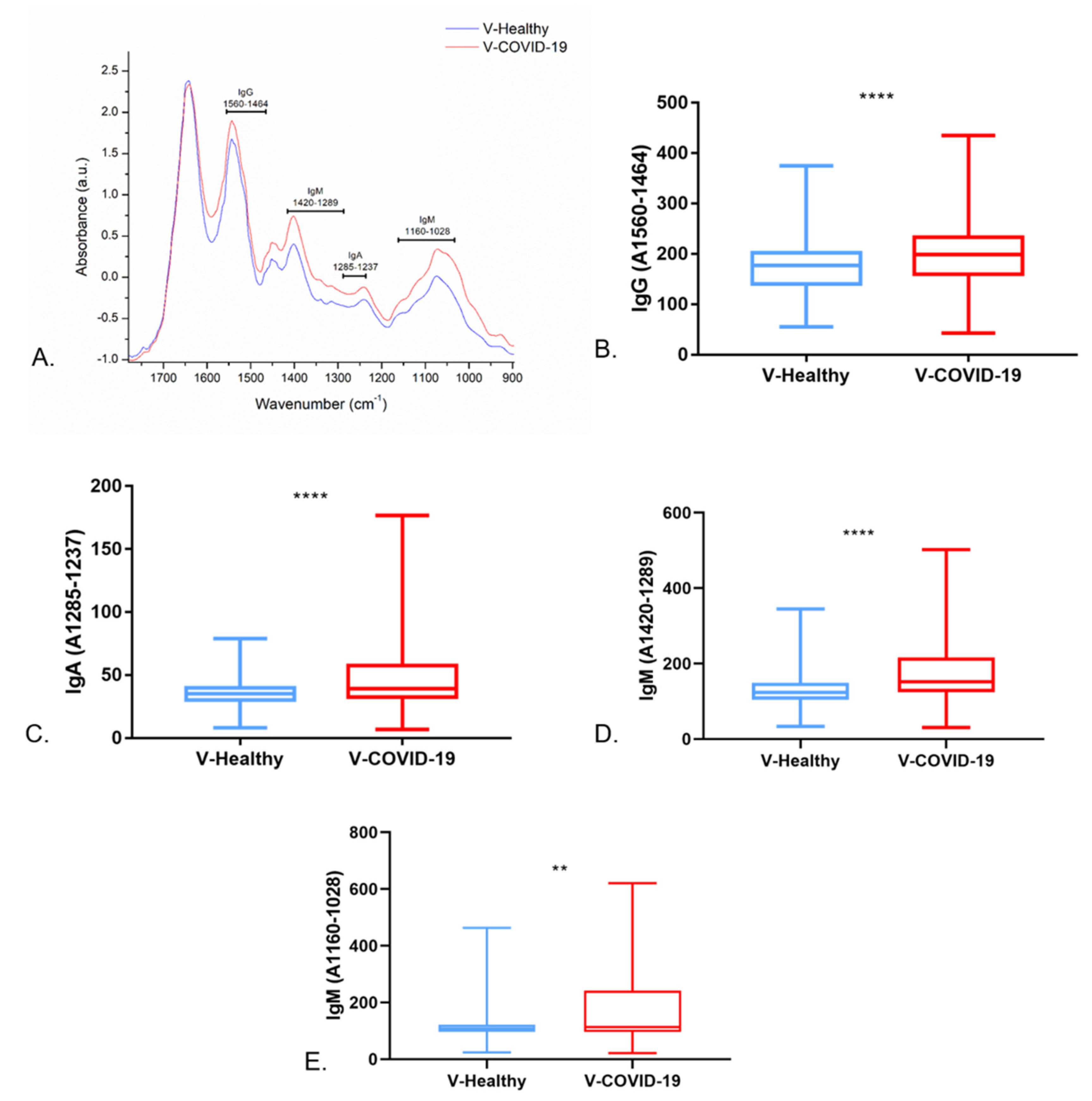
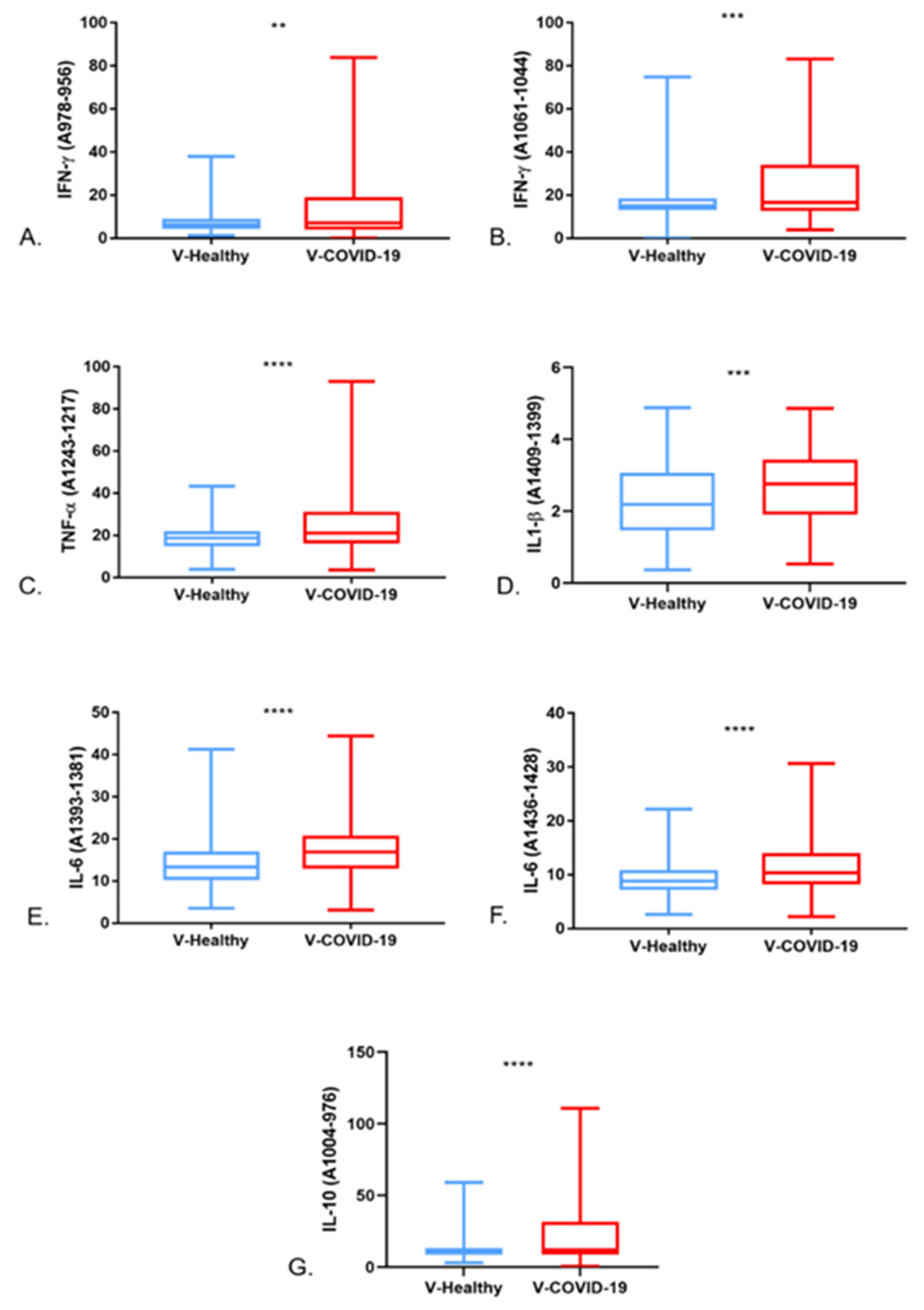
3.3. Saliva FTIR Spectra Analysis and Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciaccio, M.; Agnello, L. Biochemical biomarkers alterations in Coronavirus Disease 2019 (COVID-19). Diagnosis 2020, 7, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Güler, G.; Özdemir, H.; Omar, D.; Akdoğan, G. Coronavirus disease 2019 (COVID-19): Biophysical and biochemical aspects of SARS-CoV-2 and general characteristics. Prog. Biophys. Mol. Biol. 2021, 164, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Ashwlayan, V.D.; Antlash, C.; Imran, M.; Asdaq, S.M.B.; Alshammari, M.K.; Alomani, M.; Alzahrani, E.; Sharma, D.; Tomar, R.; Arora, M.K. Insight into the biological impact of COVID-19 and its vaccines on human health. Saudi J. Biol. Sci. 2022, 29, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Vardhana, S.; Baldo, L.; Morice, W.G.; Wherry, E.J. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci. Immunol. 2022, 7, eabo1303. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, S.K.; Maurya, V.K.; Tripathi, A.K. Progress and Challenges Toward Generation and Maintenance of Long-Lived Memory T Lymphocyte Responses During COVID-19. Front. Immunol. 2022, 12, 804808. [Google Scholar] [CrossRef]
- Toor, S.M.; Saleh, R.; Nair, V.S.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2020, 162, 30–43. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Board on Health Promotion and Disease Prevention. Vaccine Safety Forum: Summaries of Two Workshops; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Howard, F.H.N.; Kwan, A.; Winder, N.; Mughal, A.; Collado-Rojas, C.; Muthana, M. Understanding Immune Responses to Viruses—Do Underlying Th1/Th2 Cell Biases Predict Outcome? Viruses 2022, 14, 1493. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.-D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Karaba, A.H.; Zhu, X.; Benner, S.E.; Akinde, O.; Eby, Y.; Wang, K.H.; Saraf, S.; Garonzik-Wang, J.M.; Klein, S.L.; Bailey, J.R.; et al. Higher Proinflammatory Cytokines Are Associated With Increased Antibody Titer After a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients. Transplantation 2022, 106, 835–841. [Google Scholar] [CrossRef]
- Kruse, R.; Essén-Gustavsson, B.; Fossum, C.; Jensen-Waern, M. Blood concentrations of the cytokines IL-1beta, IL-6, IL-10, TNF-alpha and IFN-gamma during experimentally induced swine dysentery. Acta Vet. Scand. 2008, 50, 32. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Chapter 24, The Adaptive Immune System; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Al-Ibraheemi, J.S.S.; Al-Saeedi, A.-S. The relationship between IgG and IgM levels and severity of symptoms in COVID-19 patients confirmed by rapid antigen test. J. Med. Life 2021, 14, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Scurr, M.J.; Zelek, W.M.; Lippiatt, G.; Somerville, M.; Burnell, S.E.A.; Capitani, L.; Davies, K.; Lawton, H.; Tozer, T.; Rees, T.; et al. Whole blood-based measurement of SARS-CoV-2-specific T cells reveals asymptomatic infection and vaccine immunogenicity in healthy subjects and patients with solid-organ cancers. Immunology 2021, 165, 250–259. [Google Scholar] [CrossRef]
- Pourgholaminejad, A.; Pahlavanneshan, S.; Basiri, M. COVID-19 immunopathology with emphasis on Th17 response and cell-based immunomodulation therapy: Potential targets and challenges. Scand. J. Immunol. 2021, 95, e13131. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, Z.; Tan, C.; Zhou, H.; Hu, Y.; Shen, G.; Zhu, P.; Yang, G.; Xie, X. Changes of serum IL-10, IL-1β, IL-6, MCP-1, TNF-α, IP-10 and IL-4 in COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14462. [Google Scholar] [CrossRef]
- Brümmer, L.E.; Katzenschlager, S.; McGrath, S.; Schmitz, S.; Gaeddert, M.; Erdmann, C.; Bota, M.; Grilli, M.; Larmann, J.; Weigand, M.A.; et al. Accuracy of rapid point-of-care antigen-based diagnostics for SARS-CoV-2: An updated systematic review and meta-analysis with meta-regression analyzing influencing factors. PLOS Med. 2022, 19, e1004011. [Google Scholar] [CrossRef]
- Chow, F.W.-N.; Chan, T.T.-Y.; Tam, A.R.; Zhao, S.; Yao, W.; Fung, J.; Cheng, F.K.-K.; Lo, G.C.-S.; Chu, S.; Aw-Yong, K.L.; et al. A Rapid, Simple, Inexpensive, and Mobile Colorimetric Assay COVID-19-LAMP for Mass On-Site Screening of COVID-19. Int. J. Mol. Sci. 2020, 21, 5380. [Google Scholar] [CrossRef]
- Martinez-Cuazitl, A.; Vazquez-Zapien, G.J.; Sanchez-Brito, M.; Limon-Pacheco, J.H.; Guerrero-Ruiz, M.; Garibay-Gonzalez, F.; Delgado-Macuil, R.J.; de Jesus, M.G.G.; Corona-Perezgrovas, M.A.; Pereyra-Talamantes, A.; et al. ATR-FTIR spectrum analysis of saliva samples from COVID-19 positive patients. Sci. Rep. 2021, 11, 19980. [Google Scholar] [CrossRef] [PubMed]
- Benezzeddine-Boussaidi, L.; Cazorla, G.; Melin, A.-M. Validation for quantification of immunoglobulins by Fourier transform infrared spectrometry. Clin. Chem. Lab. Med. 2009, 47, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, M.; Wang, Y.; Peng, S.; Chen, Y.; Zhang, D.; Zhang, D.; Guo, Y.; Wang, X.; Luo, H.; et al. Fast Screening and Primary Diagnosis of COVID-19 by ATR–FT-IR. Anal. Chem. 2021, 93, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Paschotto, D.R.; Pupin, B.; Bhattacharjee, T.T.; Soares, L.E.S. Saliva Preparation Method Exploration for ATR-FTIR Spectroscopy: Towards Bio-fluid Based Disease Diagnosis. Anal. Sci. 2020, 36, 1059–1064. [Google Scholar] [CrossRef]
- Ferreira, I.C.C.; Aguiar, E.M.G.; Silva, A.T.F.; Santos, L.L.D.; Cardoso-Sousa, L.; Araújo, T.G.; Santos, D.W.; Goulart, L.R.; Sabino-Silva, R.; Maia, Y.C.P. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy Analysis of Saliva for Breast Cancer Diagnosis. J. Oncol. 2020, 2020, 4343590. [Google Scholar] [CrossRef]
- Available online: https://es.statista.com/estadisticas/1207394/dosis-confirmadas-vacunas-covid-mexico/ (accessed on 12 December 2021).
- Huang, I.; Pranata, R.; Lim, M.A.; Oehadian, A.; Alisjahbana, B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: A meta-analysis. Ther. Adv. Respir. Dis. 2020, 14, 1753466620937175. [Google Scholar] [CrossRef]
- Zurac, S.; Nichita, L.; Mateescu, B.; Mogodici, C.; Bastian, A.; Popp, C.; Cioplea, M.; Socoliuc, C.; Constantin, C.; Neagu, M. COVID-19 vaccination and IgG and IgA antibody dynamics in healthcare workers. Mol. Med. Rep. 2021, 24, 578. [Google Scholar] [CrossRef]
- Wisnewski, A.V.; Campillo Luna, J.; Redlich, C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef]
- Speletas, M.; Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Mouchtouri, V.A.; Nikoulis, D.J.; Tsakona, M.; Kyritsi, M.A.; Peristeri, A.-M.; Avakian, I.; et al. Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies. Vaccines 2022, 10, 316. [Google Scholar] [CrossRef]
- Bertoletti, A.; Le Bert, N.; Qui, M.; Tan, A.T. SARS-CoV-2-specific T cells in infection and vaccination. Cell. Mol. Immunol. 2021, 18, 2307–2312. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Brown, C.M.; McMahan, K.A.; Yu, J.; Liu, J.; Jacob-Dolan, C.; Chandrashekar, A.; Tierney, D.; Ansel, J.L.; Rowe, M.; et al. Characterization of immune responses in fully vaccinated individuals after breakthrough infection with the SARS-CoV-2 delta variant. Sci. Transl. Med. 2022, 14, eabn6150. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front. Public Health 2022, 10, 833967. [Google Scholar] [CrossRef] [PubMed]
- Kudlay, D.; Kofiadi, I.; Khaitov, M. Peculiarities of the T Cell Immune Response in COVID-19. Vaccines 2022, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.-W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2020, 42, 3–5. [Google Scholar] [CrossRef]
- Cauchois, R.; Koubi, M.; Delarbre, D.; Manet, C.; Carvelli, J.; Blasco, V.B.; Jean, R.; Fouche, L.; Bornet, C.; Pauly, V.; et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 18951–18953. [Google Scholar] [CrossRef]
- Su, C.-M.; Wang, L.; Yoo, D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 2021, 11, 13464. [Google Scholar] [CrossRef]
- Bell, L.C.; Meydan, C.; Kim, J.; Foox, J.; Butler, D.; Mason, C.E.; Shapira, S.D.; Noursadeghi, M.; Pollara, G. Transcriptional response modules characterize IL-1β and IL-6 activity in COVID-19. iScience 2021, 24, 101896. [Google Scholar] [CrossRef]

| Group | Gender | Age (Years) | |
|---|---|---|---|
| Category | Sample | ||
| V-COVID-19 | Male | 28 | 52.2 ± 17 |
| Female | 22 | ||
| V-Healthy | Male | 77 | 48 ± 15 |
| Female | 58 | ||
| Vaccine | V-COVID-19 n (%) | V-Healthy n (%) |
|---|---|---|
| Oxford, AstraZeneca | 15 (30) | 40 (29.6) |
| Cansino | 12 (24) | 39 (28.9) |
| BioNTech, Pfizer | 9 (18) | 32 (23.7) |
| Sputnik V | 2 (4) | 12 (8.9) |
| CoronaVac | 4 (8) | 2 (1.5) |
| Covaxin | 0 (0) | 2 (1.5) |
| Johnson & Johnson | 1 (2) | 1 (0.7) |
| Disown | 7 (14) | 7 (5.2) |
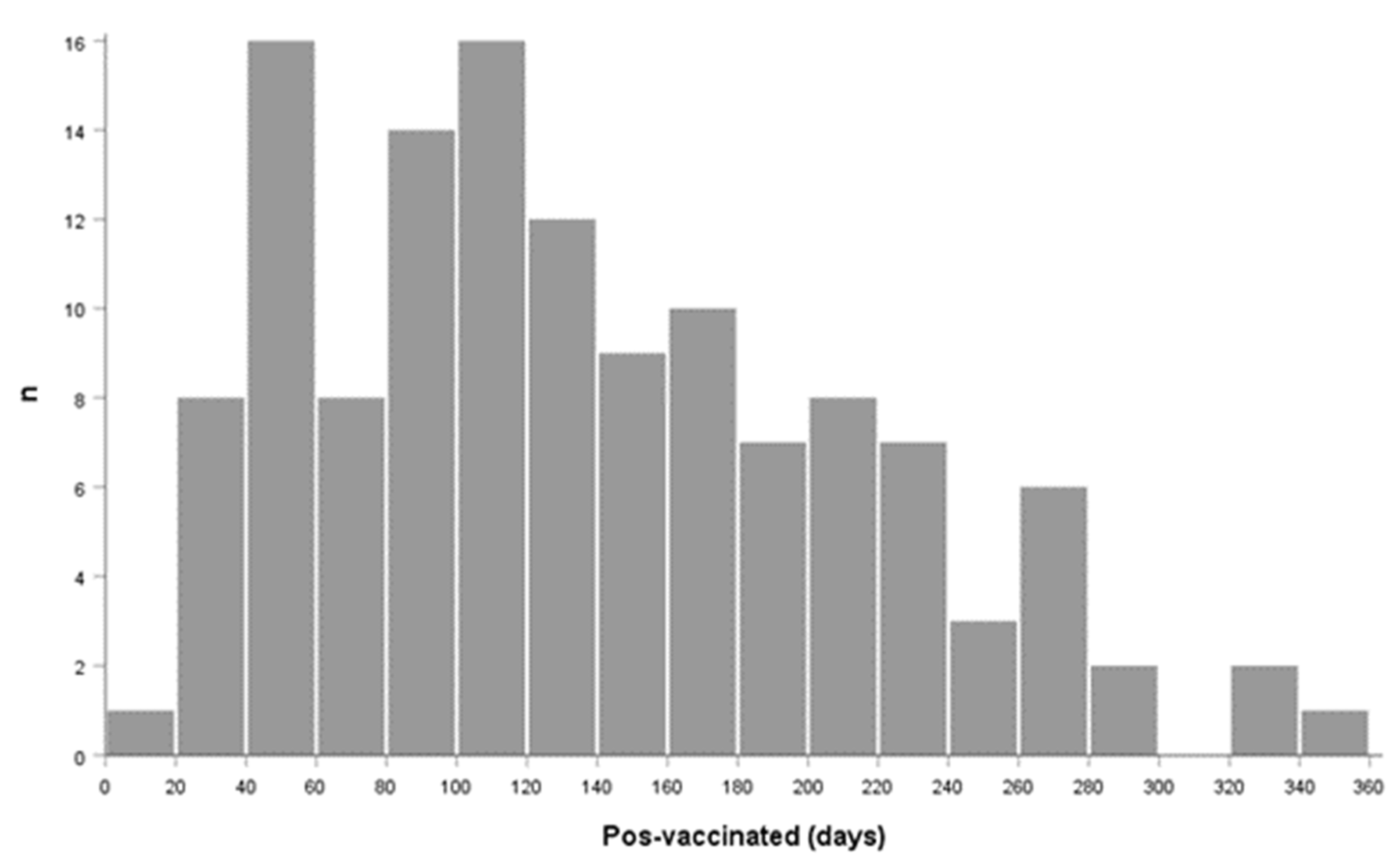
| Population | Min–Max (Days) | Median (IQR) |
|---|---|---|
| V-COVID-19 | 17–324 | 118 (74, 203) |
| V-Healthy | 20–357 | 120 (77, 185) |
| Total (N = 130) | 17–357 | 120 (79, 197) |
| Symptoms | V-COVID-19 n (%) | V-Healthy n (%) | p |
|---|---|---|---|
| Dyspnea | 23 (46) | 0 (0) | 0.0001 |
| Cough | 16 (32) | 2 (1.5) | 0.0001 |
| Asymptomatic | 14 (28) | 119 (88.1) | 0.0001 |
| Headache | 11 (22) | 10 (7.4) | 0.005 |
| Runny nose | 11 (22) | 7 (5.2) | 0.001 |
| Odynophagia | 9 (18) | 4 (3) | 0.0001 |
| Fever | 9 (18) | 3 (2.2) | 0.0001 |
| Tiredness | 9 (18) | 2 (1.5) | 0.0001 |
| Myalgia | 4 (8) | 4 (3) | 0.135 |
| Nausea | 3 (6) | 0 (0) | 0.004 |
| Arthralgia | 1 (2) | 4 (3) | 0.720 |
| Diarrhea | 1 (2) | 3 (2.2) | 0.926 |
| Anosmia | 1 (2) | 1 (0.7) | 0.462 |
| Ageusia | 1 (2) | 0 (0) | 0.099 |
| Comorbidity | V-COVID-19 n (%) | V-Healthy n (%) | p |
|---|---|---|---|
| Hypertension | 21 (42) | 32 (23.7) | 0.015 |
| Diabetes | 16 (32) | 16 (11.9) | 0.001 |
| Obesity | 4 (8) | 3 (2.2) | 0.067 |
| Overweight | 3 (6) | 3 (2.2) | 0.198 |
| Asthma | 3 (6) | 0 (0) | 0.004 |
| COPD | 3 (6) | 0 (0) | 0.004 |
| CKD | 3 (6) | 2 (1.5) | 0.092 |
| No comorbidities | 21 (42) | 89 (65.9) | 0.003 |
| Others | 0 (0) | 7 (5.18) | 0.101 |
| Laboratory Blood Tests | n | Median (IQR) |
|---|---|---|
| Hematic biometry | ||
| Leukocytes (103/μL) | 45 | 7.6 (5.9, 10.5) |
| Neutrophils (103/μL) | 5.5 (2.9, 9) | |
| Lymphocytes (103/μL) | 1.2 (0.5, 1.7) | |
| Hemoglobin (g/dL) | 13.1 (10.2, 14.8) | |
| Platelets (103/μL) | 249 (162, 368.5) | |
| Blood chemistry test | ||
| Creatinine (mg/dL) | 42 | 1 (0.8, 1.3) |
| Urea (mg/dL) | 36.4 (30, 62.5) | |
| Glucose (mg/dL) | 103 (81, 129) | |
| Serum electrolytes | ||
| Na (mmol/L) | 44 | 139.5 (137, 142) |
| K (mmol/L) | 4.1 (3.6, 4.6) | |
| Hepatic-function test | ||
| ALT (U/L) | 29 | 28 (16.5, 51.5) |
| AST (U/L) | 30 | 31.5 (21.8, 53) |
| ALP (U/L) | 15 | 114 (70, 128) |
| Bilirubin, total (mg/dL) | 10 | 0.8 (0.7, 0.9) |
| Albumin (g/dL) | 13 | 3.2 (0.3, 3.6) |
| Blood gas test | ||
| pH | 29 | 7.45 (7.4, 7.48) |
| PaO2 (mmHg) | 59 (50, 71.5) | |
| PaCO2 (mmHg) | 34 (30.5, 38) | |
| HCO3− (mmol/L) | 24.8 (19.3, 28) | |
| Lactate (mmol/L) | 1.2 (0.9, 2.3) | |
| Other | ||
| CRP (mg/dL) | 33 | 34.2 (7.5, 69.9) |
| LDH (UI/L) | 32 | 240 (182.3, 391.3) |
| Fibrinogen (mg/dL) | 31 | 531 (427, 664) |
| D-dimer (ng/mL) | 27 | 991 (436, 1927) |
| Ferritin (ng/mL) | 17 | 287 (144, 737.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazquez-Zapien, G.J.; Martinez-Cuazitl, A.; Sanchez-Brito, M.; Delgado-Macuil, R.J.; Atriano-Colorado, C.; Garibay-Gonzalez, F.; Sanchez-Monroy, V.; Lopez-Reyes, A.; Mata-Miranda, M.M. Comparison of the Immune Response in Vaccinated People Positive and Negative to SARS-CoV-2 Employing FTIR Spectroscopy. Cells 2022, 11, 3884. https://doi.org/10.3390/cells11233884
Vazquez-Zapien GJ, Martinez-Cuazitl A, Sanchez-Brito M, Delgado-Macuil RJ, Atriano-Colorado C, Garibay-Gonzalez F, Sanchez-Monroy V, Lopez-Reyes A, Mata-Miranda MM. Comparison of the Immune Response in Vaccinated People Positive and Negative to SARS-CoV-2 Employing FTIR Spectroscopy. Cells. 2022; 11(23):3884. https://doi.org/10.3390/cells11233884
Chicago/Turabian StyleVazquez-Zapien, Gustavo Jesus, Adriana Martinez-Cuazitl, Miguel Sanchez-Brito, Raul Jacobo Delgado-Macuil, Consuelo Atriano-Colorado, Francisco Garibay-Gonzalez, Virginia Sanchez-Monroy, Alberto Lopez-Reyes, and Monica Maribel Mata-Miranda. 2022. "Comparison of the Immune Response in Vaccinated People Positive and Negative to SARS-CoV-2 Employing FTIR Spectroscopy" Cells 11, no. 23: 3884. https://doi.org/10.3390/cells11233884
APA StyleVazquez-Zapien, G. J., Martinez-Cuazitl, A., Sanchez-Brito, M., Delgado-Macuil, R. J., Atriano-Colorado, C., Garibay-Gonzalez, F., Sanchez-Monroy, V., Lopez-Reyes, A., & Mata-Miranda, M. M. (2022). Comparison of the Immune Response in Vaccinated People Positive and Negative to SARS-CoV-2 Employing FTIR Spectroscopy. Cells, 11(23), 3884. https://doi.org/10.3390/cells11233884








