1. Introduction
Inflammasomes are multiprotein platforms involved in innate immune response. They are activated following danger signal recognition in a two-step process called “priming” and “activation”, leading to the cleavage of the pro-caspase-1 in activated caspase-1. NLRP3 is the most studied canonical inflammasome. It is composed of a sensor (NLRP3), recruiting a central adaptor protein known as ASC (apoptosis-associated speck-like protein containing a caspase activating and recruitment domain), which polymerizes after activation to a supramolecular signaling platform called the ASC speck. This leads to the recruitment and activation of caspase-1, responsible for pro-IL1-β and pro-IL-18 cleavages into their pro-inflammatory active forms [
1].
Aberrant activation of NLRP3 inflammasome has been implicated in the pathophysiology of many inflammatory diseases such as auto-inflammatory periodic fever syndromes, including cryopyrin-associated periodic syndromes [
2] and familial Mediterranean fever [
3] but also in cancer, diabetes, gout, cardiovascular [
4] and neurological disorders [
5]. Conversely, altered NLRP3 inflammasome activation capacity called NLRP3 inflammasome exhaustion has been described in infectious diseases such as sepsis [
6] or severe COVID-19 [
7] in association with deleterious outcomes. This highlights the necessity to develop robust immunomonitoring assays to evaluate NLRP3 inflammation activation and exhaustion in clinical samples.
Monitoring of NRLP3 inflammasome activation in human samples has mainly been performed through detection of IL1-β, IL-18 secretion or caspase-1 activation after cell stimulation ex vivo. However, these techniques require lengthy cell purification and/or stimulation steps, which may have limited their use in clinical studies. More recently, it has been shown that NLRP3 activation can be monitored through the detection of the intracytoplasmic polymerization of the ASC protein (i.e., speck formation) [
8], which might represent a more specific read-out of NLRP3 inflammasome activation than cytokine secretion measurement [
9]. Indeed, before activation, a weak and diffuse ASC fluorescence is observed in cells. After activation, ASC aggregates into a single concentrated bright fluorescent speck in the perinuclear region, which can be detected by flow cytometry, while the rest of the cell is mostly depleted of any fluorescence signal [
10].
So far, only a few papers have described a technique to detect ASC speck formation directly in whole blood without any cell purification. In addition, to the best of our knowledge, none of these protocols included an ex vivo stimulation. This is very important as such stimulation step will allow for the detection of NLRP3 inflammasome pathway exhaustion in clinical studies in addition to the monitoring of basal NLRP3 inflammasome pathway activation in vivo.
In this context, the objective of the current work was to define a simple flow cytometry protocol for the measurement of ASC speck formation directly in human whole blood including an ex vivo stimulation step. Such refined technique will help to simultaneously assess basal NLRP3 pathway activation level in vivo and its inhibition or exhaustion following re-activation ex vivo in clinical studies.
2. Materials and Methods
2.1. Reagents
The following antibodies were used: CD45-PB, CD14-APC (both from Beckman Coulter, Brea, CA, USA, clones J33 and RMO52, respectively), CD15-AF700 and anti-ASC-PE (both from Biolegend, San Diego, CA, USA, clones W6D3 and HASC-71, respectively). Following reagents were used: phosphate-buffered saline (PBS) (Eurobio Scientific, Luxembourg, Luxembourg), Ficoll (Dutscher, Bruxelles, Belgium), RPMI HEPES (Eurobio, Les Ulis, France), penicilline/streptomycine (Clinisciences, Nanterre, France), fungizone (Cheplapharm Arzneimittel, Mesekenhagen, Germany), glutamine (Ozyme, Saint-Cyr-l’École, France), versalyse (Beckman Coulter), lipopolysaccharide (LPS) (Sigma-Aldrich, single mix of references L3137, L2637 and L3012, Saint-Louis, MO, USA), nigericin (InvivoGen, San Diego, CA, USA), water for injection (Aguettant, Lyon, France), Cytofix/Cytoperm, Fixation/Permeabilization Kit, stain buffer (both from BD Bioscience, Franklin Lakes, NJ, USA) and human AB serum (SAB) (“Etablissement Français du Sang”, Lyon, France).
2.2. Blood Sample Processing
Fresh peripheral blood from healthy donors (HD) was provided by the Etablissement Français du Sang (French National Blood Bank) from Lyon using heparin anticoagulant tubes. A written non-opposition to the use of donated blood for research purposes was obtained from the HD. PBMCs were isolated using density gradient centrifugation. Briefly, heparin blood was diluted 1:1 with PBS, carefully pipetted on the ficoll solution and centrifuged for 20 min at 770×
g with low acceleration and low brake applied. The PBMCs ring was removed by pipetting and the cells washed with PBS by centrifugation for 10 min at 800×
g, 3 times. After the final wash, cells were re-suspended in 1 mL of RPMI HEPES + penicilline/streptomycine (20 µg/mL) + fungizone (2.5 µg/mL + glutamine (2 mM). In addition, 4 septic shock patients, with blood drawn during the first 48 h after ICU admission, were tested to illustrate the potential of such whole blood protocol in clinical samples. Patients were included in the IMMUNOSEPSIS-4 cohort. Diagnostic criteria for septic shock was based on the Sepsis-3 definition [
11]. Exclusion criteria disqualified patients under 18 years of age, subjects with aplasia or pre-existent immunosuppression, pregnant women, and institutionalized patients. This project was approved by Institutional Review Board for ethics (“Comité de Protection des Personnes Ouest II”, n° RCB: 2019-A00210-57). This study is registered at the French Ministry of Research and Teaching (#DC-2008-509), at the Commission Nationale de l’Informatique et des Libertés, and on
https://clinicaltrials.gov (#NCT04067674).
2.3. ASC Staining in PBMCs
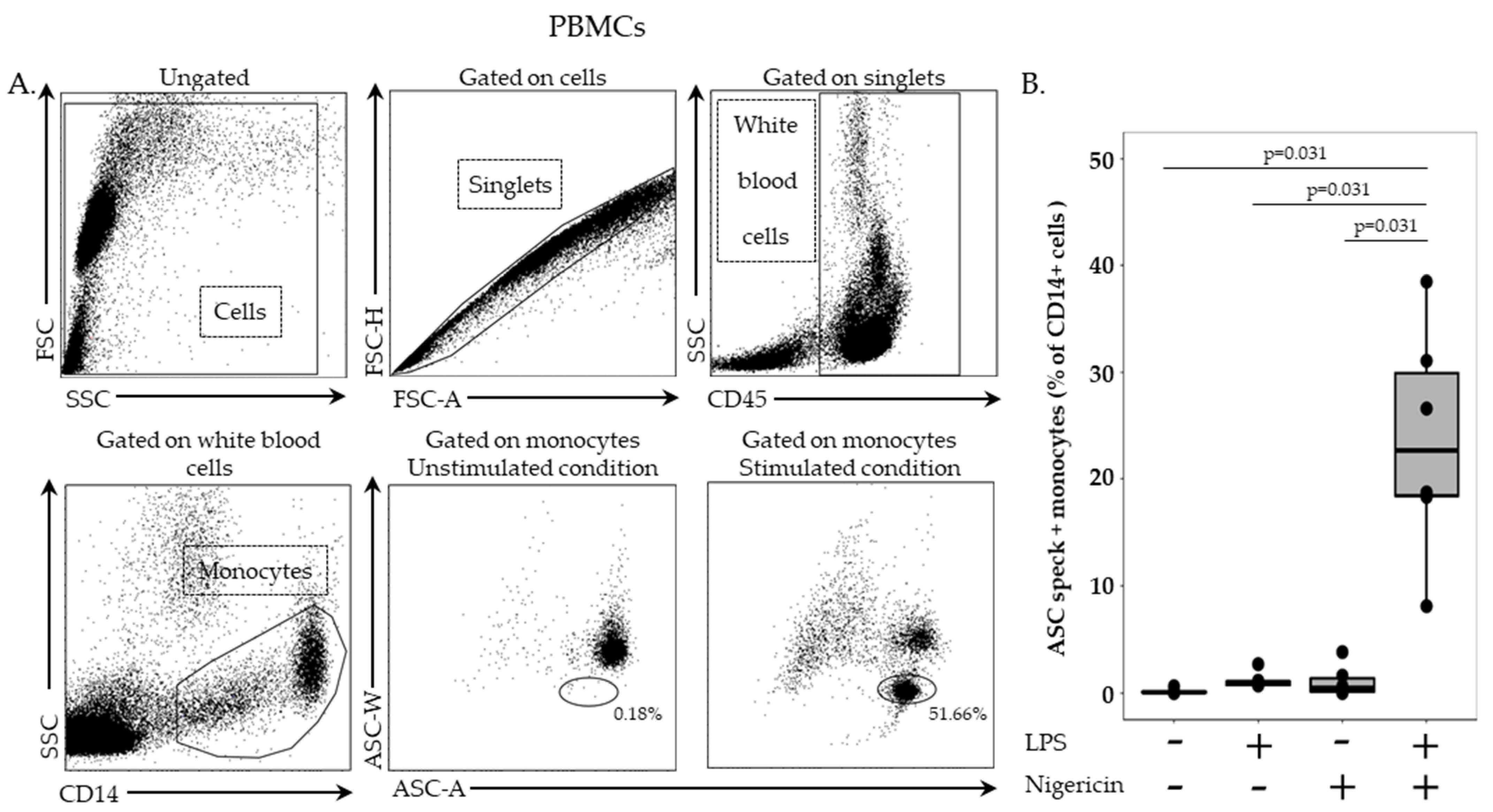
In this stage, 2.5 million cells/mL in RPMI HEPES were incubated with or without LPS at 0.1 µg/mL for 3 h, at 37 °C, followed by incubation with or without nigericin at 10 µg/mL for additional 30 min, at 37 °C. Cells were washed with 1 mL of stain buffer, centrifuged for 6 min at 300× g and supernatant removed. PBMCs were then labelled with 5 µL of PB-labelled anti-CD45 antibody and 5 µL of APC-labelled anti-CD14 antibody, incubated for 15 min, at room temperature, in the dark and washed with 1 mL of stain buffer for 6 min at 300× g. Permeabilization was performed by using 100 µL of Cytoperm reagent, incubated for 20 min, at 4 °C. After two washing steps with 1 mL of 1:10 diluted Perm/Wash buffer, cells were incubated with PE-labelled anti-ASC antibody for 20 min, at room temperature, in the dark. A final washing was performed with diluted Perm/Wash buffer, supernatant removed, and cells re-suspended in 300 µL of PBS. Samples were run on a BD FACS ARIA II (BD Bioscience, Franklin Lakes, NJ, USA) and results expressed as percentage of ASC speck-positive cells among respective cell populations.
2.4. Cell Sorting and Confocal Microscopy
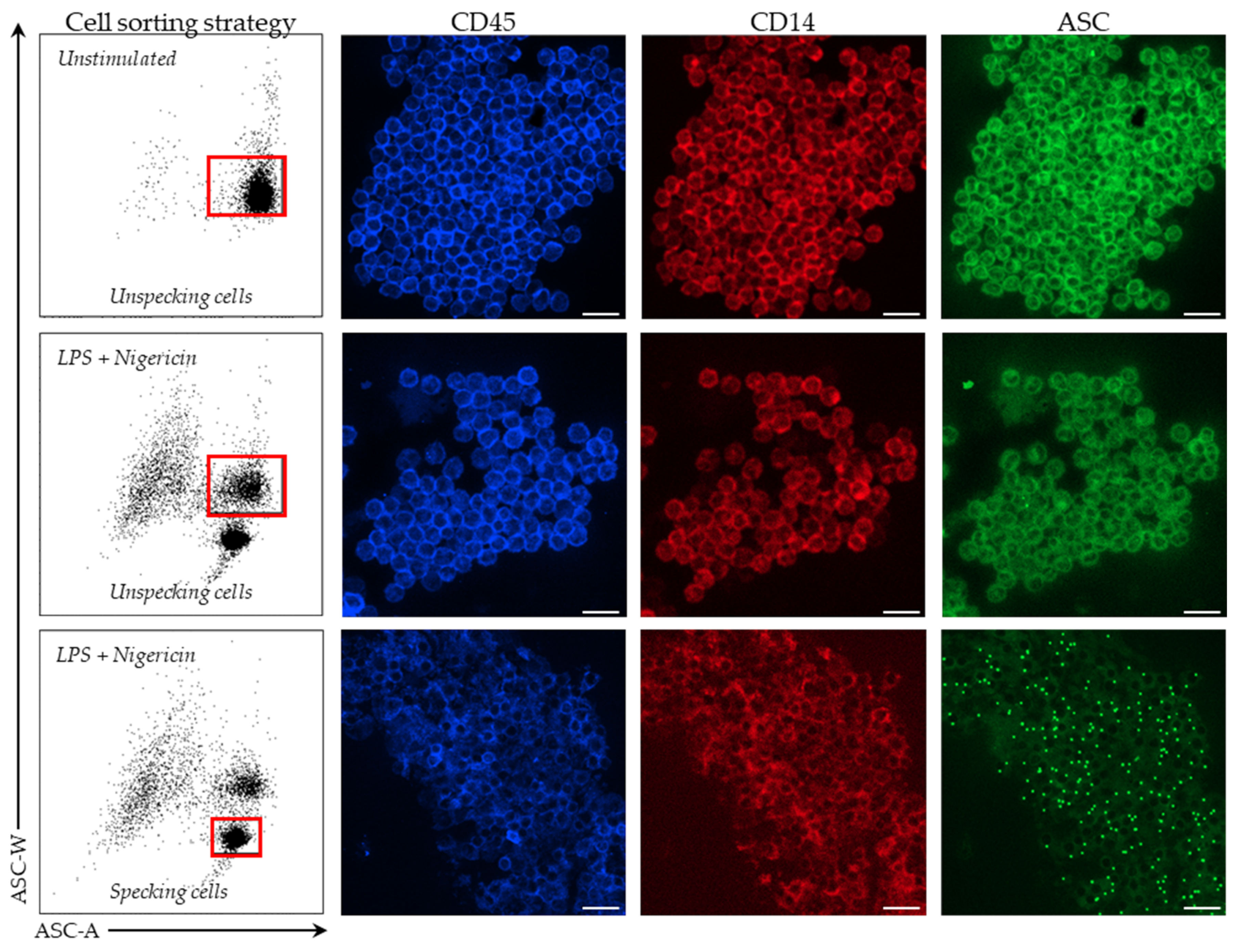
In stimulated and unstimulated conditions, monocytes were sorted on a BD FACS ARIA II cytometer (BD Bioscience). Among PBMCs, monocytes were first selected based on CD14 expression. Cells were then sorted based on ASC staining profile with a homogeneous ASC-staining profile of unstimulated monocytes and a bi-modal ASC staining in monocytes after LPS and nigericin stimulations. Sorted cells were centrifuged for 6 min at 2200× g, supernatant removed and cells re-suspended in 200 µL of SAB. Finally, cells were fixed on a glass slide using a cytospin for 5 min at 3000 rpm. Cells were imaged using Confocal Zeiss LSM980 (Carl Zeiss, Marly le Roi, France) and 10x objectives. Images were analyzed using ZEN lite software (version 3.4, Carl Zeiss, Marly le Roi, France).
2.5. ASC Staining in Whole Blood
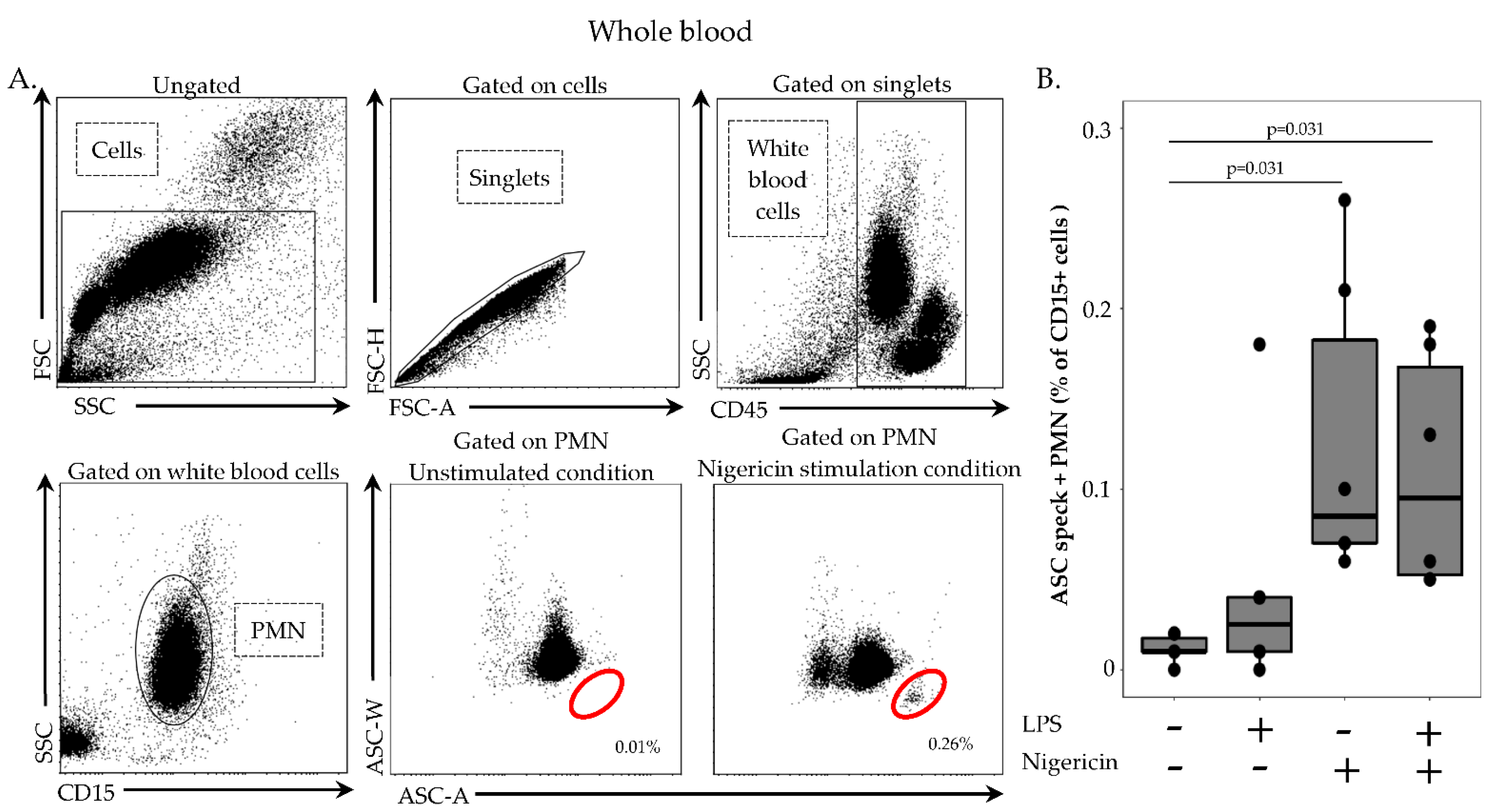
In order to remove red blood cells, 100 µL of whole heparin blood was first diluted in 1 mL of lysis solution (Versalyse) for 10 min, at room temperature, in the dark. Cells were then washed with 1 mL of PBS for 10 min, at room temperature, in the dark and centrifuged for 6 min at 300× g. After supernatant removal, cells were re-suspended in 500 µL of PBS and incubated with or without LPS at 0.1 µg/mL for 3 h, at 37 °C, then with or without nigericin at 10 µg/mL for 30 min, at 37 °C. Cells were washed with 1 mL of stain buffer, centrifuged for 6 min at 300× g and supernatant removed. Cells were then labelled with 5 µL of PB-labelled anti CD45-antibody, 5 µL of APC-labelled anti-CD14 antibody and 5 µL of AF700-labelled anti-CD15 antibody, incubated 15 min, at room temperature, in the dark and washed with 1 mL of stain buffer for 6 min at 300× g. A permeabilization step was performed by using 250 µL of Cytoperm, incubated for 20 min, at 4 °C. After two washings steps with 1 mL of 1:10 diluted Perm/Wash buffer, cells were labelled with 5 µL of PE-labelled anti-ASC antibody for 20 min, at room temperature, in the dark. A final wash was performed with 1 mL of diluted Perm/Wash buffer, supernatant removed and cells re-suspended in 300 µL of PBS. Samples were run on a FACS ARIA II cytometer and results expressed as percentage of ASC speck-positive cells among respective cell population.
2.6. Statistical Analysis
Results were presented as individual values and boxplots. Statistical analysis were performed using the non-parametric Wilcoxon paired test. Data were analyzed using R Studio software (version 1.2.5001; R studio, Boston, MA, USA).
4. Discussion
NLRP3 inflammasome is implicated in a number of highly prevalent clinical conditions and has been consequently largely investigated by different techniques both in experimental set-up and at clinical levels. A first approach consists of detecting inflammasomes activation through cytokines production (namely IL-1β and IL-18) commonly detected by ELISA or immunoblot analysis in plasma or after ex vivo stimulation of purified cells. However, IL-1β cleavage and release may occur through inflammasome-independent processes [
12]. Thus, this approach may not be specific to inflammasomes. An additional method is based on the detection of caspase 1 activation through immunoblot of purified cells or by flow cytometry in whole blood [
13]. This latest technology presents the benefit of studying the caspase 1 activation in specific cell types and subpopulations and has been used to investigate the NLRP3 inflammasome activation in COVID-19 patients [
13]. However, as for cytokine release assays, caspase 1 activation may not be fully specific of inflammasome activation but may be confounded with other non-inflammasome-related caspase or protease activities [
9]. Another way of studying inflammasomes activation relies on cell death detection, but the distinction of pyroptosis (specific to the inflammasome related cell death) from other forms of cell death remains challenging [
14].
Overall, direct assessment of the ASC polymerization at the cellular level appears as the most specific technique to monitor inflammasomes activation. In addition, the use of nigericin stimulation provides a specific activation for NLRP3 inflammasome pathway among other inflammasomes. ASC speck formation could be assessed by microscopy [
15]. However, this approach is not suitable for large-scale human studies. To circumvent microscopy practical drawbacks in clinical research, Sester and colleagues took advantage of flow cytometry to assess ASC speck polymerization in PBMCs following stimulation ex vivo [
8]. With this approach, Martínez-García et al. reported the profoundly altered NLRP3 activation in septic patients in association with mortality [
6]. Recently, after evaluation of various pre-analytical conditions (type of anticoagulant, temperature and sample storage time, etc.), Wittman and his team provided technical recommendations regarding ASC specks detection in human PBMCs [
16]. In order to facilitate clinical explorations, Cui et al. reported a whole blood protocol without any subsequent ex vivo stimulation to study in vivo NLRP3 inflammasome activation level [
17]. Consequently, the objective of the present study was to set up a refined whole blood protocol for ASC speck formation monitoring in primary human cells including an ex vivo stimulation step to follow both in vivo NLRP3 inflammasome activation level and ex vivo re-activation capacity. To the best of our knowledge, no study has, so far, described such a technique.
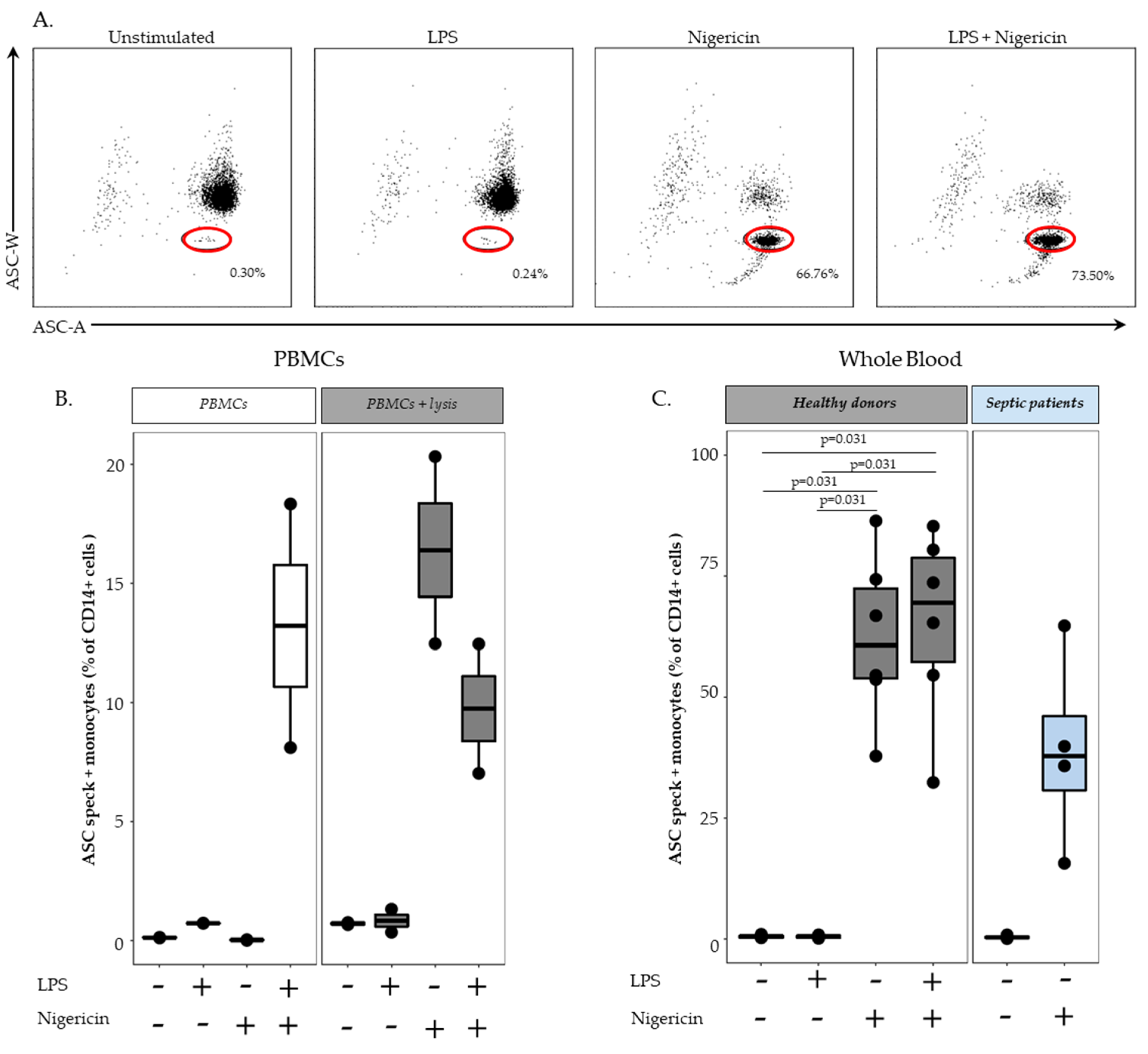
First, we demonstrated that the characteristic signal of ASC speck formation by flow cytometry (i.e., diminished fluorescence width) observed in PBMCs was also observed in whole blood. By using confocal microscopy on flow cytometry-sorted cells, we confirmed that cells identified by diminished ASC-width by flow cytometry show ASC speck. Based on this, we were able to monitor NLRP3 activation in whole blood from both HD and septic patients. In line with data reported by Martínez-García et al., our preliminary results showed a marked decrease in ASC speck formation in cells from patients suffering sepsis when sampled within the first 48 h after ICU admission.
In whole blood assay, high amount of ASC speck-positive monocytes were detected after LPS and nigericin stimulation as expected. Surprisingly, a similar level of ASC speck-positive monocytes was detected when stimulated with nigericin alone. Although the priming step of NLRP3 activation for cytokine release has been demonstrated to be sometimes dispensable [
18,
19]; priming (with LPS) preceding activation (with ATP or nigericin) considerably increases the activation of the inflammasome. It is noteworthy that we added a lysis pre-treatment step in the whole blood assay to remove red blood cells. We confirmed experimentally by using lysis buffer prior to NLRP3 inflammasome stimulation of PBMCs that cell stress caused by the initial use of lysis solution or that damage-associated molecular pattern molecules (DAMPs) released from lysed red blood cells could provide the priming signal. As a result, stimulation with nigericin alone following lysis buffer treatment enabled us to detect an increased percentages of monocytes with speck compared with results in PBMCs without lysis buffer treatment. Such a removal of the LPS stimulation step resulted in an easier and time-saving protocol.
Another asset of the described protocol is to allow the exploration of neutrophil present in whole blood but discarded upon the PBMCs purification step. Although inflammasomes were discovered and mainly studied in monocytes and macrophages, recent studies suggest that the importance of the neutrophil inflammasome has been underestimated [
20]. Here, as observed in monocytes, we noticed an increase in ASC speck-positive neutrophils after stimulation. To note, although percentages of ASC speck-positive neutrophils were much lower than in monocytes upon stimulation; ASC speck formation in these cells may nevertheless be clinically relevant considering the high number of circulating neutrophils in healthy donors and their increased number in certain clinical conditions such as sepsis. In addition, these results are consistent with the study of Boucher et al., in which neutrophils assembled less ASC speck than macrophages [
21]. Thus, the current protocol should allow for the robust monitoring of ASC speck formation in neutrophils in clinical samples.