The Efficacy of Faecal Microbiota Transplant and Rectal Bacteriotherapy in Patients with Recurrent Clostridioides difficile Infection: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
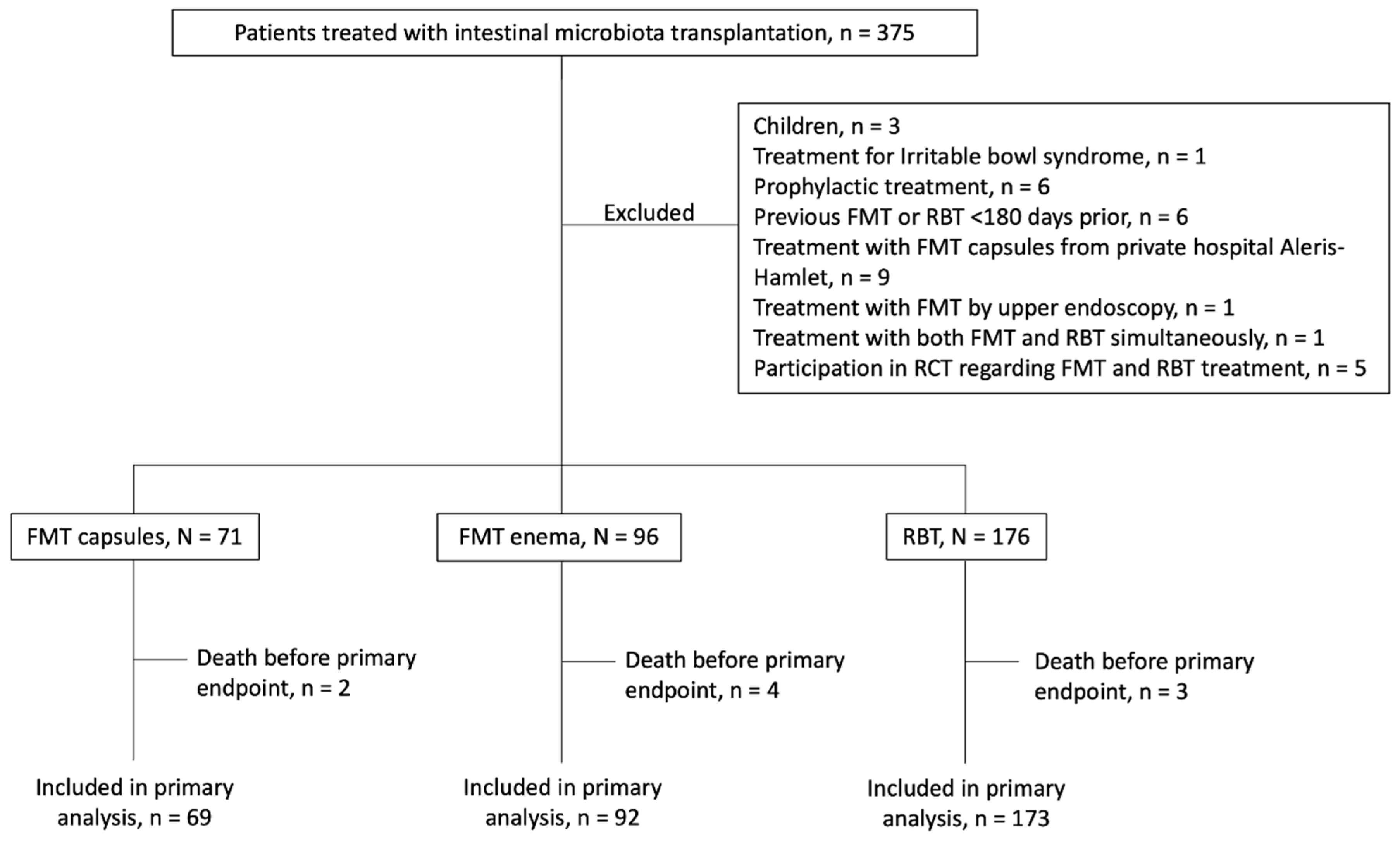
2.1. Study Population
2.2. Demographics and Clinical Information
2.3. Outcome
2.4. Statistical Analyses
2.5. Treatments
3. Results
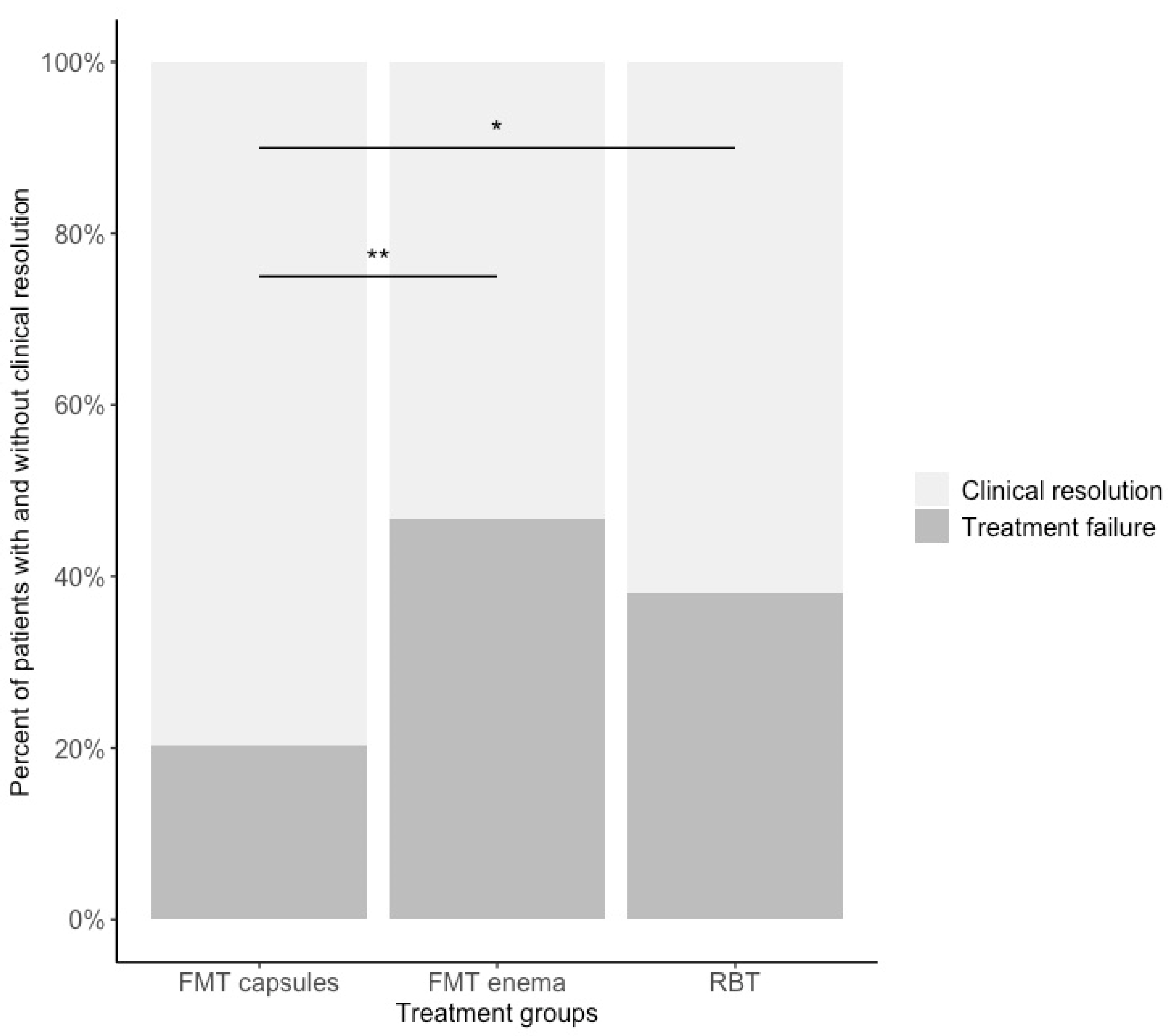
3.1. Clinical Resolution
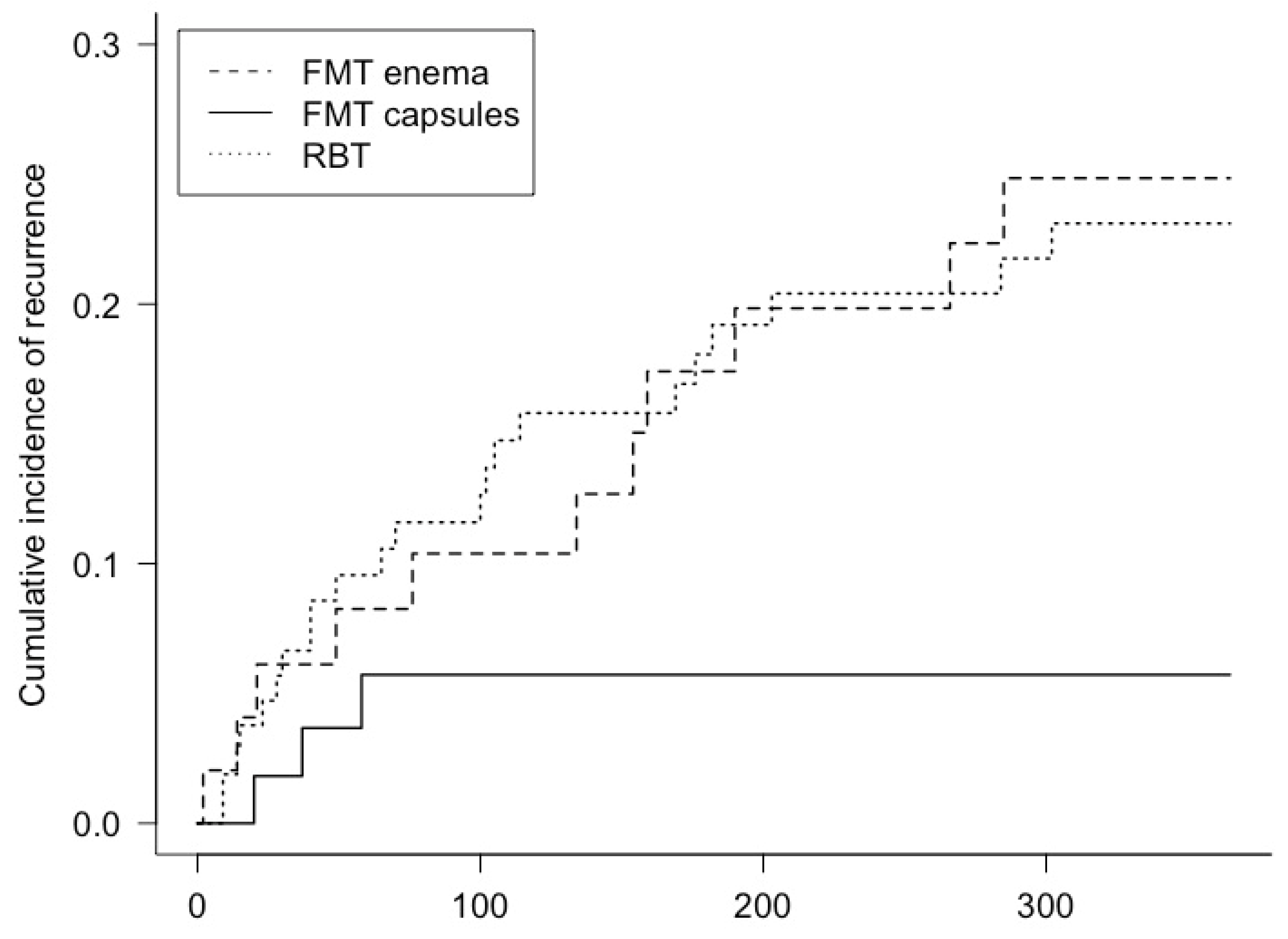
3.2. Recurrence
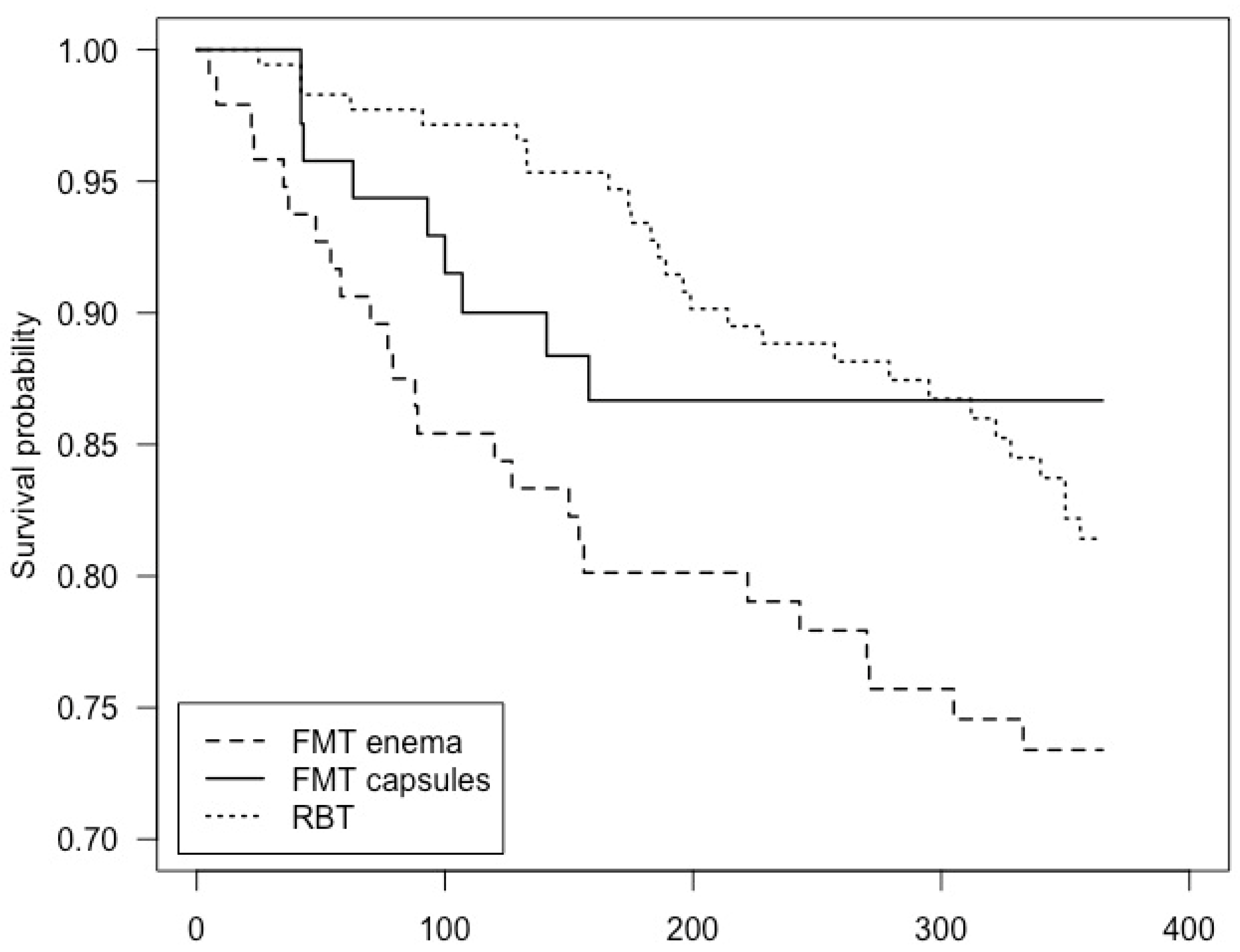
3.3. Mortality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Magee, G.; Strauss, M.E.; Thomas, S.M.; Brown, H.; Baumer, D.; Broderick, K.C. Impact of Clostridium difficile-associated diarrhea on acute care length of stay, hospital costs, and readmission: A multicenter retrospective study of inpatients, 2009–2011. Am. J. Infect. Control 2015, 43, 1148–1153. [Google Scholar] [CrossRef]
- McFarland, L. Epidemiology, Risk Factors and Treatments for Antibiotic-Associated Diarrhea. Dig. Dis. 1998, 16, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Reveles, K.R.; Lee, G.C.; Boyd, N.K.; Frei, C.R. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001–2010. Am. J. Infect. Control 2014, 42, 1028–1032. [Google Scholar] [CrossRef]
- Le Monnier, A.; Duburcq, A.; Zahar, J.-R.; Corvec, S.; Guillard, T.; Cattoir, V.; Woerther, P.-L.; Fihman, V.; Lalande, V.; Jacquier, H.; et al. Hospital cost of Clostridium difficile infection including the contribution of recurrences in French acute-care hospitals. J. Hosp. Infect. 2015, 91, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Braae, U.C.; Møller, F.T.; Ibsen, R.; Ethelberg, S.; Kjellberg, J.; Mølbak, K. The Economic Burden of Clostridioides difficile in Denmark: A Retrospective Cohort Study. Front. Public Health 2020, 8, 562957. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin. Microbiol. Infect. 2012, 18 (Suppl. 6), 21–27. [Google Scholar] [CrossRef]
- Ianiro, G.; Maida, M.; Burisch, J.; Simonelli, C.; Hold, G.; Ventimiglia, M.; Gasbarrini, A.; Cammarota, G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 1232–1244. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Widlak, M.; Bhala, N.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T.H. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. 2), S1–S21. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. eClinicalMedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef]
- Jiang, Z.-D.; Jenq, R.R.; Ajami, N.J.; Petrosino, J.F.; Alexander, A.A.; Ke, S.; Iqbal, T.; Dupont, A.W.; Muldrew, K.; Shi, Y.; et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: A randomized clinical trial. PLoS ONE 2018, 13, e0205064. [Google Scholar] [CrossRef]
- Youngster, I.; Sauk, J.; Pindar, C.; Wilson, R.G.; Kaplan, J.L.; Smith, M.B.; Alm, E.J.; Gevers, D.; Russell, G.H.; Hohmann, E.L. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin. Infect. Dis. 2014, 58, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Cold, F.; Baunwall, S.M.D.; Dahlerup, J.F.; Petersen, A.M.; Hvas, C.L.; Hansen, L.H. Systematic review with meta-analysis: Encapsulated faecal microbiota transplantation—Evidence for clinical efficacy. Ther. Adv. Gastroenterol. 2021, 14, 17562848211041004. [Google Scholar] [CrossRef]
- Hvas, C.L.; Dahl Jørgensen, S.M.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology 2019, 156, 1324–1332. [Google Scholar] [CrossRef]
- Tvede, M.; Tinggaard, M.; Helms, M. Rectal bacteriotherapy for recurrent Clostridium difficile-associated diarrhoea: Results from a case series of 55 patients in Denmark 2000–2012. Clin. Microbiol. Infect. 2015, 21, 48–53. [Google Scholar] [CrossRef]
- Tvede, M.; Rask-Madsen, J. Bacteriotherapy for chronic relapsing clostridium difficile diarrhoea in six patients. Lancet 1989, 1, 1156–1160. [Google Scholar] [CrossRef]
- Rode, A.A.; Chehri, M.; Krogsgaard, L.R.; Heno, K.K.; Svendsen, A.T.; Ribberholt, I.; Helms, M.; Engberg, J.; Schønning, K.; Tvede, M.; et al. Randomised clinical trial: A 12-strain bacterial mixture versus faecal microbiota transplantation versus vancomycin for recurrent Clostridioides difficile infections. Aliment. Pharmacol. Ther. 2021, 53, 999–1009. [Google Scholar]
- Cold, F.; Svensson, C.K.; Petersen, A.M.; Hansen, L.H.; Helms, M. Long-Term Safety Following Faecal Microbiota Transplantation as a Treatment for Recurrent Clostridioides difficile Infection Compared with Patients Treated with a Fixed Bacterial Mixture: Results from a Retrospective Cohort Study. Cells 2022, 11, 435. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Dahlerup, J.F.; Engberg, J.H.; Erikstrup, C.; Helms, M.; Juel, M.A.; Kjeldsen, J.; Nielsen, H.L.; Nilsson, A.C.; Rode, A.A.; et al. Danish national guideline for the treatment of Clostridioides difficile infection and use of faecal microbiota transplantation (FMT). Scand. J. Gastroenterol. 2021, 56, 1056–1077. [Google Scholar] [CrossRef] [PubMed]
- Cold, F.; Svensson, C.K.; Christensen, A.H.; Günther, S.; Petersen, A.M.; Hansen, L.H.; Helms, M. Successful treatment of Clostridioides difficile infection with single-donor faecal microbiota transplantation capsules. Dan. Med. J. 2022, 69, A09210712. [Google Scholar] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Lauridsen, H.C. Capsule Comprising a Faecal Composition. International Patent Publication Number WO 2021/130182A1, 1 July 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021130182 (accessed on 10 October 2022).
- Jørgensen, S.M.D.; Rubak, T.M.M.; Damsgaard, E.M.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation as a home therapy to frail older people. Age Ageing 2020, 49, 1093–1096. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Rode, A.A.; Bytzer, P.; Pedersen, O.B.; Engberg, J. Establishing a donor stool bank for faecal microbiota transplantation: Methods and feasibility. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.M.D.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation: Establishment of a clinical application framework. Eur. J. Gastroenterol. Hepatol. 2017, 29, e36–e45. [Google Scholar] [CrossRef]
- Ajami, N.J.; Cope, J.L.; Wong, M.C.; Petrosino, J.F.; Chesnel, L. Impact of Oral Fidaxomicin Administration on the Intestinal Microbiota and Susceptibility to Clostridium difficile Colonization in Mice. Antimicrob. Agents Chemother. 2018, 62, e02112-17. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Andreasen, S.E.; Hansen, M.M.; Kelsen, J.; Høyer, K.L.; Rågård, N.; Eriksen, L.L.; Støy, S.; Rubak, T.; Damsgaard, E.M.S.; et al. Faecal microbiota transplantation for first or second Clostridioides difficile infection (EarlyFMT): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2022, 22. [Google Scholar] [CrossRef]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Luo, Y.; Walsh, S.; Grinspan, A.M. S0203 Oral Fecal Microbiota Transplantation Capsules Are Effective and Safe for Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2020, 115, S68. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Zellmer, C.; A Sater, M.R.; Huntley, M.H.; Osman, M.; Olesen, S.W.; Ramakrishna, B. Shiga Toxin–Producing Escherichia coli Transmission via Fecal Microbiota Transplant. Clin. Infect. Dis. 2020, 72, e876–e880. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Dahlerup, J.F.; Hvas, C.L.; Licht, T.R. Gut microbiota differs between treatment outcomes early after fecal microbiota transplantation against recurrent Clostridioides difficile infection. Gut Microbes 2022, 14, e2084306-13. [Google Scholar] [CrossRef]
- Staley, C.; Vaughn, B.P.; Graiziger, C.T.; Singroy, S.; Hamilton, M.J.; Yao, D.; Chen, C.; Khoruts, A.; Sadowsky, M.J. Community dynamics drive punctuated engraftment of the fecal microbiome following transplantation using freeze-dried, encapsulated fecal microbiota. Gut Microbes 2017, 8, 276–288. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018, 67, 634–643. [Google Scholar]
- Haifer, C.; Paramsothy, S.; Borody, T.J.; Clancy, A.; Leong, R.W.; Kaakoush, N.O. Long-Term Bacterial and Fungal Dynamics following Oral Lyophilized Fecal Microbiota Transplantation in Clostridioides difficile Infection. mSystems 2021, 6, e00905-20. [Google Scholar] [CrossRef]
| FMT Capsules N = 71 | FMT Enema N = 96 | RBT N = 176 | p | |
|---|---|---|---|---|
| Male gender, n (%) | 25 (35.2) | 46 (47.9) | 72 (40.9) | 0.14 a, 0.49 b, 0.32 c |
| Age, years | 64.1 ± 18.6 | 69.0 ± 18.1 | 69.1 ± 17.0 | 0.09 a, 0.04 b, 0.94 c |
| CCI | 5.0 [3.5] | 5.0 [3.0] | 5.0 [3.0] | 0.51 a, 0.47 b, 0.96 c |
| Previous CDI, n | 3.0 [1.0] | 2.0 [1.0] | 3.0 [1.0] | 0.18 a, 0.06 b, 0.73 c |
| Severity | 0.61 a, 0.49 b, 0.07 c | |||
| Mild/moderate, n (%) | 70 (98.6) | 92 (95.8) | 175 (99.4) | |
| Severe, n (%) | 1 (1.4) | 2 (2.08) | 1 (0.6) | |
| Fulminant, n (%) | 0 | 2 (2.08) | 0 | |
| Clostridioides difficile subtype | 0.66 a, 0.49 b, 0.08 c | |||
| CD027, n (%) | 4 (5.6) | 16 (16.7) | 35 (19.9) | |
| Non-027, n (%) | 45 (63.4) | 64 (66.7) | 99 (56.2) | |
| Not specified, n (%) | 22 (31.0) | 16 (16.7) | 42 (23.9) | |
| Proton-pump inhibitor, n (%) | 31 (43.7) | 41 (42.7) | 80 (45.5) | 1.00 a, 0.91 b, 0.76 c |
| Immunosuppression, n (%) * | 18 (25.4) | 19 (19.8) | 41 (23.3) | 0.50 a, 0.86 b, 0.61 c |
| Previous treatment with either FMT or RBT, n (%) ** | 5 (7.0) | 8 (8.3) | 12 (6.8) | 0.99 a, 0.07 b, 0.83 c |
| Most recent antibiotics for CDI | 1.00 a, 0.054 b, <0.01 c | |||
| Vancomycin, n (%) | 67 (95.4) | 89 (92.7) | 174 (98.9) | |
| Fidaxomicin, n (%) | 3 (4.2) | 5 (5.2) | 2 (1.1) | |
| Duration of antibiotics, days | 58.0 [54.5] | 46.5 [65.3] | 81.0 [41.6] | 0.29 a, <0.001 b, <0.001 c |
| Amount of faecal material, g | 50.0 [0.0] | 50.0 [0.0] | - | 0.001 a |
| Related donor, n (%) | 0 | 9 (9.4) | - | 0.01 a |
| Follow-up time, days | 179.0 [173.5] | 64.0 [458.5] | 123.5 [500.0] | 0.30 a, 1.00 b, 0.41 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svensson, C.K.; Cold, F.; Ribberholt, I.; Zangenberg, M.; Mirsepasi-Lauridsen, H.C.; Petersen, A.M.; Helms, M. The Efficacy of Faecal Microbiota Transplant and Rectal Bacteriotherapy in Patients with Recurrent Clostridioides difficile Infection: A Retrospective Cohort Study. Cells 2022, 11, 3272. https://doi.org/10.3390/cells11203272
Svensson CK, Cold F, Ribberholt I, Zangenberg M, Mirsepasi-Lauridsen HC, Petersen AM, Helms M. The Efficacy of Faecal Microbiota Transplant and Rectal Bacteriotherapy in Patients with Recurrent Clostridioides difficile Infection: A Retrospective Cohort Study. Cells. 2022; 11(20):3272. https://doi.org/10.3390/cells11203272
Chicago/Turabian StyleSvensson, Camilla Kara, Frederik Cold, Iben Ribberholt, Mike Zangenberg, Hengameh Chloé Mirsepasi-Lauridsen, Andreas Munk Petersen, and Morten Helms. 2022. "The Efficacy of Faecal Microbiota Transplant and Rectal Bacteriotherapy in Patients with Recurrent Clostridioides difficile Infection: A Retrospective Cohort Study" Cells 11, no. 20: 3272. https://doi.org/10.3390/cells11203272
APA StyleSvensson, C. K., Cold, F., Ribberholt, I., Zangenberg, M., Mirsepasi-Lauridsen, H. C., Petersen, A. M., & Helms, M. (2022). The Efficacy of Faecal Microbiota Transplant and Rectal Bacteriotherapy in Patients with Recurrent Clostridioides difficile Infection: A Retrospective Cohort Study. Cells, 11(20), 3272. https://doi.org/10.3390/cells11203272






