A Disturbed Siderophore Transport Inhibits Myxobacterial Predation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Media
2.2. Predation Assay on Agar Plates
2.3. Bacterial Competition Assay
2.4. Iron Content Determination
2.5. Chromeazurol S Overlay (O-CAS) Assay
2.6. Transcriptome Analysis
2.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.8. Isolation of OMVs
2.9. Data Availability
3. Results
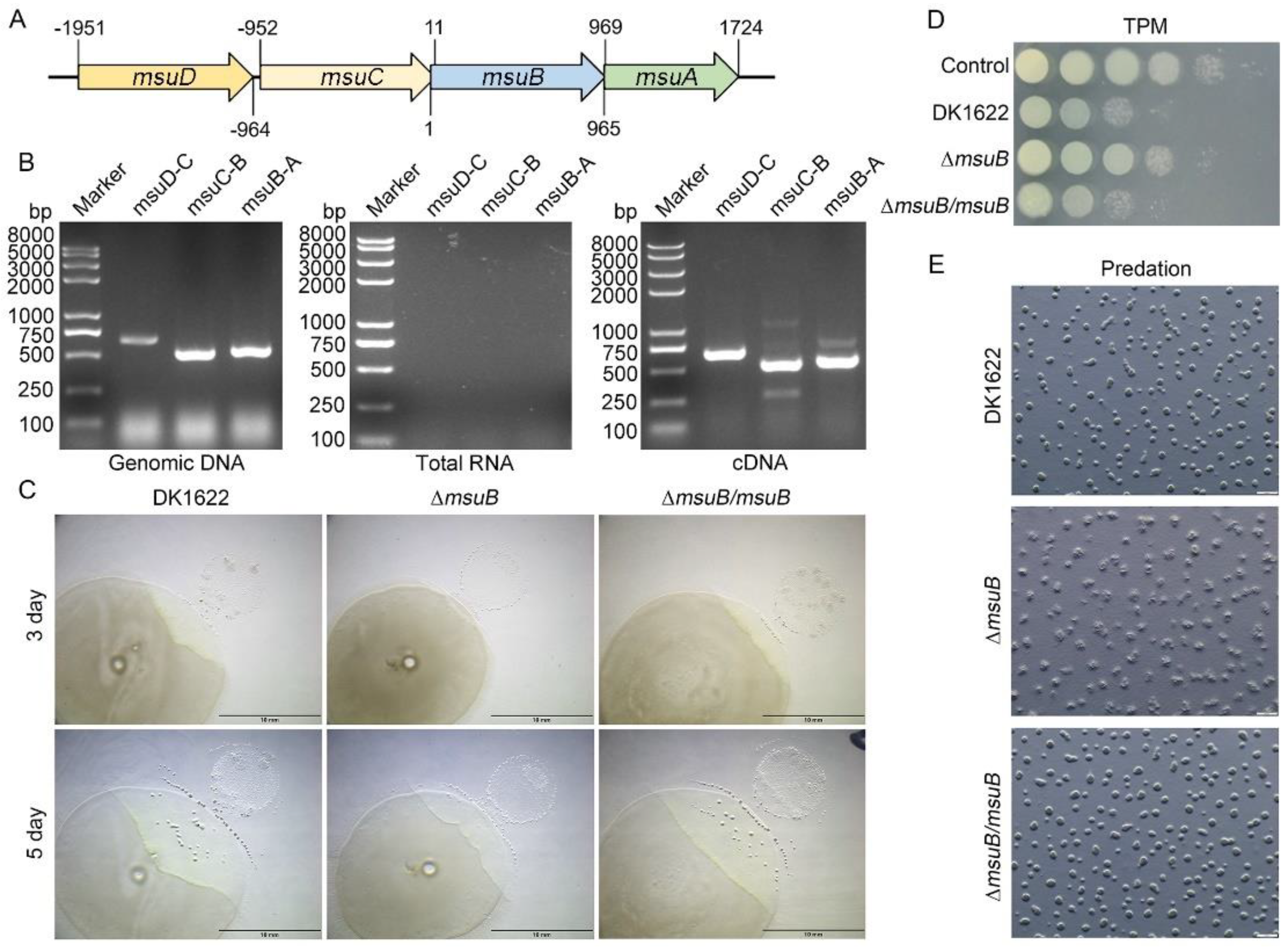
3.1. Loss of msuB Function Affects Myxobacterial Predation
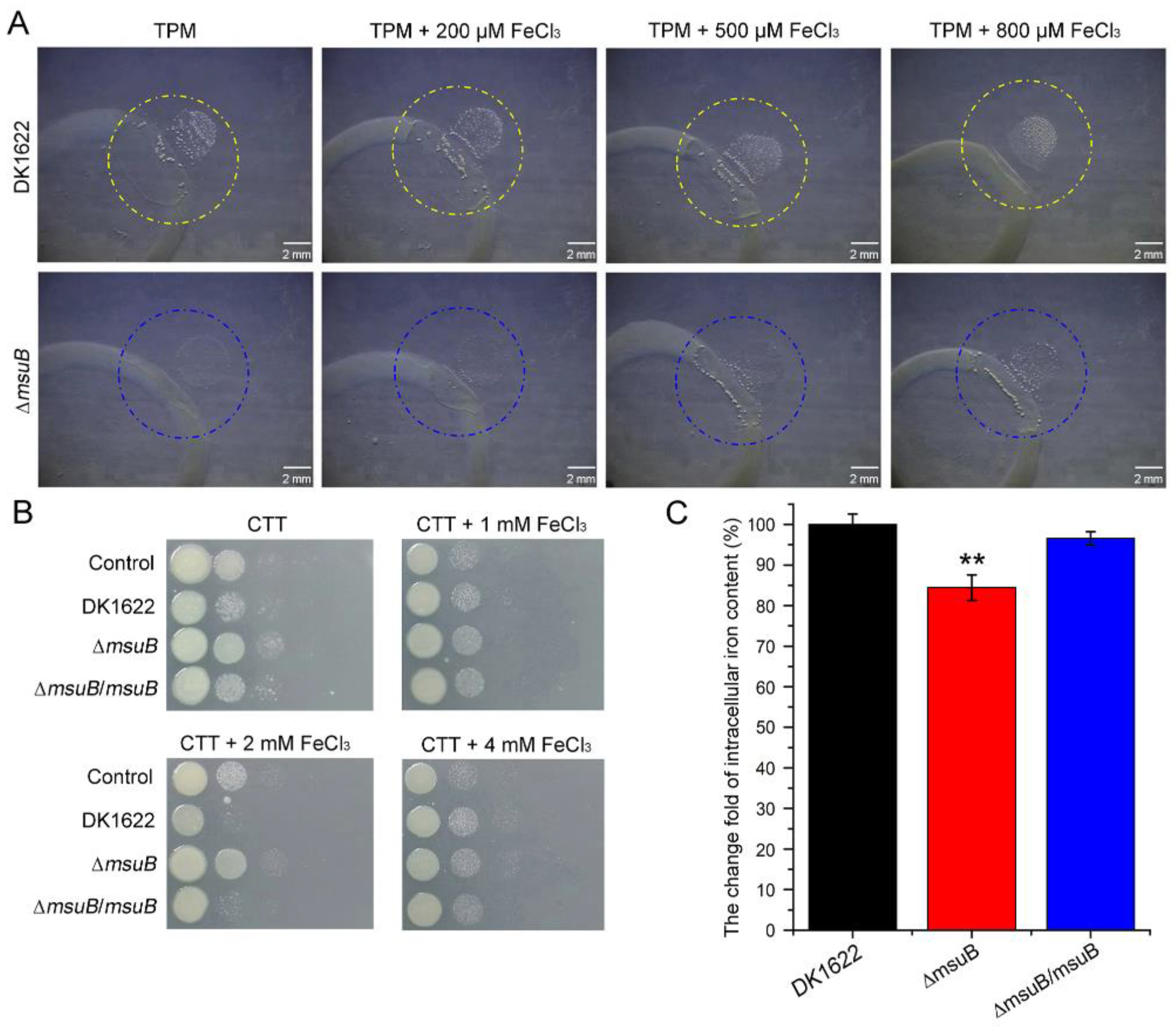
3.2. Deletion of msuB Leads to a Significant Decrease in Intracellular Iron Levels
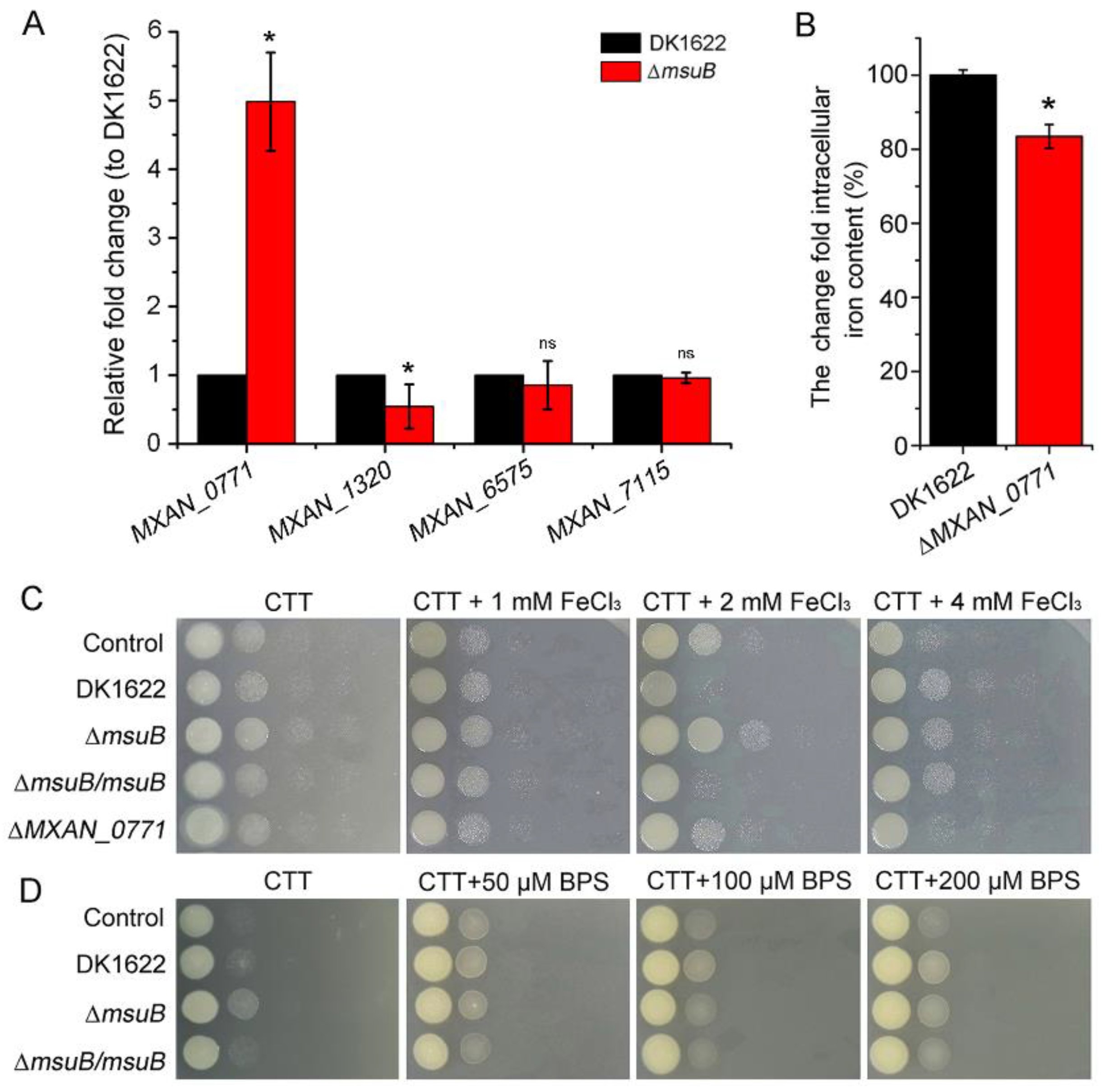
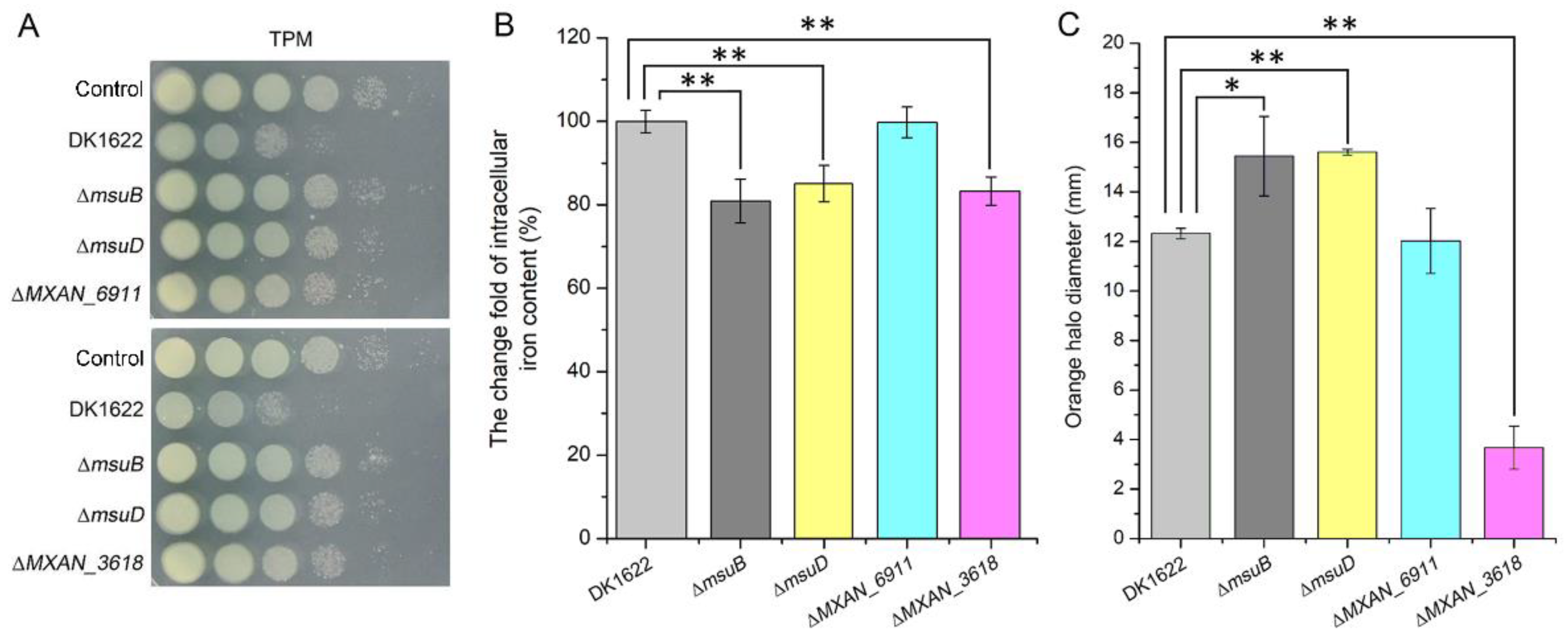
3.3. MsuB Is Involved in the Maintenance of Myxobacterial Intracellular Iron Homeostasis
3.4. Disruption of Siderophore Synthesis and Transport Decreased Predation in M. xanthus
3.5. MsuABCD Is Closely Related to the Secretion of Lytic Enzymes
3.6. Interruption of Siderophore Transport Decreased the Expression of OMV-Specific Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, T.A.; Ahmad, S.; Whitney, J.C. Contact-dependent interbacterial antagonism mediated by protein secretion machines. Trends Microbiol. 2020, 28, 387–400. [Google Scholar] [CrossRef]
- Stubbendieck, R.M.; Straight, P.D. Multifaceted interfaces of bacterial competition. J. Bacteriol. 2016, 198, 2145–2155. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Ghoul, M.; Mitri, S. The ecology and evolution of microbial competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef]
- Kamal, F.; Liang, X.; Manera, K.; Pei, T.T.; Kim, H.; Lam, L.G.; Pun, A.; Hersch, S.J.; Dong, T.G. Differential cellular response to translocated toxic effectors and physical penetration by the Type VI Secretion System. Cell Rep. 2020, 31, 107766. [Google Scholar] [CrossRef]
- Kramer, J.; Ozkaya, O.; Kummerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Kurth, C.; Kage, H.; Nett, M. Siderophores as molecular tools in medical and environmental applications. Org. Biomol. Chem. 2016, 14, 8212–8227. [Google Scholar] [CrossRef]
- Gorska, A.; Sloderbach, A.; Marszall, M.P. Siderophore-drug complexes: Potential medicinal applications of the ‘Trojan horse’ strategy. Trends Pharmacol. Sci. 2014, 35, 442–449. [Google Scholar] [CrossRef]
- Clarke, T.E.; Tari, L.W.; Vogel, H.J. Structural biology of bacterial iron uptake systems. Curr. Top. Med. Chem. 2001, 1, 7–30. [Google Scholar] [CrossRef]
- Eickhoff, M.J.; Bassler, B.L. Vibrio fischeri siderophore production drives competitive exclusion during dual-species growth. Mol. Microbiol. 2020, 114, 244–261. [Google Scholar] [CrossRef]
- Leinweber, A.; Weigert, M.; Kummerli, R. The bacterium Pseudomonas aeruginosa senses and gradually responds to interspecific competition for iron. Evolution 2018, 72, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.; Livingstone, P.G.; Furness, E.; Swain, M.T.; Whitworth, D.E. Genome-wide identification of myxobacterial predation genes and demonstration of formaldehyde secretion as a potentially predation-resistant trait of Pseudomonas aeruginosa. Front. Microbiol. 2019, 10, 2650. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Phillips, K.E.; Misra, S.K.; Sharp, J.S.; Stevens, D.C. Differential response to prey quorum signals indicates predatory specialization of myxobacteria and ability to predate Pseudomonas aeruginosa. Environ. Microbiol. 2022, 24, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, P.G.; Morphew, R.M.; Whitworth, D.E. Myxobacteria are able to prey broadly upon clinically-relevant pathogens, exhibiting a prey range which cannot be explained by phylogeny. Front. Microbiol. 2017, 8, 1593. [Google Scholar] [CrossRef]
- Thiery, S.; Kaimer, C. The predation strategy of Myxococcus xanthus. Front. Microbiol. 2020, 11, 2. [Google Scholar] [CrossRef]
- Morgan, A.D.; MacLean, R.C.; Hillesland, K.L.; Velicer, G.J. Comparative analysis of Myxococcus predation on soil bacteria. Appl. Environ. Microbiol. 2010, 76, 6920–6927. [Google Scholar] [CrossRef]
- Keane, R.; Berleman, J. The predatory life cycle of Myxococcus xanthus. Microbiology 2016, 162, 1–11. [Google Scholar] [CrossRef]
- Velicer, G.J.; Kroos, L.; Lenski, R.E. Developmental cheating in the social bacterium Myxococcus xanthus. Nature 2000, 404, 598–601. [Google Scholar] [CrossRef]
- Stolle, A.S.; Meader, B.T.; Toska, J.; Mekalanos, J.J. Endogenous membrane stress induces T6SS activity in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2018365118. [Google Scholar] [CrossRef]
- Kunze, B.; Bedorf, N.; Kohl, W.; Hofle, G.; Reichenbach, H. Myxochelin A, a new iron-chelating compound from Angiococcus disciformis (Myxobacterales). Production, isolation, physico-chemical and biological properties. J. Antibiot. 1989, 42, 14–27. [Google Scholar] [CrossRef]
- Silakowski, B.; Kunze, B.; Nordsiek, G.; Blocker, H.; Hofle, G.; Muller, R. The myxochelin iron transport regulon of the myxobacterium Stigmatella aurantiaca Sg a15. Eur. J. Biochem. 2000, 267, 6476–6485. [Google Scholar] [CrossRef]
- Gaitatzis, N.; Kunze, B.; Muller, R. Novel insights into siderophore formation in myxobacteria. Chembiochem 2005, 6, 365–374. [Google Scholar] [CrossRef]
- Findlay, B.L. The chemical ecology of predatory soil bacteria. ACS Chem. Biol. 2016, 11, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Krug, D.; Zurek, G.; Revermann, O.; Vos, M.; Velicer, G.J.; Muller, R. Discovering the hidden secondary metabolome of Myxococcus xanthus: A study of intraspecific diversity. Appl. Environ. Microbiol. 2008, 74, 3058–3068. [Google Scholar] [CrossRef]
- Weissman, K.J.; Muller, R. Myxobacterial secondary metabolites: Bioactivities and modes-of-action. Nat. Prod. Rep. 2010, 27, 1276–1295. [Google Scholar] [CrossRef]
- Sester, A.; Winand, L.; Pace, S.; Hiller, W.; Werz, O.; Nett, M. Myxochelin- and Pseudochelin-derived lipoxygenase inhibitors from a genetically engineered Myxococcus xanthus strain. J. Nat. Prod. 2019, 82, 2544–2549. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.; Chung, J.; Lee, Y.; Cho, S.; Jang, K.S.; Kim, S.C.; Palsson, B.; Cho, B.K. Iron competition triggers antibiotic biosynthesis in Streptomyces coelicolor during coculture with Myxococcus xanthus. ISME J. 2020, 14, 1111–1124. [Google Scholar] [CrossRef]
- Dhurve, G.; Madikonda, A.K.; Jagannadham, M.V.; Siddavattam, D. Outer membrane vesicles of Acinetobacter baumannii DS002 are selectively enriched with TonB-Dependent transporters and play a key role in iron acquisition. Microbiol. Spectr. 2022, 10, e00293-22. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Wang, D.; Wei, Z.; Hao, X.; Wang, Z.; Li, T.; Zhang, L.; Lu, Z.; Long, M.; et al. T6SS secretes an LPS-binding effector to recruit OMVs for exploitative competition and horizontal gene transfer. ISME J. 2022, 16, 500–510. [Google Scholar] [CrossRef]
- Evans, A.G.L.; Davey, H.M.; Cookson, A.; Currinn, H.; Cooke-Fox, G.; Stanczyk, P.J.; Whitworth, D.E. Predatory activity of Myxococcus xanthus outer-membrane vesicles and properties of their hydrolase cargo. Microbiology 2012, 158, 2742–2752. [Google Scholar] [CrossRef]
- Berleman, J.E.; Allen, S.; Danielewicz, M.A.; Remis, J.P.; Gorur, A.; Cunha, J.; Hadi, M.Z.; Zusman, D.R.; Northen, T.R.; Witkowska, H.E.; et al. The lethal cargo of Myxococcus xanthus outer membrane vesicles. Front. Microbiol. 2014, 5, 474. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 1979, 76, 5952–5956. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Wang, Y.; Li, Z.F.; Gong, Y.; Zhang, P.; Hu, W.C.; Sheng, D.H.; Li, Y.Z. Increasing on-target cleavage efficiency for CRISPR/Cas9-induced large fragment deletion in Myxococcus xanthus. Microb. Cell Fact. 2017, 16, 142. [Google Scholar] [CrossRef]
- Julien, B.; Kaiser, A.D.; Garza, A. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 2000, 97, 9098–9103. [Google Scholar] [CrossRef]
- Wu, S.S.; Wu, J.; Kaiser, D. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol. Microbiol. 1997, 23, 109–121. [Google Scholar] [CrossRef]
- Dong, H.; Xu, X.; Gao, R.; Li, Y.; Li, A.; Yao, Q.; Zhu, H. Myxococcus xanthus R31 suppresses tomato bacterial wilt by inhibiting the pathogen Ralstonia solanacearum with secreted proteins. Front. Microbiol. 2021, 12, 801091. [Google Scholar] [CrossRef]
- Berleman, J.E.; Chumley, T.; Cheung, P.; Kirby, J.R. Rippling is a predatory behavior in Myxococcus xanthus. J. Bacteriol. 2006, 188, 5888–5895. [Google Scholar] [CrossRef]
- Basler, M.; Ho, B.T.; Mekalanos, J.J. Tit-for-tat: Type VI secretion system counterattack during bacterial cell-cell interactions. Cell 2013, 152, 884–894. [Google Scholar] [CrossRef]
- Hsu, P.C.; Yang, C.Y.; Lan, C.Y. Candida albicans Hap43 is a repressor induced under low-iron conditions and is essential for iron-responsive transcriptional regulation and virulence. Eukaryot. Cell 2011, 10, 207–225. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, D.; Yu, Q.; Zhao, Q.; Xiao, C.; Zhang, K.; Jia, C.; Chen, S.; Zhang, B.; Zhang, B.; et al. Loss of Ssq1 leads to mitochondrial dysfunction, activation of autophagy and cell cycle arrest due to iron overload triggered by mitochondrial iron-sulfur cluster assembly defects in Candida albicans. Int. J. Biochem. Cell Biol. 2017, 85, 44–55. [Google Scholar] [CrossRef]
- Kominek, J.; Doering, D.T.; Opulente, D.A.; Shen, X.X.; Zhou, X.; DeVirgilio, J.; Hulfachor, A.B.; Groenewald, M.; McGee, M.A.; Karlen, S.D.; et al. Eukaryotic acquisition of a bacterial operon. Cell 2019, 176, 1356–1366. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Niehus, R.; Picot, A.; Oliveira, N.M.; Mitri, S.; Foster, K.R. The evolution of siderophore production as a competitive trait. Evolution 2017, 71, 1443–1455. [Google Scholar] [CrossRef]
- Lee, W.; van Baalen, M.; Jansen, V.A. Siderophore production and the evolution of investment in a public good: An adaptive dynamics approach to kin selection. J. Theor. Biol. 2016, 388, 61–71. [Google Scholar] [CrossRef]
- Popp, P.F.; Mascher, T. Coordinated cell death in isogenic bacterial populations: Sacrificing some for the benefit of many? J. Mol. Biol. 2019, 431, 4656–4669. [Google Scholar] [CrossRef] [PubMed]
- Goldman, B.S.; Nierman, W.C.; Kaiser, D.; Slater, S.C.; Durkin, A.S.; Eisen, J.A.; Ronning, C.M.; Barbazuk, W.B.; Blanchard, M.; Field, C.; et al. Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl. Acad. Sci. USA 2006, 103, 15200–15205. [Google Scholar] [CrossRef]
- Arend, K.I.; Schmidt, J.J.; Bentler, T.; Luchtefeld, C.; Eggerichs, D.; Hexamer, H.M.; Kaimer, C. Myxococcus xanthus predation of Gram-positive or Gram-negative bacteria is mediated by different bacteriolytic mechanisms. Appl. Environ. Microbiol. 2020, 87, e02382-20. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Nunez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Knauf, G.A.; Powers, M.J.; Herrera, C.M.; Trent, M.S.; Davies, B.W. Acinetobactin-mediated inhibition of commensal bacteria by Acinetobacter baumannii. mSphere 2022, 7, e00016–e00022. [Google Scholar] [CrossRef]
- Dziewanowska, K.; Settles, M.; Hunter, S.; Linquist, I.; Schilkey, F.; Hartzell, P.L. Phase variation in Myxococcus xanthus yields cells specialized for iron sequestration. PLoS ONE 2014, 9, e95189. [Google Scholar] [CrossRef]
- Huang, W.; Wilks, A. Extracellular heme uptake and the challenge of bacterial cell membranes. Annu. Rev. Biochem. 2017, 86, 799–823. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.C.; Robinson, A.K.; Rodriguez-Quinones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Yan, J.; Bradley, M.D.; Friedman, J.; Welch, R.D. Phenotypic profiling of ABC transporter coding genes in Myxococcus xanthus. Front. Microbiol. 2014, 5, 352. [Google Scholar] [CrossRef]
- McHugh, C.A.; Fontana, J.; Nemecek, D.; Cheng, N.; Aksyuk, A.A.; Heymann, J.B.; Winkler, D.C.; Lam, A.S.; Wall, J.S.; Steven, A.C.; et al. A virus capsid-like nanocompartment that stores iron and protects bacteria from oxidative stress. EMBO J. 2014, 33, 1896–1911. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.; Duong, F. The SecY complex: Conducting the orchestra of protein translocation. Trends Cell Biol. 2011, 21, 506–514. [Google Scholar] [CrossRef]
- Cranford-Smith, T.; Huber, D. The way is the goal: How SecA transports proteins across the cytoplasmic membrane in bacteria. FEMS Microbiol. Lett. 2018, 365, fny093. [Google Scholar] [CrossRef]
- Costa, T.R.; Felisberto-Rodrigues, C.; Meir, A.; Prevost, M.S.; Redzej, A.; Trokter, M.; Waksman, G. Secretion systems in Gram-negative bacteria: Structural and mechanistic insights. Nat. Rev. Microbiol. 2015, 13, 343–359. [Google Scholar] [CrossRef]
- Goosens, V.J.; van Dijl, J.M. Twin-Arginine protein translocation. Curr. Top. Microbiol. Immunol. 2017, 404, 69–94. [Google Scholar] [CrossRef]
- Sebastian, S.; Agarwal, S.; Murphy, J.R.; Genco, C.A. The gonococcal fur regulon: Identification of additional genes involved in major catabolic, recombination, and secretory pathways. J. Bacteriol. 2002, 184, 3965–3974. [Google Scholar] [CrossRef]
- Zusman, D.R.; Scott, A.E.; Yang, Z.; Kirby, J.R. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat. Rev. Microbiol. 2007, 5, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, A.M.; Huemer, M.; Zinsli, L.V.; Meile, S.; Keller, A.P.; Rohrig, C.; Eichenseher, F.; Shen, Y.; Zinkernagel, A.S.; Loessner, M.J.; et al. Engineering of long-circulating peptidoglycan hydrolases enables efficient treatment of systemic Staphylococcus aureus infection. mBio 2020, 11, e01781-20. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ye, X.; Liu, M.; Xia, C.; Zhang, L.; Luo, X.; Wang, T.; Chen, Y.; Zhao, Y.; Qiao, Y.; et al. A novel outer membrane β-1,6-glucanase is deployed in the predation of fungi by myxobacteria. ISME J. 2019, 13, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.J.; Schubotz, F.; Roggensack, S.E.; Thompson, A.W.; Summons, R.E.; Chisholm, S.W. Bacterial vesicles in marine ecosystems. Science 2014, 343, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Cordero, R.J.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef]
- Guerrero-Mandujano, A.; Hernandez-Cortez, C.; Ibarra, J.A.; Castro-Escarpulli, G. The outer membrane vesicles: Secretion system type zero. Traffic 2017, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, P.G.; Millard, A.D.; Swain, M.T.; Whitworth, D.E. Transcriptional changes when Myxococcus xanthus preys on Escherichia coli suggest myxobacterial predators are constitutively toxic but regulate their feeding. Microb. Genom. 2018, 4, e000152. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, W.; Cheng, J.; Yang, X.; Zhu, K.; Wang, Y.; Wei, G.; Qian, P.Y.; Luo, Z.Q.; Shen, X. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 2017, 8, 14888. [Google Scholar] [CrossRef]
- Behrens, H.M.; Lowe, E.D.; Gault, J.; Housden, N.G.; Kaminska, R.; Weber, T.M.; Thompson, C.M.A.; Mislin, G.L.A.; Schalk, I.J.; Walker, D.; et al. Pyocin S5 import into Pseudomonas aeruginosa reveals a generic mode of bacteriocin transport. mBio 2020, 11, e03230-19. [Google Scholar] [CrossRef]
- Lee, E.Y.; Bang, J.Y.; Park, G.W.; Choi, D.S.; Kang, J.S.; Kim, H.J.; Park, K.S.; Lee, J.O.; Kim, Y.K.; Kwon, K.H.; et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [Google Scholar] [CrossRef]
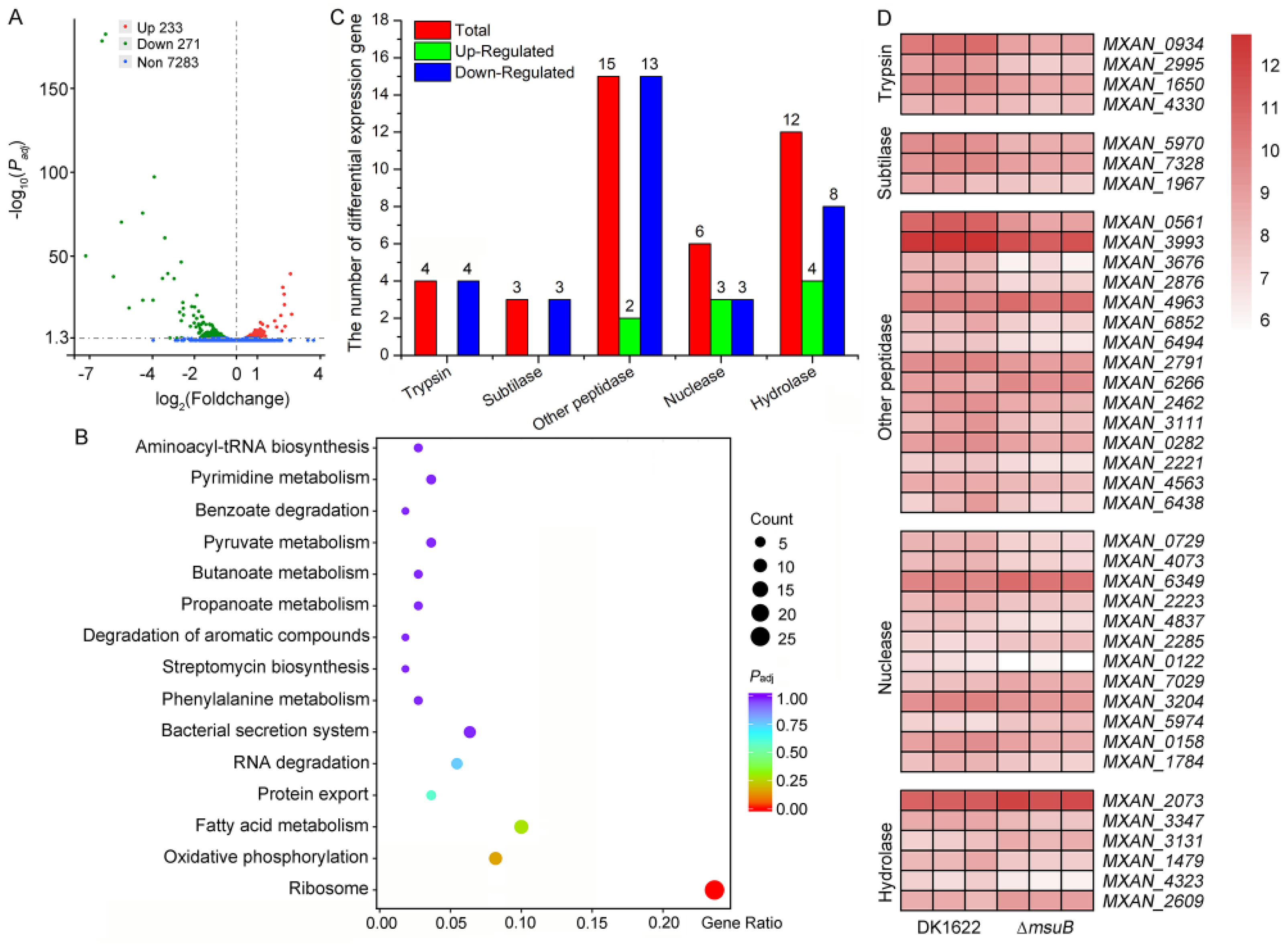
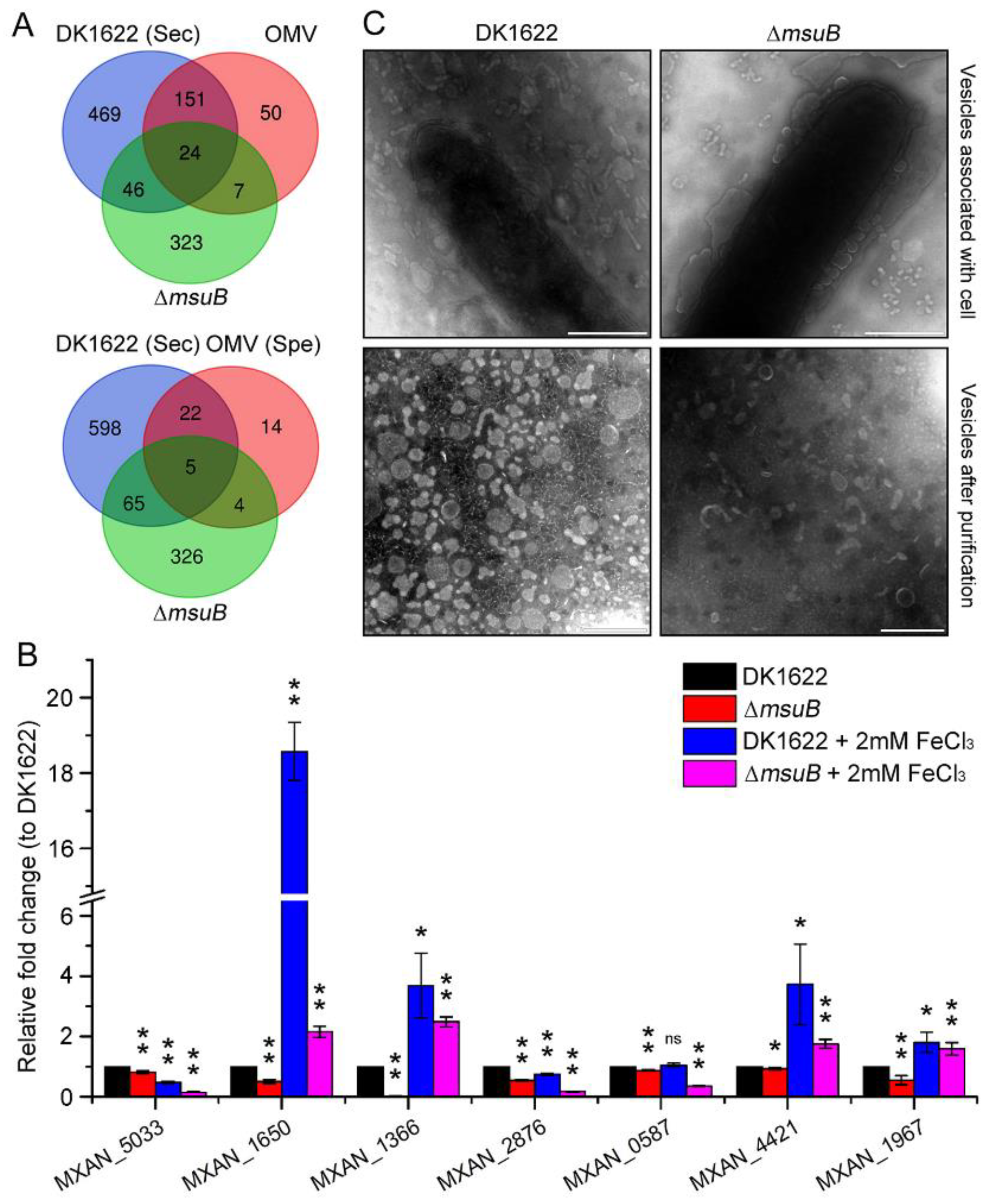
| No. | Old Locus | Locus | Gene Function |
|---|---|---|---|
| 1 | MXAN_0587 | MXAN_RS02845 | trypsin-like serine protease: Trypsin |
| 2 | MXAN_1967 | MXAN_RS09535 | S8 family serine peptidase: Subtilase family |
| 3 | MXAN_1650 | MXAN_RS08015 | trypsin-like serine protease: Trypsin |
| 4 | MXAN_2876 | MXAN_RS13930 | endopeptidase |
| 5 | MXAN_5970 | MXAN_RS28960 | S8 family serine peptidase: Subtilase family |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Dong, H.; Feng, Z.; Wang, X.; Yao, Q.; Zhu, H. A Disturbed Siderophore Transport Inhibits Myxobacterial Predation. Cells 2022, 11, 3718. https://doi.org/10.3390/cells11233718
Dong Y, Dong H, Feng Z, Wang X, Yao Q, Zhu H. A Disturbed Siderophore Transport Inhibits Myxobacterial Predation. Cells. 2022; 11(23):3718. https://doi.org/10.3390/cells11233718
Chicago/Turabian StyleDong, Yijie, Honghong Dong, Zengwei Feng, Xing Wang, Qing Yao, and Honghui Zhu. 2022. "A Disturbed Siderophore Transport Inhibits Myxobacterial Predation" Cells 11, no. 23: 3718. https://doi.org/10.3390/cells11233718
APA StyleDong, Y., Dong, H., Feng, Z., Wang, X., Yao, Q., & Zhu, H. (2022). A Disturbed Siderophore Transport Inhibits Myxobacterial Predation. Cells, 11(23), 3718. https://doi.org/10.3390/cells11233718






