Prolyl Isomerase, Pin1, Controls Meiotic Progression in Mouse Oocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Oocyte Collection and In Vitro Maturation
2.3. Pin1 Inhibition
2.4. Immunoblotting Analysis
2.5. Immunofluorescence Staining
2.6. Statistical Analysis
3. Results
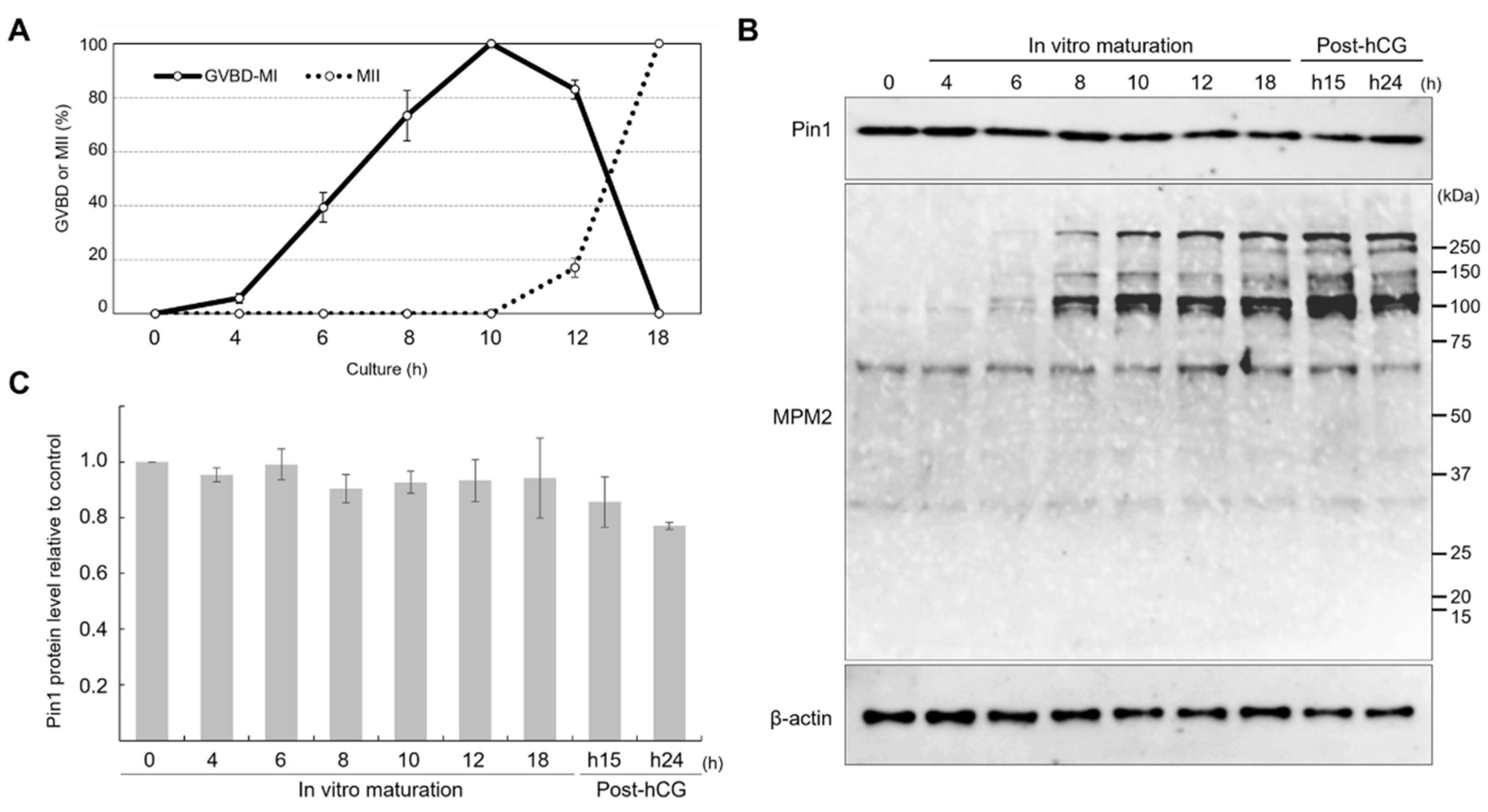
3.1. Expression of Pin1 Remains Constant during Meiotic Maturation, but That of MPM2 Changes with Meiotic Progression
3.2. Localization of Pin1 Is Altered during Maturation of Mouse Oocytes
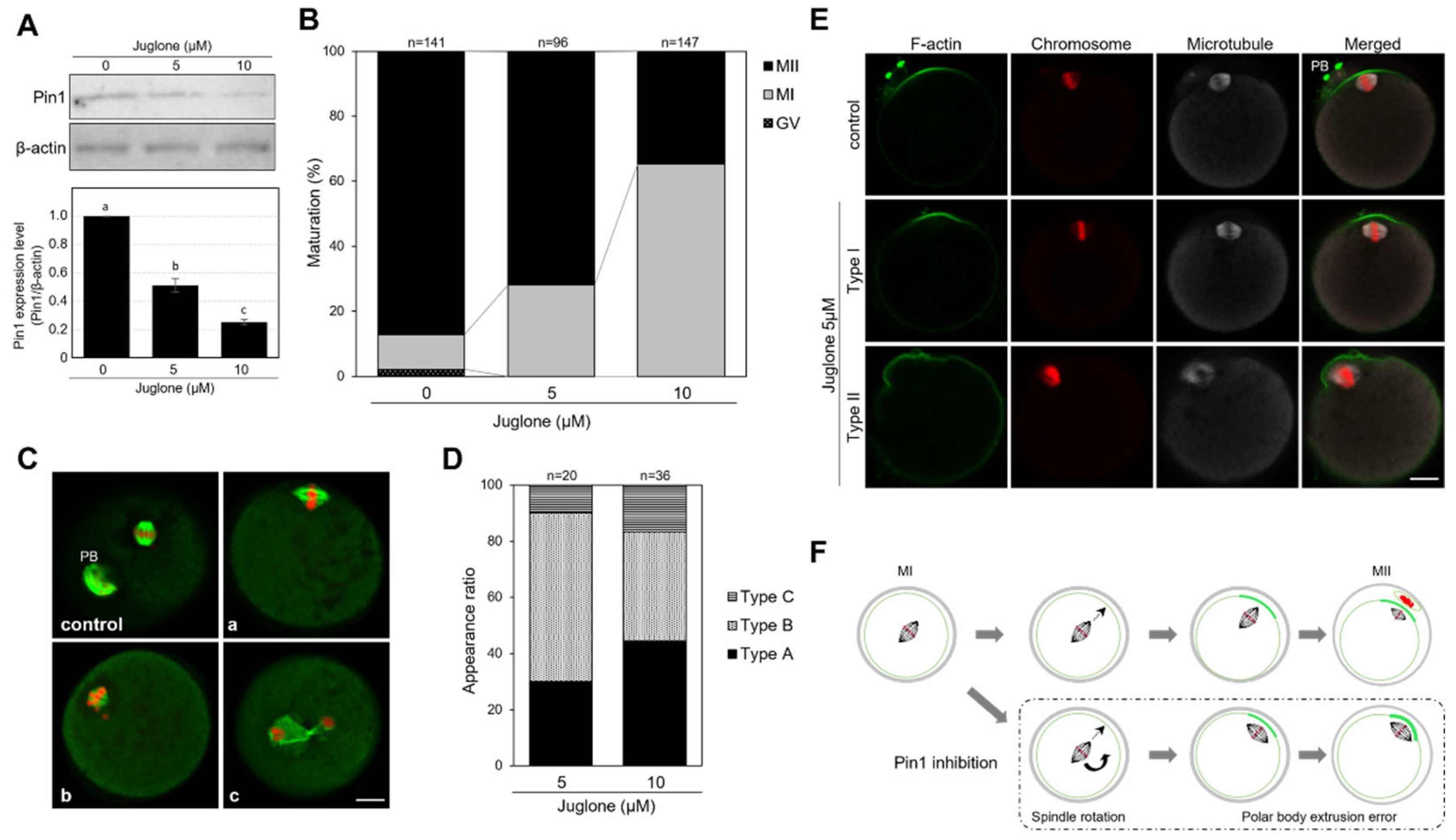
3.3. Juglone Inhibits the Extrusion of the First Polar Body in Mouse Oocytes
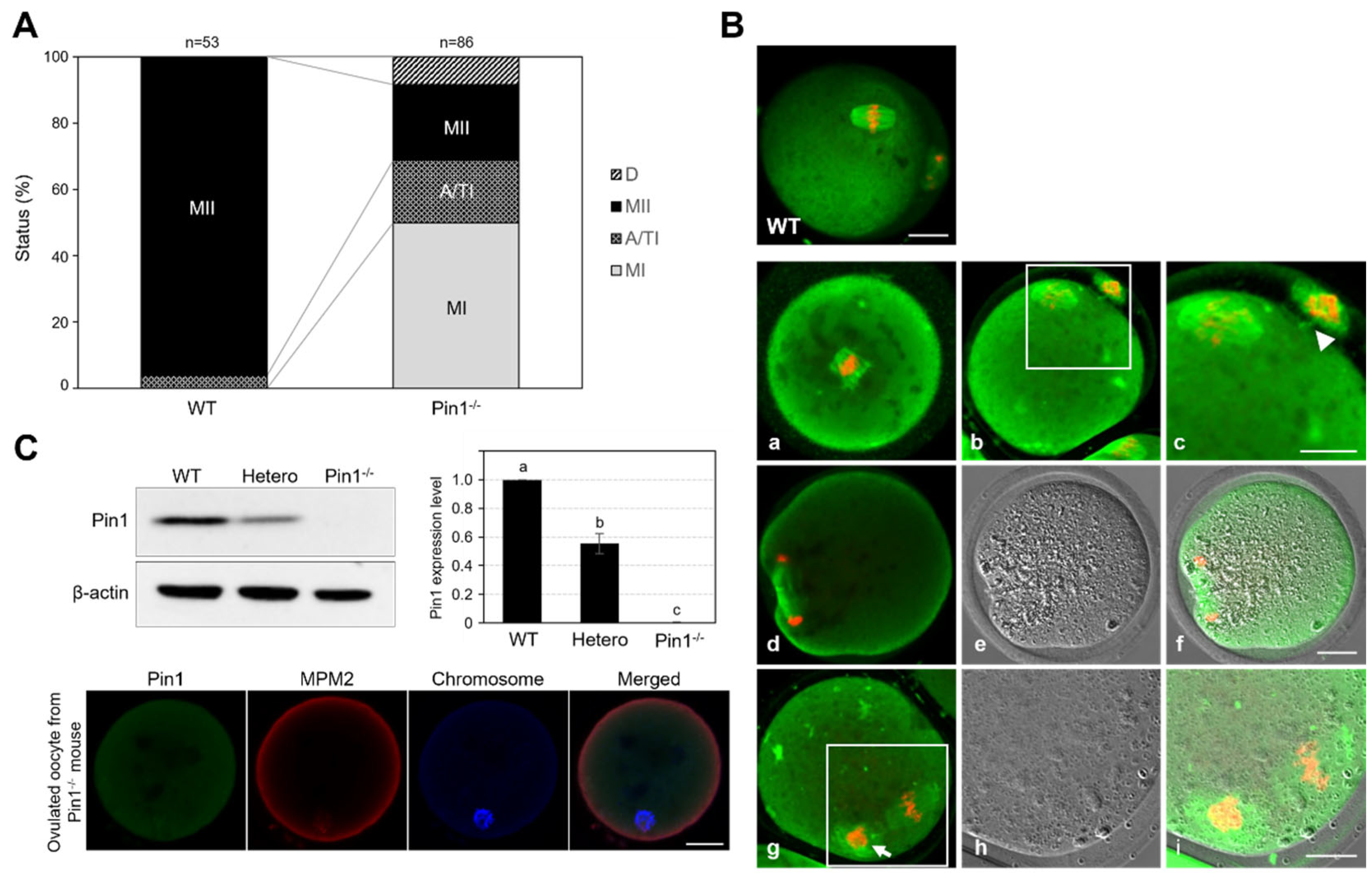
3.4. Oocytes from Pin1−/− Mice Show Frequent Failure of Extrusion of the First Polar Body
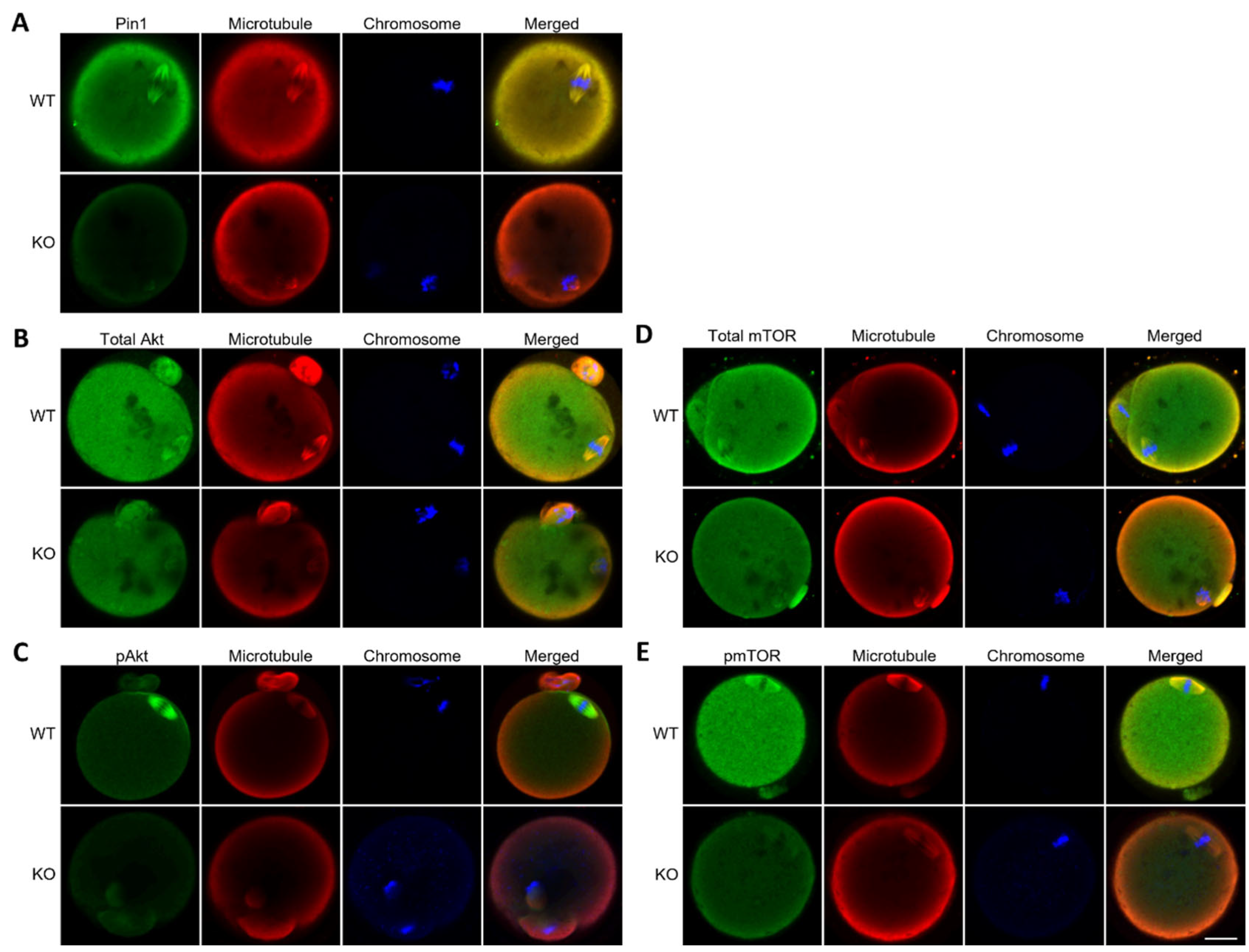
3.5. Oocytes from Pin1−/− Mice Do Not Show Specific Localization of Akt and mTOR
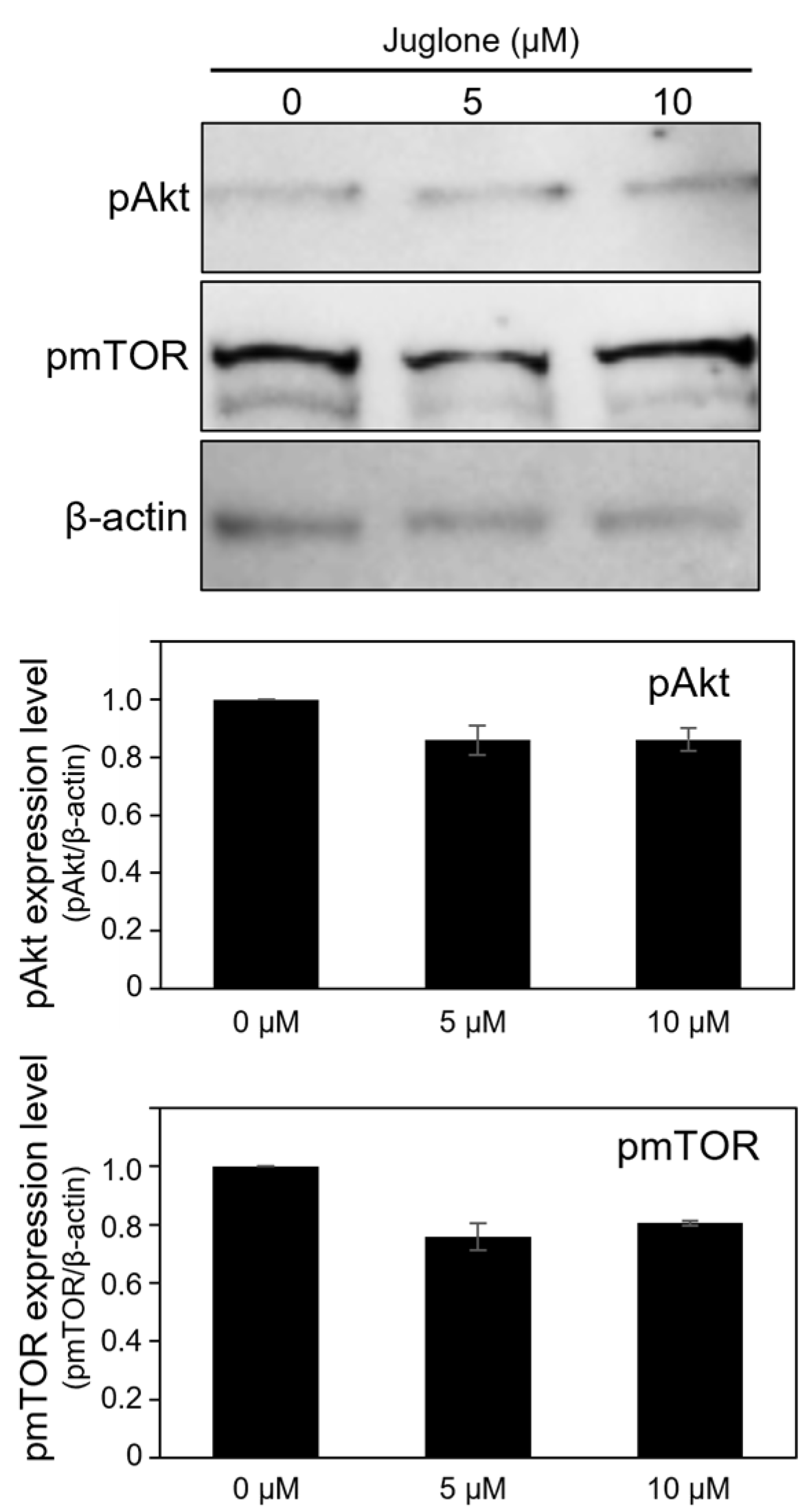
3.6. Juglone Reduces the Expression of Phospho-Akt and Phospho-mTOR Slightly
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Tiwari, M.; Prasad, S.; Chaube, S.K. Role of cyclic nucleotide phosphodiesterases during meiotic resumption from diplotene arrest in mammalian oocytes. J. Cell Biochem. 2017, 118, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Gupta, A.; Sharma, A.; Prasad, S.; Pandey, A.N.; Yadav, P.K.; Pandey, A.K.; Shrivastav, T.G.; Chaube, S.K. Role of mitogen activated protein kinase and maturation promoting factor during the achievement of meiotic competency in mammalian oocytes. J. Cell Biochem. 2018, 119, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Solc, P.; Schultz, R.M.; Motlik, J. Prophase I arrest and progression to metaphase I in mouse oocytes: Comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol. Hum. Reprod. 2010, 16, 654–664. [Google Scholar] [CrossRef]
- Adhikari, D.; Liu, K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol. Cell Endocrinol. 2014, 382, 480–487. [Google Scholar] [CrossRef]
- Hoshino, Y.; Sato, E. Protein kinase B (PKB/Akt) is required for the completion of meiosis in mouse oocytes. Dev. Biol. 2008, 314, 215–223. [Google Scholar] [CrossRef]
- Sun, S.C.; Kim, N.H. Molecular mechanisms of asymmetric division in oocytes. Microsc. Microanal. 2013, 19, 883–897. [Google Scholar] [CrossRef]
- Kogasaka, Y.; Hoshino, Y.; Hiradate, Y.; Tanemura, K.; Sato, E. Distribution and association of mTOR with its cofactors, raptor and rictor, in cumulus cells and oocytes during meiotic maturation in mice. Mol. Reprod. Dev. 2013, 80, 334–348. [Google Scholar] [CrossRef]
- Downs, S.M. Regulation of the G2/M transition in rodent oocytes. Mol. Reprod. Dev. 2010, 77, 566–585. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Brandl, C.J.; Deber, C.M. Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Li, H.Y.; Lee, Y.C.; Calkins, M.J.; Lee, K.H.; Yang, C.N.; Lu, P.J. Landscape of Pin1 in the cell cycle. Exp. Biol. Med. 2015, 240, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.P.; Hanes, S.D.; Hunter, T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 1996, 380, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.P.; Zhou, X.Z. The prolyl isomerase PIN1: A pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 904–916. [Google Scholar] [CrossRef]
- Wulf, G.; Finn, G.; Suizu, F.; Lu, K.P. Phosphorylation-specific prolyl isomerization: Is there an underlying theme? Nat. Cell Biol. 2005, 7, 435–441. [Google Scholar] [CrossRef]
- Fujimori, F.; Takahashi, K.; Uchida, C.; Uchida, T. Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G(0) arrest. Biochem. Biophys. Res. Commun. 1999, 265, 658–663. [Google Scholar] [CrossRef]
- Hoshino, Y.; Yokoo, M.; Yoshida, N.; Sasada, H.; Matsumoto, H.; Sato, E. Phosphatidylinositol 3-kinase and Akt participate in the FSH-induced meiotic maturation of mouse oocytes. Mol. Reprod. Dev. 2004, 69, 77–86. [Google Scholar] [CrossRef]
- Sakai, C.; Hoshino, Y.; Sato, Y.; Sato, E. Evaluation of maturation competence of metaphase II oocytes in mice based on the distance between pericentriolar materials of meiotic spindle: Distance of PCM during oocyte maturation. J. Assist. Reprod. Genet. 2011, 28, 157–166. [Google Scholar] [CrossRef]
- Zhong, Z.S.; Huo, L.J.; Liang, C.G.; Chen, D.Y.; Sun, Q.Y. Small GTPase RhoA is required for ooplasmic segregation and spindle rotation, but not for spindle organization and chromosome separation during mouse oocyte maturation, fertilization, and early cleavage. Mol. Reprod. Dev. 2005, 71, 256–261. [Google Scholar] [CrossRef]
- Na, J.; Zernicka-Goetz, M. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr. Biol. 2006, 16, 1249–1254. [Google Scholar] [CrossRef]
- Ma, C.; Benink, H.A.; Cheng, D.; Montplaisir, V.; Wang, L.; Xi, Y.; Zheng, P.P.; Bement, W.M.; Liu, X.J. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr. Biol. 2006, 16, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.L.; Kikuchi, K.; Sun, Q.Y.; Schatten, H. Oocyte aging: Cellular and molecular changes, developmental potential and reversal possibility. Hum. Reprod. Update 2009, 15, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Chen, D.Y.; Li, J.S.; Lian, L.; Lei, L.; Han, Z.M.; Sun, Q.Y. Rotation of meiotic spindle is controlled by microfilaments in mouse oocytes. Biol. Reprod. 2003, 68, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, A.S.; Ellefson, M.L.; McNally, F.J. Nuclear and spindle positioning during oocyte meiosis. Curr. Opin. Cell Biol. 2011, 23, 78–84. [Google Scholar] [CrossRef]
- Bao, L.; Kimzey, A.; Sauter, G.; Sowadski, J.M.; Lu, K.P.; Wang, D.G. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 2004, 164, 1727–1737. [Google Scholar] [CrossRef]
- Driver, J.A.; Zhou, X.Z.; Lu, K.P. Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer’s disease. Biochim. Biophys. Acta 2015, 1850, 2069–2076. [Google Scholar] [CrossRef]
- Shimizu, T.; Akiyama, H.; Abe, Y.; Sasada, H.; Sato, E.; Miyamoto, A.; Uchida, T. Expression of Pin1, a peptidyl-prolyl isomerase, in the ovaries of eCG/hCG-treated immature female mice. J. Reprod. Dev. 2006, 52, 287–291. [Google Scholar] [CrossRef]
- Shimizu, T.; Tetsuka, M.; Miyamoto, A.; Uchida, T. Follicle-stimulating hormone (FSH) stimulates the expression of Pin1, a peptidyl-prolyl isomerase, in the bovine granulosa cells. Domest. Anim. Endocrinol. 2007, 32, 226–234. [Google Scholar] [CrossRef]
- Albert, A.L.; Lavoie, S.B.; Vincent, M. Multisite phosphorylation of Pin1-associated mitotic phosphoproteins revealed by monoclonal antibodies MPM-2 and CC-3. BMC Cell Biol. 2004, 5, 22. [Google Scholar] [CrossRef]
- Xu, Y.X.; Manley, J.L. The prolyl isomerase Pin1 functions in mitotic chromosome condensation. Mol. Cell 2007, 26, 287–300. [Google Scholar] [CrossRef]
- Goldberg, M.W.; Fiserova, J.; Huttenlauch, I.; Stick, R. A new model for nuclear lamina organization. Biochem. Soc. Trans. 2008, 36, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Arnault, E.; Doussau, M.; Pesty, A.; Lefevre, B.; Courtot, A.M. Review: Lamin A/C, caspase-6, and chromatin configuration during meiosis resumption in the mouse oocyte. Reprod. Sci. 2010, 17, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, J.; Webel, R.; Auerochs, S.; Sticht, H.; Marschall, M. Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus. J. Biol. Chem. 2010, 285, 13979–13989. [Google Scholar] [CrossRef] [PubMed]
- Napoletano, F.; Ferrari Bravo, G.; Voto, I.A.P.; Santin, A.; Celora, L.; Campaner, E.; Dezi, C.; Bertossi, A.; Valentino, E.; Santorsola, M.; et al. The prolyl-isomerase PIN1 is essential for nuclear Lamin-B structure and function and protects heterochromatin under mechanical stress. Cell Rep. 2021, 36, 109694. [Google Scholar] [CrossRef]
- Mao, L.; Lou, H.; Lou, Y.; Wang, N.; Jin, F. Behaviour of cytoplasmic organelles and cytoskeleton during oocyte maturation. Reprod. Biomed. Online 2014, 28, 284–299. [Google Scholar] [CrossRef]
- Leader, B.; Lim, H.; Carabatsos, M.J.; Harrington, A.; Ecsedy, J.; Pellman, D.; Maas, R.; Leder, P. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat. Cell Biol. 2002, 4, 921–928. [Google Scholar] [CrossRef]
- Almonacid, M.; Ahmed, W.W.; Bussonnier, M.; Mailly, P.; Betz, T.; Voituriez, R.; Gov, N.S.; Verlhac, M.H. Active diffusion positions the nucleus in mouse oocytes. Nat. Cell Biol. 2015, 17, 470–479. [Google Scholar] [CrossRef]
- Maro, B.; Johnson, M.H.; Webb, M.; Flach, G. Mechanism of polar body formation in the mouse oocyte: An interaction between the chromosomes, the cytoskeleton and the plasma membrane. J. Embryol. Exp. Morphol. 1986, 92, 11–32. [Google Scholar] [CrossRef]
- Schuh, M.; Ellenberg, J. A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 2008, 18, 1986–1992. [Google Scholar] [CrossRef]
- Brunn, G.J.; Fadden, P.; Haystead, T.A.; Lawrence, J.C., Jr. The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J. Biol. Chem. 1997, 272, 32547–32550. [Google Scholar] [CrossRef]
- Liao, Y.; Wei, Y.; Zhou, X.; Yang, J.Y.; Dai, C.; Chen, Y.J.; Agarwal, N.K.; Sarbassov, D.; Shi, D.; Yu, D. Peptidyl-prolyl cis/trans isomerase Pin1 is critical for the regulation of PKB/Akt stability and activation phosphorylation. Oncogene 2009, 28, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Pfender, S.; Kuznetsov, V.; Pleiser, S.; Kerkhoff, E.; Schuh, M. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr. Biol. 2011, 21, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Racowsky, C.; Deng, M. Mechanism of the chromosome-induced polar body extrusion in mouse eggs. Cell Div. 2011, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Hodgman, R.; Tay, J.; Mendez, R.; Richter, J.D. CPEB phosphorylation and cytoplasmic polyadenylation are catalyzed by the kinase IAK1/Eg2 in maturing mouse oocytes. Development 2001, 128, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Nechama, M.; Lin, C.L.; Richter, J.D. An unusual two-step control of CPEB destruction by Pin1. Mol. Cell Biol. 2013, 33, 48–58. [Google Scholar] [CrossRef]
- Tay, J.; Hodgman, R.; Richter, J.D. The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev. Biol. 2000, 221, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Melton, C.; Suh, N.; Oh, J.S.; Horner, K.; Xie, F.; Sette, C.; Blelloch, R.; Conti, M. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011, 25, 755–766. [Google Scholar] [CrossRef]
- Berger, M.; Stahl, N.; Del Sal, G.; Haupt, Y. Mutations in proline 82 of p53 impair its activation by Pin1 and Chk2 in response to DNA damage. Mol. Cell Biol. 2005, 25, 5380–5388. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshino, Y.; Uchida, T. Prolyl Isomerase, Pin1, Controls Meiotic Progression in Mouse Oocytes. Cells 2022, 11, 3772. https://doi.org/10.3390/cells11233772
Hoshino Y, Uchida T. Prolyl Isomerase, Pin1, Controls Meiotic Progression in Mouse Oocytes. Cells. 2022; 11(23):3772. https://doi.org/10.3390/cells11233772
Chicago/Turabian StyleHoshino, Yumi, and Takafumi Uchida. 2022. "Prolyl Isomerase, Pin1, Controls Meiotic Progression in Mouse Oocytes" Cells 11, no. 23: 3772. https://doi.org/10.3390/cells11233772
APA StyleHoshino, Y., & Uchida, T. (2022). Prolyl Isomerase, Pin1, Controls Meiotic Progression in Mouse Oocytes. Cells, 11(23), 3772. https://doi.org/10.3390/cells11233772







