Iron Oxide Nanoparticles Combined with Static Magnetic Fields in Bone Remodeling
Abstract
1. Introduction
2. Effects of Iron Oxide Nanoparticles on Bone Remodeling
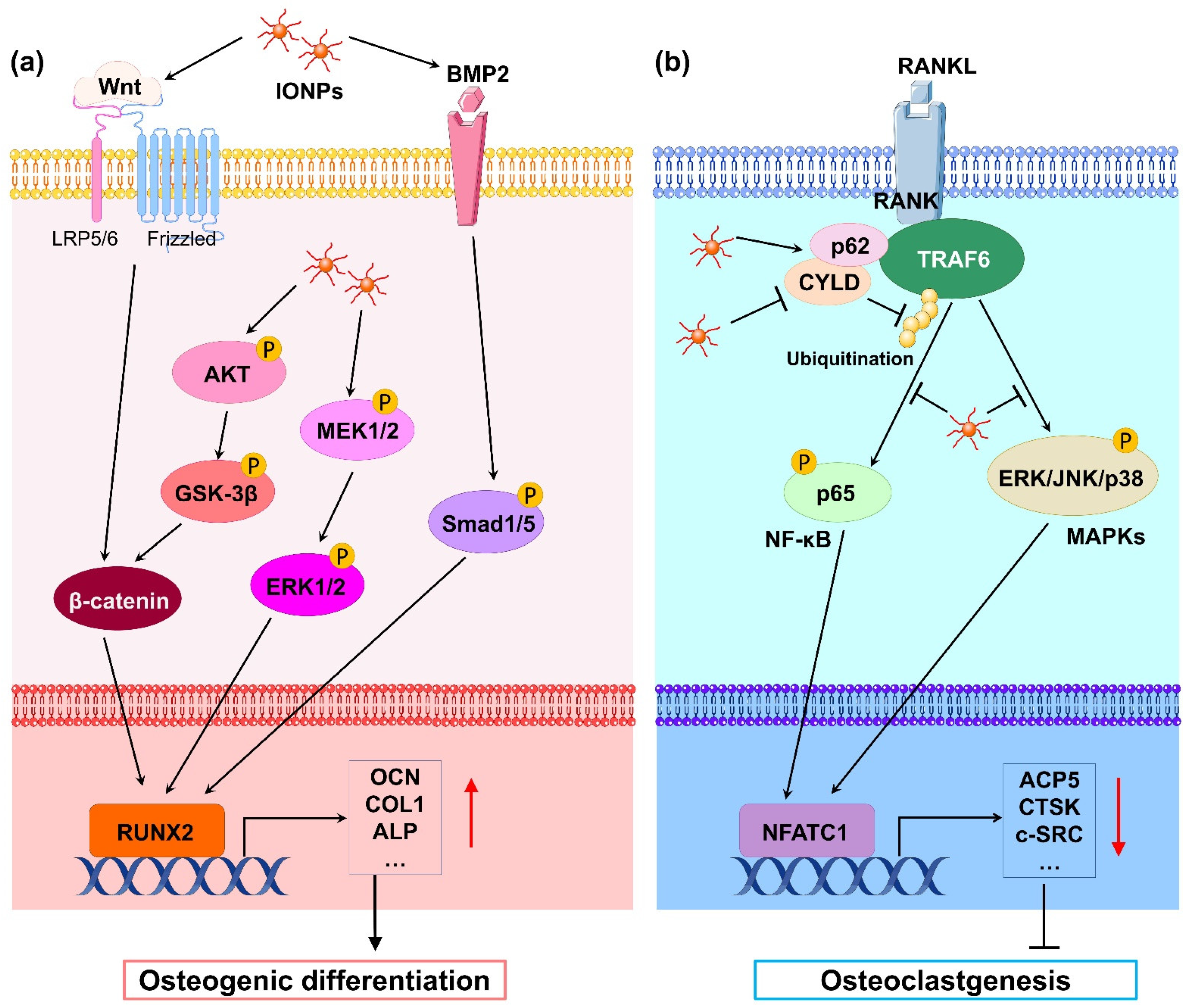
2.1. Effects of Iron Oxide Nanoparticles on Osteoblasts
2.2. Effects of Iron Oxide Nanoparticles on Osteoclasts
3. Effects of Static Magnetic Fields on Bone Remodeling
3.1. Effects of Static Magnetic Fields on Osteoblasts
3.2. Effects of Static Magnetic Fields on Osteoclasts
4. Effects of Iron Oxide Nanoparticles Combined with Static Magnetic Fields on Bone Remodeling
4.1. Effects of Iron Oxide Nanoparticles Combined with Static Magnetic Fields on Osteoblasts
4.2. Effects of Iron Oxide Nanoparticles Combined with Static Magnetic Fields on Osteoclasts
5. Mechanism of Static Magnetic Field Enhanced the Biological Effects of Iron Oxide Nanoparticles
5.1. Micromagnetic Field Effects
5.2. Mechanical Stimulation
5.3. Increases in Intracellular Iron Oxide Nanoparticles
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Ye, D.; Li, M.; Ma, M.; Gu, N. Adaptive Materials Based on Iron Oxide Nanoparticles for Bone Regeneration. Chemphyschem 2018, 19, 1965–1979. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics 2022, 12, 796–816. [Google Scholar] [CrossRef]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef]
- Yang, J.; Shang, P.; Zhang, H. Effect of static magnetic field on bone and its molecular mechanism. Chin. Sci. Bull. 2020, 65, 1238–1250. [Google Scholar] [CrossRef]
- Marycz, K.; Sobierajska, P.; Roecken, M.; Kornicka-Garbowska, K.; Kępska, M.; Idczak, R.; Nedelec, J.M.; Wiglusz, R.J. Iron oxides nanoparticles (IOs) exposed to magnetic field promote expression of osteogenic markers in osteoblasts through integrin alpha-3 (INTa-3) activation, inhibits osteoclasts activity and exerts anti-inflammatory action. J. Nanobiotechnol. 2020, 18, 1–24. [Google Scholar]
- Pareta, R.A.; Taylor, E.; Webster, T.J. Increased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticles. Nanotechnology 2008, 19, 265101. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater. 2011, 7, 1298–1306. [Google Scholar] [CrossRef]
- Tran, N.; Hall, D.; Webster, T.J. Mechanisms of enhanced osteoblast gene expression in the presence of hydroxyapatite coated iron oxide magnetic nanoparticles. Nanotechnology 2012, 23, 455104. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Understanding magnetic nanoparticle osteoblast receptor-mediated endocytosis using experiments and modeling. Nanotechnology 2013, 24, 185102. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.F.; Jia, J.F.; Guo, X.K.; Zhao, Y.P.; Chen, D.S.; Guo, Y.Y.; Cheng, T.; Zhang, X.L. Biocompatibility of chitosan-coated iron oxide nanoparticles with osteoblast cells. Int. J. Nanomed. 2012, 7, 5593–5602. [Google Scholar]
- Yin, G.; Huang, Z.; Deng, M.; Zeng, J.; Gu, J. Preparation and cell response of bio-mineralized Fe3O4 nanoparticles. J. Colloid Interface Sci. 2011, 363, 393–402. [Google Scholar] [CrossRef]
- Xiao, H.T.; Wang, L.; Yu, B. Superparamagnetic iron oxide promotes osteogenic differentiation of rat adipose-derived stem cells. Int. J. Clin. Exp. Med. 2015, 8, 698–705. [Google Scholar]
- Xia, Y.; Chen, H.; Zhang, F.; Wang, L.; Chen, B.; Reynolds, M.A.; Ma, J.; Schneider, A.; Gu, N.; Xu, H.H.K. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 423–433. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C-Mater. 2019, 98, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med. 1995, 18, 775–794. [Google Scholar] [CrossRef]
- Huang, D.M.; Hsiao, J.K.; Chen, Y.C.; Chien, L.Y.; Yao, M.; Chen, Y.K.; Ko, B.S.; Hsu, S.C.; Tai, L.A.; Cheng, H.Y.; et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M.; Jho, E.H. Wnt/β-catenin signalling: From plasma membrane to nucleus. Biochem. J. 2013, 450, 9–21. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, Y.; Yang, Z.; Chen, H.; Ren, K.; Weir, M.D.; Chow, L.C.; Reynolds, M.A.; Zhang, F.; Gu, N.; et al. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/beta-catenin signaling. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109955. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Styner, M.; Xie, Z.; Case, N.; Rubin, C.T.; Rubin, J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J. Biol. Chem. 2009, 284, 34607–34617. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Jiang, D.; Wang, T.; Wang, Y.; Lou, Y.; Zhang, Y.; Ma, H.; Kang, Y. Mechanical Stress Regulates Osteogenesis and Adipogenesis of Rat Mesenchymal Stem Cells through PI3K/Akt/GSK-3β/β-Catenin Signaling Pathway. Biomed. Res. Int. 2017, 2017, 6027402. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zheng, L.; Wang, P.; Chai, S.; Zhang, Y.; Shi, T.; Zhang, L.; Peng, R.; Huang, C.; Guo, B.; et al. Development of a novel polysaccharide-based iron oxide nanoparticle to prevent iron accumulation-related osteoporosis by scavenging reactive oxygen species. Int. J. Biol. Macromol. 2020, 165, 1634–1645. [Google Scholar] [CrossRef]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, C.; Shin, D.Y.; Kim, J.M.; Lalani, S.; Li, N.; Yang, Y.S.; Liu, Y.; Eiseman, M.; Davis, R.J.; et al. c-Jun N-Terminal Kinases (JNKs) Are Critical Mediators of Osteoblast Activity In Vivo. J. Bone Miner. Res. 2017, 32, 1811–1815. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Cao, M.; Sun, J.; Wu, H.; Zhao, P.; Xing, J.; Yang, Y.; Zhang, X.; Ji, M.; et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11–20. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Lu, J.-W.; Yang, F.; Ke, Q.-F.; Xie, X.-T.; Guo, Y.-P. Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 811–822. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H.K. Enhanced bone regeneration and visual monitoring via super-paramagnetic iron oxide nanoparticle-scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef]
- Liao, W.; Lu, J.; Wang, Q.; Yan, S.; Li, Y.; Zhang, Y.; Wang, P.; Jiang, Q.; Gu, N. Osteogenesis of Iron Oxide Nanoparticles-Labeled Human Precartilaginous Stem Cells in Interpenetrating Network Printable Hydrogel. Front. Bioeng. Biotechnol. 2022, 10, 872149. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Patel, K.D.; Lee, J.H.; Lee, E.J.; Kim, J.H.; Kim, T.H.; Kim, H.W. Potential of magnetic nanofiber scaffolds with mechanical and biological properties applicable for bone regeneration. PLoS ONE 2014, 9, e91584. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Russo, A.; Giavaresi, G.; Sartori, M.; Veronesi, F.; Fini, M.; Salter, D.M.; Ortolani, A.; Strazzari, A.; Visani, A.; et al. Innovative magnetic scaffolds for orthopedic tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020, 185, 108275. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Tan, Y.; Jie, L.; Wu, X.; Yu, R.; Zhang, M. Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Res. Ther. 2013, 4, 44. [Google Scholar] [CrossRef]
- Shrestha, S.; Jiang, P.; Sousa, M.H.; Morais, P.C.; Mao, Z.; Gao, C. Citrate-capped iron oxide nanoparticles impair the osteogenic differentiation potential of rat mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 245–256. [Google Scholar] [CrossRef]
- Guimarães, L.F.; Fidalgo, T.K.; Menezes, G.C.; Primo, L.G.; Costa e Silva-Filho, F. Effects of citric acid on cultured human osteoblastic cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 665–669. [Google Scholar] [CrossRef]
- Li, M.; Fu, S.; Cai, Z.; Li, D.; Liu, L.; Deng, D.; Jin, R.; Ai, H. Dual regulation of osteoclastogenesis and osteogenesis for osteoporosis therapy by iron oxide hydroxyapatite core/shell nanocomposites. Regen Biomater. 2021, 8, rbab027. [Google Scholar] [CrossRef]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, 587–594. [Google Scholar] [CrossRef]
- Liu, L.; Jin, R.; Duan, J.; Yang, L.; Cai, Z.; Zhu, W.; Nie, Y.; He, J.; Xia, C.; Gong, Q.; et al. Bioactive Iron Oxide Nanoparticles Suppress Osteoclastogenesis and Ovariectomy-induced Bone Loss through Regulating the TRAF6-p62-CYLD Signaling Complex. Acta Biomater. 2019, 103, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhuang, Z.; Li, Y.; Shi, T.; Fu, K.; Yan, W.; Zhang, L.; Wang, P.; Li, L.; Jiang, Q. Bone targeting antioxidative nano-iron oxide for treating postmenopausal osteoporosis. Bioact. Mater. 2022, 14, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, Q.; Zeng, Y. Melatonin: Potential avenue for treating iron overload disorders. Ageing Res. Rev. 2022, 81, 101717. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Jin, W.; Chang, M.; Paul, E.M.; Babu, G.; Lee, A.J.; Reiley, W.; Wright, A.; Zhang, M.; You, J.; Sun, S.C. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J. Clin. Investig. 2008, 118, 1858–1866. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, C.; Ren, L.; Zhou, Y.; Shang, P. The effects of static magnetic fields on bone. Prog. Biophys. Mol. Biol. 2014, 114, 146–152. [Google Scholar] [CrossRef]
- Lew, W.Z.; Feng, S.W.; Lee, S.Y.; Huang, H.M. The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine. Cells 2021, 10, 2662. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Ding, C.; Dong, D.; Shang, P. Regulation of Osteoblast Differentiation and Iron Content in MC3T3-E1 Cells by Static Magnetic Field with Different Intensities. Biol. Trace Elem. Res. 2018, 184, 214–225. [Google Scholar] [CrossRef]
- Kim, E.C.; Leesungbok, R.; Lee, S.W.; Lee, H.W.; Park, S.H.; Mah, S.J.; Ahn, S.J. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics 2015, 36, 267–276. [Google Scholar] [CrossRef]
- Chuo, W.; Ma, T.; Saito, T.; Sugita, Y.; Maeda, H.; Zhang, G.; Li, J.; Liu, J.; Lu, L. A preliminary study of the effect of static magnetic field acting on rat bone marrow mesenchymal stem cells during osteogenic differentiation in vitro. J. Hard Tissue Biol. 2013, 22, 227–232. [Google Scholar] [CrossRef]
- Chen, G.; Zhuo, Y.; Tao, B.; Liu, Q.; Shang, W.; Li, Y.; Wang, Y.; Li, Y.; Zhang, L.; Fang, Y.; et al. Moderate SMFs attenuate bone loss in mice by promoting directional osteogenic differentiation of BMSCs. Stem Cell Res. Ther. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, L.; Chen, L.; Jiang, J.; Zhou, X.; Wang, M.; Fan, Y. Static magnetic field regulates proliferation, migration, differentiation and YAP/TAZ activation of human dental pulp stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ohsaki, Y.; Goto, T.; Nakasima, A.; Iijima, T. Effects of static magnetic fields on bone formation in rat osteoblast cultures. J. Dent. Res. 2003, 82, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Lee, S.Y.; Yao, W.C.; Lin, C.T.; Yeh, C.Y. Static magnetic fields up-regulate osteoblast maturity by affecting local differentiation factors. Clin. Orthop. Relat. Res. 2006, 447, 201–208. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Ozawa, S.; Hirukawa, K.; Togari, A.; Tanaka, Y. Effects of a Static Magnetic Field on Mineralization of MC3T3-E1 Cells. Prosthod. Res. Pract. 2007, 6, 87–92. [Google Scholar] [CrossRef]
- Nowogrodzki, A. The world’s strongest MRI machines are pushing human imaging to new limits. Nature 2018, 563, 24–26. [Google Scholar] [CrossRef]
- Nagel, A.M.; Umathum, R.; Rösler, M.B.; Ladd, M.E.; Litvak, I.; Gor’kov, P.L.; Brey, W.W.; Schepkin, V.D. 39K and 23Na relaxation times and MRI of rat head at 21.1 T. NMR Biomed. 2016, 29, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kotani, H.; Kawaguchi, H.; Shimoaka, T.; Iwasaka, M.; Ueno, S.; Ozawa, H.; Nakamura, K.; Hoshi, K. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Miner. Res. 2002, 17, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, S.; Zhang, G.; Fang, Y.; Fang, Z.; Shang, P.; Zhang, H. Static Magnetic Field (2-4 T) Improves Bone Microstructure and Mechanical Properties by Coordinating Osteoblast/Osteoclast Differentiation in Mice. Bioelectromagnetics 2021, 42, 200–211. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Ning, K.; Yang, B.; Acosta, F.M.; Shang, P.; Jiang, J.X.; Xu, H. Osteocytic Connexin43 Channels Regulate Bone-Muscle Crosstalk. Cells 2021, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, G.; Li, Q.; Tang, Q.; Feng, Y.; Shang, P.; Zeng, Y. Effect of High Static Magnetic Fields on Biological Activities and Iron Metabolism in MLO-Y4 Osteocyte-like Cells. Cells 2021, 10, 3519. [Google Scholar] [CrossRef] [PubMed]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, X.; Ding, C.; Xie, L.; Yang, P.; Shang, P. Regulation of osteoclast differentiation by static magnetic fields. Electromagn. Biol. Med. 2017, 36, 8–19. [Google Scholar] [CrossRef]
- Kim, E.C.; Park, J.; Noh, G.; Park, S.J.; Noh, K.; Kwon, I.K.; Ahn, S.J. Effects of moderate intensity static magnetic fields on osteoclastic differentiation in mouse bone marrow cells. Bioelectromagnetics 2018, 39, 394–404. [Google Scholar] [CrossRef]
- Dong, D.; Yang, J.; Zhang, G.; Huyan, T.; Shang, P. 16 T high static magnetic field inhibits receptor activator of nuclear factor kappa-Β ligand-induced osteoclast differentiation by regulating iron metabolism in Raw264.7 cells. J. Tissue Eng. Regen. Med. 2019, 13, 2181–2190. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, Y.; Qi, X.; Kong, H.; Wang, C.; Xu, Z.; Xie, S.; Gu, N.; Xu, H. Paramagnetic nanofibrous composite films enhance the osteogenic responses of pre-osteoblast cells. Nanoscale 2010, 2, 2565–2569. [Google Scholar] [CrossRef]
- Zeng, X.B.; Hu, H.; Xie, L.Q.; Lan, F.; Jiang, W.; Wu, Y.; Gu, Z.W. Magnetic responsive hydroxyapatite composite scaffolds construction for bone defect reparation. Int. J. Nanomed. 2012, 7, 3365–3378. [Google Scholar] [CrossRef]
- Meng, J.; Xiao, B.; Zhang, Y.; Liu, J.; Xue, H.; Lei, J.; Kong, H.; Huang, Y.; Jin, Z.; Gu, N.; et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci. Rep. 2013, 3, 2655. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Huang, J.; Song, L.; Chen, Z.; Liu, H.; Li, Y.; Zhang, Y.; Gu, N. Magnetic assembly-mediated enhancement of differentiation of mouse bone marrow cells cultured on magnetic colloidal assemblies. Sci. Rep. 2014, 4, 5125. [Google Scholar] [CrossRef]
- Cai, Q.; Shi, Y.; Shan, D.; Jia, W.; Duan, S.; Deng, X.; Yang, X. Osteogenic differentiation of MC3T3-E1 cells on poly(L-lactide)/Fe3O4 nanofibers with static magnetic field exposure. Mater. Sci. Eng. C-Mater. 2015, 55, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, Y.; Zhu, C.; Zhang, W.; Mao, Z.; Gao, C. Fe3O4/BSA particles induce osteogenic differentiation of mesenchymal stem cells under static magnetic field. Acta Biomater. 2016, 46, 141–150. [Google Scholar] [CrossRef]
- Yun, H.M.; Ahn, S.J.; Park, K.R.; Kim, M.J.; Kim, J.J.; Jin, G.Z.; Kim, H.W.; Kim, E.C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, H.; Zeng, X.; Lan, F.; Wu, F.; Wu, Y. A magnetic hydroxyapatite composite scaffold-based magnetic therapy for bone repair: An experimental study in canis lupus familiaris. Regener. Biomater. 2017, 4, 97–103. [Google Scholar] [CrossRef][Green Version]
- Zhuang, J.; Lin, S.; Dong, L.; Cheng, K.; Weng, W. Magnetically actuated mechanical stimuli on Fe3O4/mineralized collagen coatings to enhance osteogenic differentiation of the MC3T3-E1 cells. Acta Biomater. 2018, 71, 49–60. [Google Scholar] [CrossRef]
- Hao, L.; Li, L.; Wang, P.; Wang, Z.; Shi, X.; Guo, M.; Zhang, P. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale 2019, 11, 23423–23437. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Alicka, M.; Kornicka-Garbowska, K.; Polnar, J.; Lis-Bartos, A.; Wiglusz, R.J.; Roecken, M.; Nedelec, J.M. Promotion through external magnetic field of osteogenic differentiation potential in adipose-derived mesenchymal stem cells: Design of polyurethane/poly(lactic) acid sponges doped with iron oxide nanoparticles. J. Biomed. Mater. Res. B 2020, 108, 1398–1411. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Liu, Y.; Yu, Y.; Lin, S.; Wang, Q.; Miao, L.; Wei, H.; Sun, W. Fe3O4@GO magnetic nanocomposites protect mesenchymal stem cells and promote osteogenic differentiation of rat bone marrow mesenchymal stem cells. Biomater. Sci. 2020, 8, 5984–5993. [Google Scholar] [CrossRef]
- Huang, Z.; He, Y.; Chang, X.; Liu, J.; Yu, L.; Wu, Y.; Li, Y.; Tian, J.; Kang, L.; Wu, D.; et al. A Magnetic Iron Oxide/Polydopamine Coating Can Improve Osteogenesis of 3D-Printed Porous Titanium Scaffolds with a Static Magnetic Field by Upregulating the TGFβ-Smads Pathway. Adv. Healthc. Mater. 2020, 9, e2000318. [Google Scholar] [CrossRef]
- Tang, B.; Shen, X.; Yang, Y.; Xu, Z.; Yi, J.; Yao, Y.; Cao, M.; Zhang, Y.; Xia, H. Enhanced cellular osteogenic differentiation on CoFe2O4/P(VDF-TrFE) nanocomposite coatings under static magnetic field. Colloids Surf. B Biointerfaces 2020, 198, 111473. [Google Scholar] [CrossRef]
- Tang, B.; Shen, X.; Ye, G.; Yang, Y.; Jiang, Y.; Xia, H.; Chen, X. Magnetic-Field-Assisted Cellular Osteogenic Differentiation on Magnetic Zinc Ferrite Coatings via MEK/ERK Signaling Pathways. ACS Biomater. Sci. Eng. 2020, 6, 6864–6873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Chen, R.; Xue, P.P.; Luo, L.Z.; Zhong, B.; Tong, M.Q.; Chen, B.; Yao, Q.; Yuan, J.D.; Xu, H.L. Magnetic PLGA microspheres loaded with SPIONs promoted the reconstruction of bone defects through regulating the bone mesenchymal stem cells under an external magnetic field. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111877. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, G.; Li, Y.; Li, Y.; Yi, C.; Zhang, X.; Li, H.; Zeng, B.; Wang, C.; Xie, W.; et al. Effect of magnetic graphene oxide on cellular behaviors and osteogenesis under a moderate static magnetic field. Nanomedicine 2021, 37, 102435. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Turlej, E.; Kornicka-Garbowska, K.; Zachanowicz, E.; Tomaszewska, A.; Kulpa-Greszta, M.; Pązik, R. Co0.5Mn0.5Fe2O4@PMMA Nanoparticles Promotes Preosteoblast Differentiation through Activation of OPN-BGLAP2-DMP1 Axis and Modulates Osteoclastogenesis under Magnetic Field Conditions. Materials 2021, 14, 5010. [Google Scholar] [CrossRef]
- Marycz, K.; Smieszek, A.; Marcinkowska, K.; Sikora, M.; Turlej, E.; Sobierajska, P.; Patej, A.; Bienko, A.; Wiglusz, R.J. Nanohydroxyapatite (nHAp) Doped with Iron Oxide Nanoparticles (IO), miR-21 and miR-124 Under Magnetic Field Conditions Modulates Osteoblast Viability, Reduces Inflammation and Inhibits the Growth of Osteoclast—A Novel Concept for Osteoporosis Treatment: Part 1. Int. J. Nanomed. 2021, 16, 3429–3456. [Google Scholar]
- Pacakova, B.; Kubickova, S.; Salas, G.; Mantlikova, A.R.; Marciello, M.; Morales, M.P.; Niznansky, D.; Vejpravova, J. The internal structure of magnetic nanoparticles determines the magnetic response. Nanoscale 2017, 9, 5129–5140. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Karatzas, D.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 2009, 15, 208–216. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, Q.; Li, J.; Liang, X.; Chen, Y.; Wang, H.; Meng, B. Fluid shear stress changes cell morphology and regulates the expression of ATP6V1A and TCIRG1 mRNA in rat osteoclasts. Mol. Med. Rep. 2010, 3, 173–178. [Google Scholar]
- Suzuki, N.; Yoshimura, Y.; Deyama, Y.; Suzuki, K.; Kitagawa, Y. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int. J. Mol. Med. 2008, 21, 291–296. [Google Scholar] [CrossRef]
- Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol. 2008, 3, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Blümler, P. Magnetic Guiding with Permanent Magnets: Concept, Realization and Applications to Nanoparticles and Cells. Cells 2021, 10, 2708. [Google Scholar] [CrossRef]
- Li, X.; Zou, Q.; Man, Y.; Li, W. Synergistic Effects of Novel Superparamagnetic/Upconversion HA Material and Ti/Magnet Implant on Biological Performance and Long-Term In Vivo Tracking. Small 2019, 15, e1901617. [Google Scholar] [CrossRef] [PubMed]
- Boda, S.K.; Thrivikraman, G.; Basu, B. Magnetic field assisted stem cell differentiation–role of substrate magnetization in osteogenesis. J. Mater. Chem. B 2015, 3, 3150–3168. [Google Scholar] [CrossRef]
- Yuan, Z.; Memarzadeh, K.; Stephen, A.S.; Allaker, R.P.; Brown, R.A.; Huang, J. Development of a 3D Collagen Model for the In Vitro Evaluation of Magnetic-assisted Osteogenesis. Sci. Rep. 2018, 8, 16270. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Swift, J.; Ivanovska, I.; Spinler, K.R.; Buxboim, A.; Discher, D.E. Mechanobiology of bone marrow stem cells: From myosin-II forces to compliance of matrix and nucleus in cell forms and fates. Differentiation 2013, 86, 77–86. [Google Scholar] [CrossRef]
- Hamamura, K.; Swarnkar, G.; Tanjung, N.; Cho, E.; Li, J.; Na, S.; Yokota, H. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect. Tissue Res. 2012, 53, 398–406. [Google Scholar] [CrossRef]
- Katsumi, A.; Orr, A.W.; Tzima, E.; Schwartz, M.A. Integrins in mechanotransduction. J. Biol. Chem. 2004, 279, 12001–12004. [Google Scholar] [CrossRef]
- Marie, P.J. Targeting integrins to promote bone formation and repair. Nat. Rev. Endocrinol. 2013, 9, 288–295. [Google Scholar] [CrossRef]
- Kasten, A.; Müller, P.; Bulnheim, U.; Groll, J.; Bruellhoff, K.; Beck, U.; Steinhoff, G.; Möller, M.; Rychly, J. Mechanical integrin stress and magnetic forces induce biological responses in mesenchymal stem cells which depend on environmental factors. J. Cell Biochem. 2010, 111, 1586–1597. [Google Scholar] [CrossRef]
- Munger, J.S.; Sheppard, D. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb. Perspect. Biol. 2011, 3, a005017. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; He, T.; Chen, S.; Yang, D.; Yi, W.; Cao, H.; Xiao, G. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 2019, 8, e47454. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci. Rep. 2017, 7, 17696. [Google Scholar] [CrossRef]
- Tschulik, K.; Compton, R.G. Nanoparticle impacts reveal magnetic field induced agglomeration and reduced dissolution rates. Phys. Chem. Chem. Phys. 2014, 16, 13909–13913. [Google Scholar] [CrossRef]
- Shanehsazzadeh, S.; Lahooti, A.; Hajipour, M.J.; Ghavami, M.; Azhdarzadeh, M. External magnetic fields affect the biological impacts of superparamagnetic iron nanoparticles. Colloids Surf. B Biointerfaces 2015, 136, 1107–1112. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar]
- Delgado-Calle, J.M.; Bellido, T. The Osteocyte as a Signaling Cell. Physiol. Rev. 2022, 102, 379–410. [Google Scholar]
- van Tol, A.F.; Schemenz, V.; Wagermaier, W.; Roschger, A.; Razi, H.; Vitienes, I.; Fratzl, P.; Willie, B.M.; Weinkamer, R. The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. Proc. Natl. Acad. Sci. USA 2020, 117, 32251–32259. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, G.; Rux, C.; Sherk, V.D.; Heveran, C.M. Lacunar-canalicular bone remodeling: Impacts on bone quality and tools for assessment. Bone 2020, 143, 115663. [Google Scholar] [CrossRef] [PubMed]
- Gokduman, K.; Bestepe, F.; Li, L.; Yarmush, M.L.; Usta, O.B. Dose-, treatment- and time-dependent toxicity of superparamagnetic iron oxide nanoparticles on primary rat hepatocytes. Nanomedicine 2018, 13, 1267–1284. [Google Scholar] [CrossRef] [PubMed]

| Cell Type | Animal Model | Magnetic Field Intensity | IONPs | Outcome | Ref. |
|---|---|---|---|---|---|
| Pre-osteoblasts MC3T3-E1 | No application | 0.9–1.0 mT | HA- and PLA-coated γ-Fe2O3 | Promoting cell proliferation and ALP secretion | [67] |
| Pre-osteoblasts MC3T3-E1 | No application | Not clear | HA doped with Fe3O4 | Enhancing osteoblast proliferation, ALP activity, and osteocalcin synthesis | [68] |
| No application | Rabbit model of lumbar transverse defects. | 0.05–25 mT | HA and PLA doped with γ-Fe2O3 | Accelerating new bone tissue formation | [69] |
| Mouse BMSCs | No application | 20–120 mT | Bare γ-Fe2O3 | Enhancing osteogenic differentiation | [70] |
| Pre-osteoblasts MC3T3-E1 | No application | 100 mT | PLA doped with Fe3O4 | Promoting the proliferation and osteogenic differentiation | [71] |
| Rat BMSCs | No application | 1 T | BSA doped with Fe3O4 | Elevating ALP activity, calcium deposition, and expressions of osteogenic markers | [72] |
| Primary mouse calvarium osteoblasts | Mouse model of calvarium defects | 15 mT | PCL doped with Fe3O4 | Enhancing osteoblastic differentiation in vitro and the new bone formation | [73] |
| Pre-osteoblasts MC3T3-E1 | Beagle dog with femur transverse defect | 200 mT | HA doped with Fe3O4 | Increasing cell proliferation in vitro and bone healing in vivo | [74] |
| Pre-osteoblasts MC3T3-E1 | No application | 100 mT | Mineralized collagen doped with IONPs | Enhancing ALP activity, calcium deposition, and expressions of osteogenic genes | [75] |
| Pre-osteoblasts MC3T3-E1 | No application | 70–80 mT | Oleic acid and PLGA doped with IONPs | Promoting cell attachment and osteogenic differentiation | [76] |
| hDPSCs | Rat model of mandible defects | 35 ± 5 mT | CPC doped with γ-Fe2O3 | Enhancing osteogenic differentiation in vitro and bone formation in vivo | [16] |
| Pre-osteoblasts MC3T3-E1 | No application | 200 mT | α-Fe2O3/γ-Fe2O3 nanocomposite | Enhancing expression of crucial markers for osteogenesis | [7] |
| Mouse ADSCs | No application | 200 mT | TPU and PLA doped with Fe2O3 | Enhancing osteogenic differentiation of ADSCs | [77] |
| MSCs | No application | Not clear | Graphene oxide doped with Fe3O4 | Promoting osteogenic differentiation in presence of BMP2 | [78] |
| Human BMSCs | Rabbit model of femoral bone defects | 15 mT | Polydopamine doped with Fe3O4 | Enhancing cell proliferation and osteogenic differentiation in vitro and new bone formation in vivo | [79] |
| Pre-osteoblasts MC3T3-E1 | No application | 200 mT | CoFe2O4/P(VDF-TrFE) nanocomposite coatings | Enhancing cell adhesion, proliferation, and differentiation | [80] |
| Pre-osteoblasts MC3T3-E1 | No application | 200 mT | ZnFe2O4 coatings | Promoting early proliferation (3 days) and osteogenic differentiation | [81] |
| Rat BMSCs | Rat model of femoral bone defects | 50 mT | SPIONs were encapsulated into PLGA microspheres | Promoting osteogenic differentiation in vitro and repairing bone defects in vivo | [82] |
| Rat BMSCs | No application | 15 mT | Graphene oxide doped with Fe3O4 | Promoting osteogenesis in BMSCs | [83] |
| Pre-osteoblasts MC3T3-E1 and pre-osteoclasts 4B12 | No application | 200 mT | PMMA covered Co0.5Mn0.5Fe2O4 | Promoting osteoblastic differentiation and modulating osteoclastogenesis | [84] |
| Pre-osteoblasts MC3T3-E1 and pre-osteoclasts 4B12 | No application | 200 mT | Ca5(PO4)3OH/Fe3O4 functionalized with microRNAs | Activating osteogenesis and inhibiting osteoclastic differentiation | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wu, J.; Guo, Z.; Zhang, G.; Zhang, H. Iron Oxide Nanoparticles Combined with Static Magnetic Fields in Bone Remodeling. Cells 2022, 11, 3298. https://doi.org/10.3390/cells11203298
Yang J, Wu J, Guo Z, Zhang G, Zhang H. Iron Oxide Nanoparticles Combined with Static Magnetic Fields in Bone Remodeling. Cells. 2022; 11(20):3298. https://doi.org/10.3390/cells11203298
Chicago/Turabian StyleYang, Jiancheng, Jiawen Wu, Zengfeng Guo, Gejing Zhang, and Hao Zhang. 2022. "Iron Oxide Nanoparticles Combined with Static Magnetic Fields in Bone Remodeling" Cells 11, no. 20: 3298. https://doi.org/10.3390/cells11203298
APA StyleYang, J., Wu, J., Guo, Z., Zhang, G., & Zhang, H. (2022). Iron Oxide Nanoparticles Combined with Static Magnetic Fields in Bone Remodeling. Cells, 11(20), 3298. https://doi.org/10.3390/cells11203298







