IgLON4 Regulates Myogenesis via Promoting Cell Adhesion and Maintaining Myotube Orientation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. C2C12 Myoblast Cells
2.1.2. Mouse MSC Cultures
2.2. Mouse MSC Isolation
2.3. Neutralization of IgLON4
2.4. Gene Knockdown
2.5. Cell Adhesion and Proliferation Assays
2.6. Centrifugation-Based Assessment of Adhesion Force
2.7. The Animal Experiments
2.8. Immunocytochemistry
2.9. Fusion Indices
2.10. Total RNA Extraction, cDNA Synthesis, and Real-Time RT-PCR
2.11. Extraction of Lipid Rafts
2.12. Western Blot Analysis
2.13. Immunohistochemistry and Immunofluorescence
2.14. Statistical Analysis
3. Results
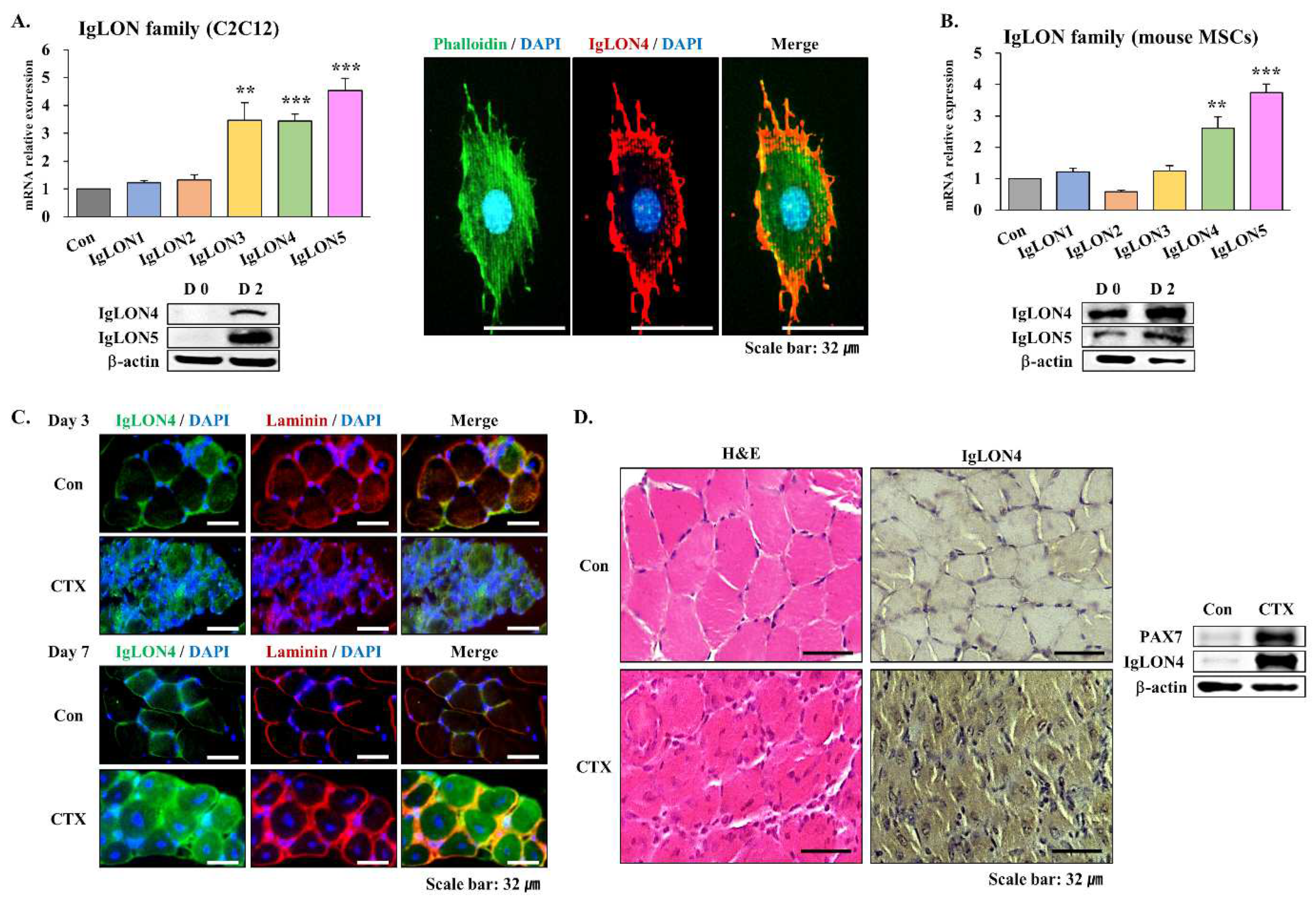
3.1. Expressions of IgLONs in Mouse Muscles
3.1.1. Expressions of IgLONs in C2C12 Cells and Primary MSCs
3.1.2. Muscle Regeneration
3.2. The Function of IgLON4 during Myoblast Differentiation
3.3. IgLON4 Expression during Myoblast Differentiation, Adhesion, and Proliferation
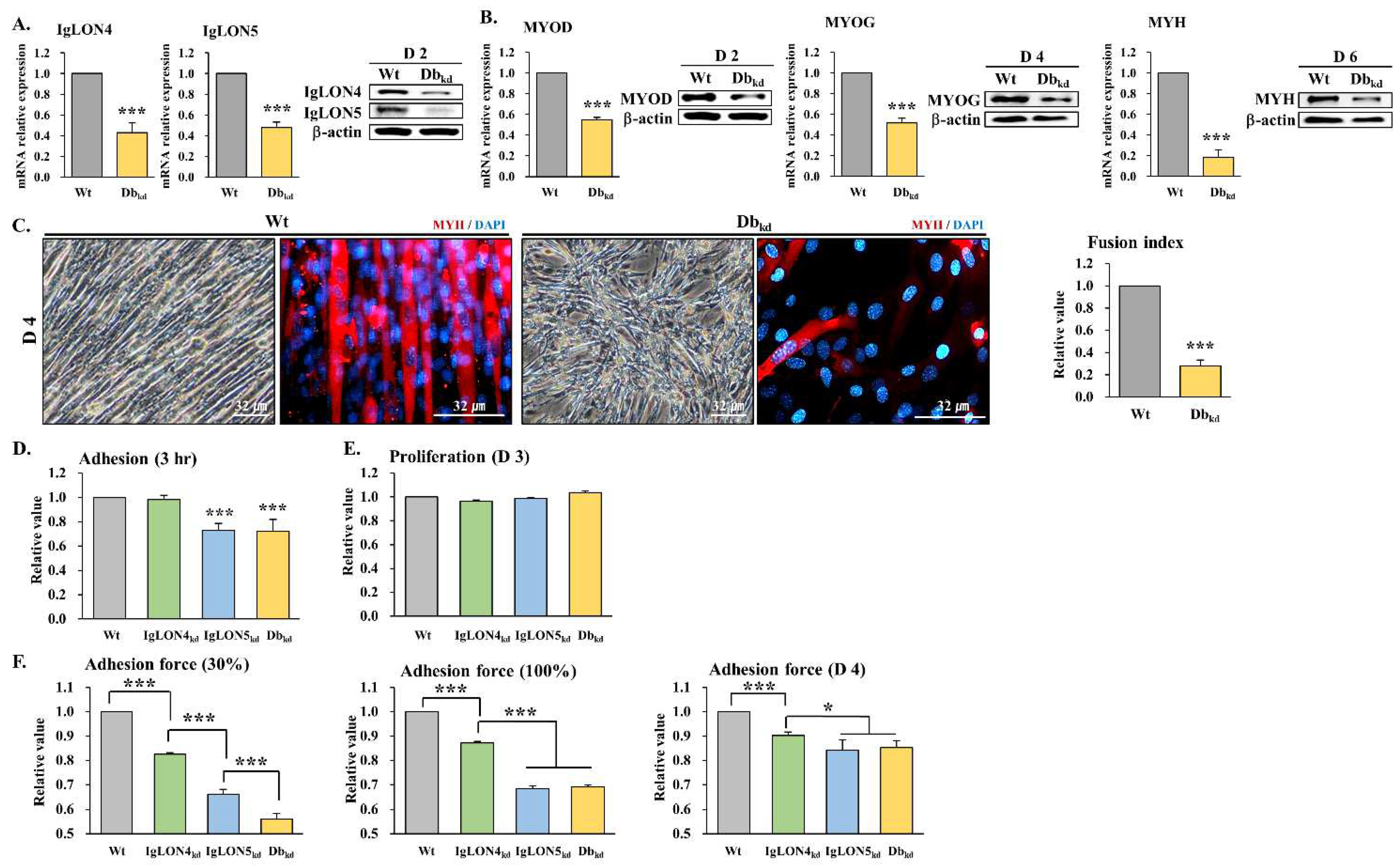
3.3.1. Effect of IgLON4 and IgLON5 Double Knockdown on Myoblast Differentiation
3.3.2. Effects of IgLON4kd, IgLON5kd, and Dbkd on Myoblast Adhesion, Proliferation, and Adhesive Force
3.4. Participation of IgLON4 in the Regulation of Myoblast Differentiation Involves Cell-to-Cell Adhesion and Alignment Functions
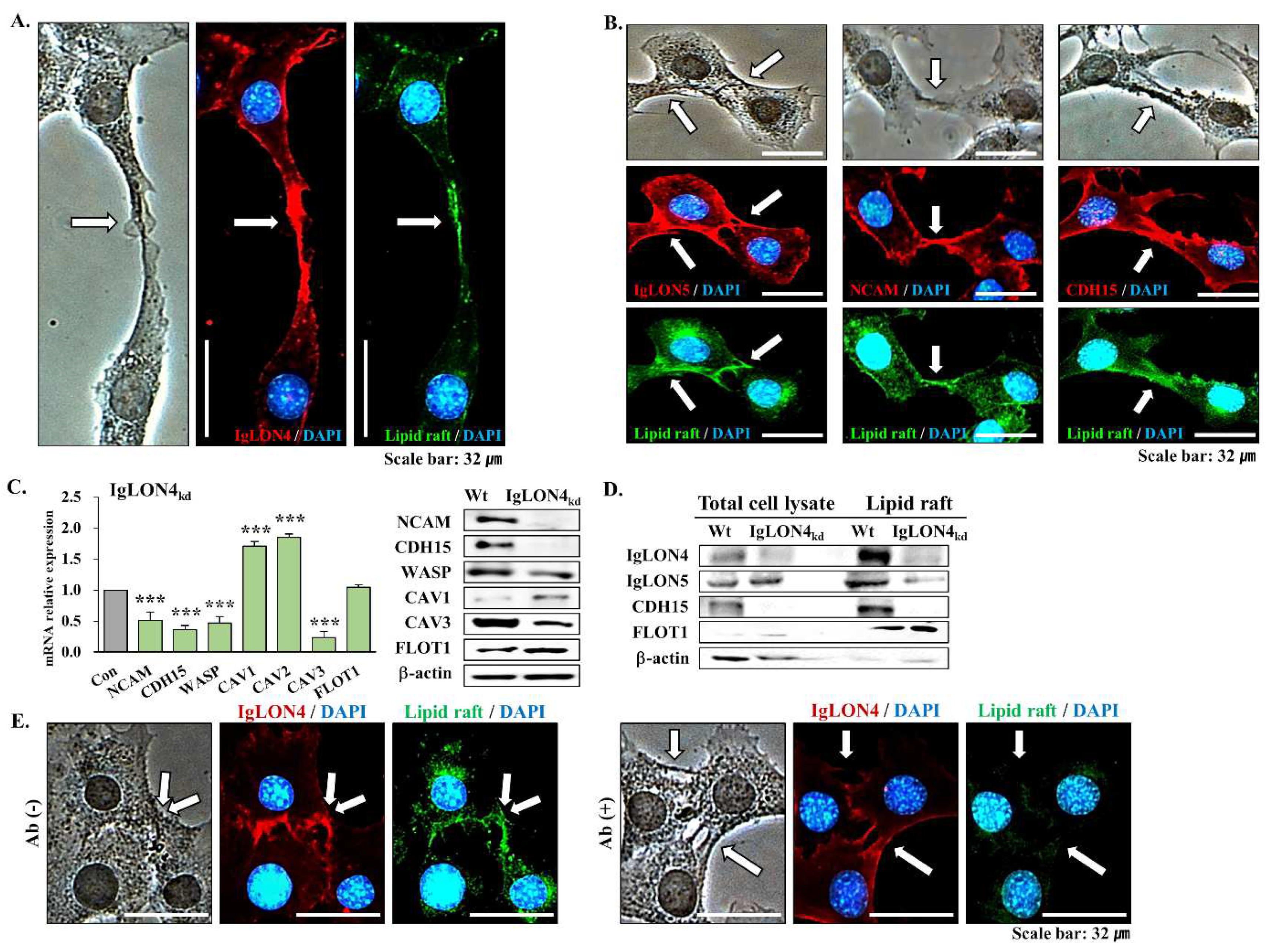
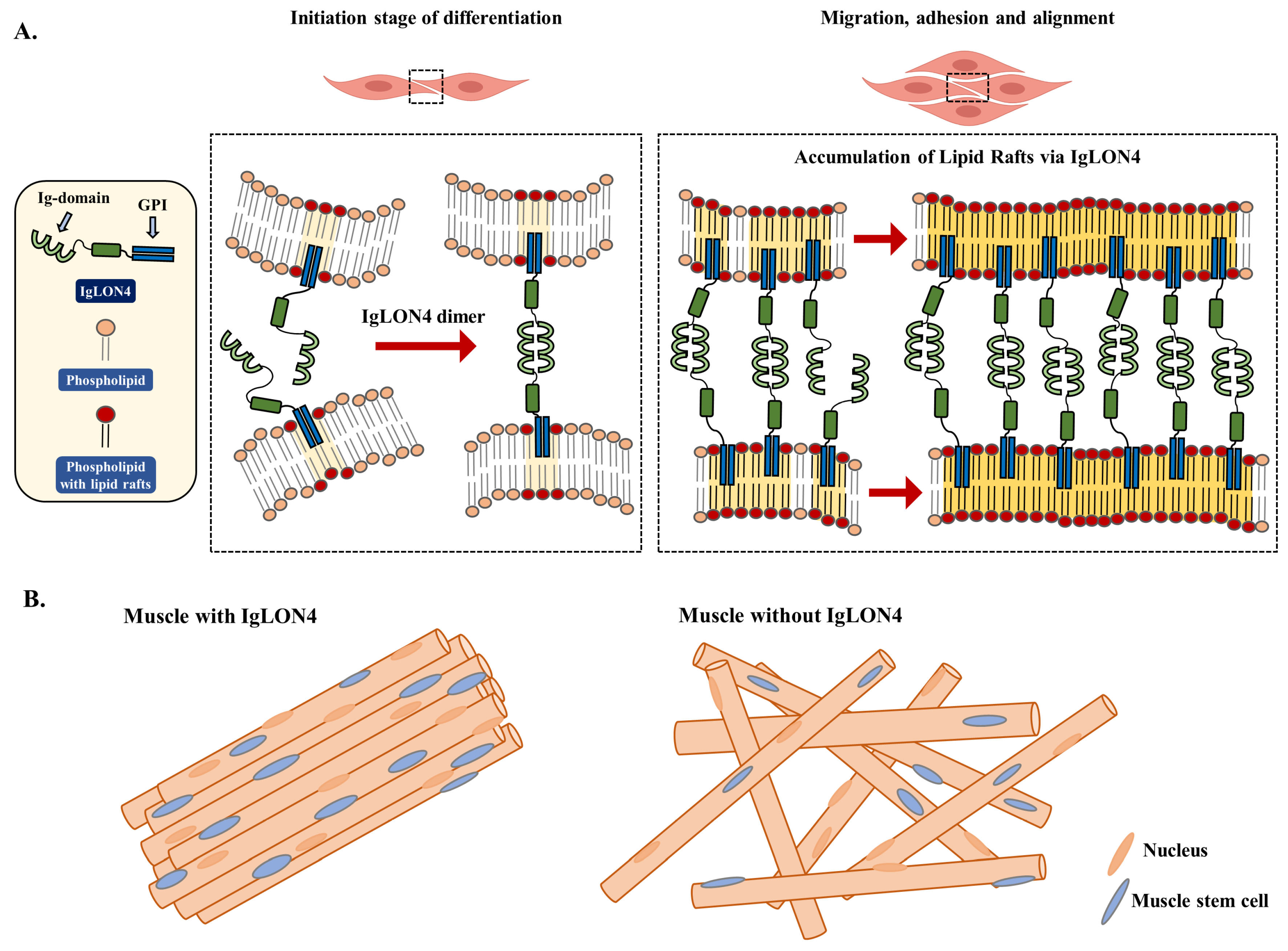
3.5. The Effect of IgLON4 on Muscle Differentiation Involved Lipid Raft Accumulation
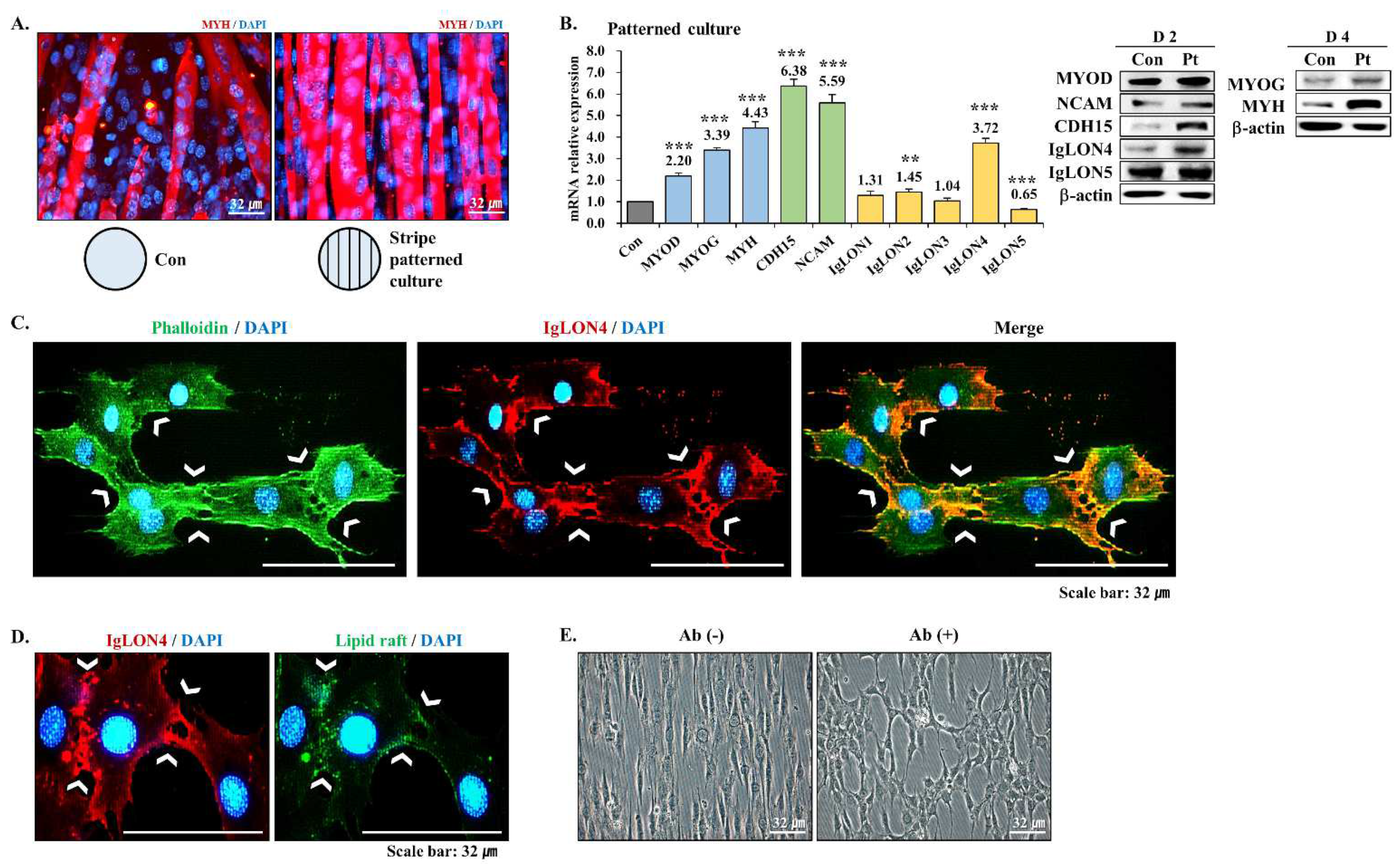
3.6. Effect of an Aligned Environment on Myogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SM | skeletal muscle |
| MSC | muscle satellite (stem) cells |
| ECM | extracellular matrix |
| FMOD | fibromodulin |
| IgLON | immunoglobulin-like cell adhesion molecule |
| GPI | glycosylphosphatidylinositol |
| CAV | caveolin |
| GAS | gastrocnemius |
References
- Yoo, A.; Joo, Y.; Cheon, Y.; Lee, S.J.; Lee, S. Neuronal growth regulator 1 promotes adipocyte lipid trafficking via interaction with CD36. J. Lipid Res. 2022, 63, 100221. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Deries, M.; Cachaco, A.S.; Bajanca, F. The extracellular matrix dimension of skeletal muscle development. Dev. Biol. 2011, 354, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Lee, E.J.; Moon, J.S.; Park, S.-Y.; Choi, I. Multifaceted interweaving between extracellular matrix, insulin resistance, and skeletal muscle. Cells 2018, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ashraf, J.M.; Nahm, S.S.; Kim, Y.W.; Park, S.Y.; Choi, I. Fibromodulin: A master regulator of myostatin controlling progression of satellite cells through a myogenic program. FASEB J. 2016, 30, 2708–2719. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ahmad, K.; Malik, A.; Rabbani, G.; Kim, T.; Lee, I.K.; Lee, Y.H.; Park, S.Y.; et al. Fibromodulin and regulation of the intricate balance between myoblast differentiation to myocytes or adipocyte-like cells. FASEB J. 2018, 32, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Ahmad, S.S.; Lim, J.H.; Ahmad, K.; Shaikh, S.; Lee, Y.-S.; Park, S.J.; Jin, J.O.; Lee, Y.-H.; Choi, I. Interaction of Fibromodulin and Myostatin to Regulate Skeletal Muscle Aging: An Opposite Regulation in Muscle Aging, Diabetes, and Intracellular Lipid Accumulation. Cells 2021, 10, 2083. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Jan, A.T.; Baig, M.H.; Lee, E.J.; Choi, I. Matrix gla protein: An extracellular matrix protein regulates myostatin expression in the muscle developmental program. Life Sci. 2017, 172, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.; Martins, G.G.; Thorsteinsdóttir, S.; Rodrigues, G. Fibronectin promotes migration, alignment and fusion in an in vitro myoblast cell model. Cell Tissue Res. 2012, 348, 569–578. [Google Scholar] [CrossRef]
- Lee, E.-J.; Ahmad, K.; Pathak, S.; Lee, S.; Baig, M.H.; Jeong, J.-H.; Doh, K.-O.; Lee, D.-M.; Choi, I. Identification of Novel FNIN2 and FNIN3 Fibronectin-Derived Peptides That Promote Cell Adhesion, Proliferation and Differentiation in Primary Cells and Stem Cells. Int. J. Mol. Sci. 2021, 22, 3042. [Google Scholar] [CrossRef]
- Kim, T.; Ahmad, K.; Shaikh, S.; Jan, A.T.; Seo, M.G.; Lee, E.J.; Choi, I. Dermatopontin in Skeletal Muscle Extracellular Matrix Regulates Myogenesis. Cells 2019, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Ezura, Y.; Chakravarti, S.; Oldberg, A.; Chervoneva, I.; Birk, D.E. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000, 151, 779–788. [Google Scholar] [CrossRef]
- Lim, J.H.; Beg, M.M.A.; Ahmad, K.; Shaikh, S.; Ahmad, S.S.; Chun, H.J.; Choi, D.; Lee, W.-J.; Jin, J.-O.; Kim, J. IgLON5 Regulates the Adhesion and Differentiation of Myoblasts. Cells 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Kubick, N.; Brosamle, D.; Mickael, M.E. Molecular Evolution and Functional Divergence of the IgLON Family. Evol. Bioinform. Online 2018, 14, 1176934318775081. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Munro, S. Lipid rafts: Elusive or illusive? Cell 2003, 115, 377–388. [Google Scholar] [CrossRef]
- Schafer, M.; Brauer, A.U.; Savaskan, N.E.; Rathjen, F.G.; Brummendorf, T. Neurotractin/kilon promotes neurite outgrowth and is expressed on reactive astrocytes after entorhinal cortex lesion. Mol. Cell. Neurosci. 2005, 29, 580–590. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, J.S.; Lee, B.; Hong, J.; Lee, S. Newly Identified Cancer-Associated Role of Human Neuronal Growth Regulator 1 (NEGR1). J Cancer 2014, 5, 598–608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joo, Y.; Kim, H.; Lee, S.; Lee, S. Neuronal growth regulator 1-deficient mice show increased adiposity and decreased muscle mass. Int. J. Obes. 2019, 43, 1769–1782. [Google Scholar] [CrossRef]
- Huang, K.T.; Chen, Y.H.; Walker, A.M. Inaccuracies in MTS assays: Major distorting effects of medium, serum albumin, and fatty acids. BioTechniques 2004, 37, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, N.; Miyata, S.; Kumanogoh, H.; Shigeta, M.; Hamada, K.; Endo, Y.; Sokawa, Y.; Maekawa, S. Characterization of a novel rat brain glycosylphosphatidylinositol-anchored protein (Kilon), a member of the IgLON cell adhesion molecule family. J. Biol. Chem. 1999, 274, 8224–8230. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, F.; Razani, B.; Lisanti, M.P. Emerging themes in lipid rafts and caveolae. Cell 2001, 106, 403–411. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Boscher, C.; Inder, K.L.; Fairbank, M.; Loo, D.; Hill, M.M.; Nabi, I.R.; Foster, L.J. Differential impact of caveolae and caveolin-1 scaffolds on the membrane raft proteome. Mol. Cell. Proteom. 2011, 10, M110.007146. [Google Scholar] [CrossRef]
- Tang, Z.; Scherer, P.E.; Okamoto, T.; Song, K.; Chu, C.; Kohtz, D.S.; Nishimoto, I.; Lodish, H.F.; Lisanti, M.P. Molecular Cloning of Caveolin-3, a Novel Member of the Caveolin Gene Family Expressed Predominantly in Muscle (∗). J. Biol. Chem. 1996, 271, 2255–2261. [Google Scholar] [CrossRef]
- Mukai, A.; Hashimoto, N. Regulation of pre-fusion events: Recruitment of M-cadherin to microrafts organized at fusion-competent sites of myogenic cells. BMC Cell Biol. 2013, 14, 37. [Google Scholar] [CrossRef]
- Sanz, R.; Ferraro, G.B.; Fournier, A.E. IgLON cell adhesion molecules are shed from the cell surface of cortical neurons to promote neuronal growth. J. Biol. Chem. 2015, 290, 4330–4342. [Google Scholar] [CrossRef]
- Struyk, A.; Canoll, P.; Wolfgang, M.; Rosen, C.; d’Eustachio, P.; Salzer, J. Cloning of neurotrimin defines a new subfamily of differentially expressed neural cell adhesion molecules. J. Neurosci. 1995, 15, 2141–2156. [Google Scholar] [CrossRef][Green Version]
- Schwarz, V.; Pan, J.; Voltmer-Irsch, S.; Hutter, H. IgCAMs redundantly control axon navigation in Caenorhabditis elegans. Neural Dev. 2009, 4, 13. [Google Scholar] [CrossRef]
- Sharma, K.; Schmitt, S.; Bergner, C.G.; Tyanova, S.; Kannaiyan, N.; Manrique-Hoyos, N.; Kongi, K.; Cantuti, L.; Hanisch, U.-K.; Philips, M.-A. Cell type–and brain region–resolved mouse brain proteome. Nat. Neurosci. 2015, 18, 1819–1831. [Google Scholar] [CrossRef]
- Vanaveski, T.; Singh, K.; Narvik, J.; Eskla, K.-L.; Visnapuu, T.; Heinla, I.; Jayaram, M.; Innos, J.; Lilleväli, K.; Philips, M.-A. Promoter-specific expression and genomic structure of IgLON family genes in mouse. Front. Neurosci. 2017, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Akeel, M.; McNamee, C.J.; Youssef, S.; Moss, D. DIgLONs inhibit initiation of neurite outgrowth from forebrain neurons via an IgLON-containing receptor complex. Brain Res. 2011, 1374, 27–35. [Google Scholar] [CrossRef]
- Alliance of Genome Resources Portal: Unified model organism research platform. Nucleic Acids Res. 2020, 48, D650–D658. [CrossRef]
- Sugimoto, C.; Maekawa, S.; Miyata, S. OBCAM, an immunoglobulin superfamily cell adhesion molecule, regulates morphology and proliferation of cerebral astrocytes. J. Neurochem. 2010, 112, 818–828. [Google Scholar] [CrossRef]
- Sugimoto, C.; Morita, S.; Miyata, S. Overexpression of IgLON cell adhesion molecules changes proliferation and cell size of cortical astrocytes. Cell Biochem. Funct. 2012, 30, 400–405. [Google Scholar] [CrossRef]
- Kuthe, C.D.; Uddanwadiker, R.V. Investigation of effect of fiber orientation on mechanical behavior of skeletal muscle. J. Appl. Biomater. Funct. Mater. 2016, 14, 154–162. [Google Scholar] [CrossRef]
- Kim, H.; Chun, Y.; Che, L.; Kim, J.; Lee, S.; Lee, S. The new obesity-associated protein, neuronal growth regulator 1 (NEGR1), is implicated in Niemann-Pick disease Type C (NPC2)-mediated cholesterol trafficking. Biochem. Biophys. Res. Commun. 2017, 482, 1367–1374. [Google Scholar] [CrossRef]
- Donalies, M.; Cramer, M.; Ringwald, M.; Starzinski-Powitz, A. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc. Natl. Acad. Sci. USA 1991, 88, 8024–8028. [Google Scholar] [CrossRef]
- Pradhan, B.S.; Prószyński, T.J. A Role for Caveolin-3 in the Pathogenesis of Muscular Dystrophies. Int. J. Mol. Sci. 2020, 21, 8736. [Google Scholar] [CrossRef]
- Song, K.S.; Scherer, P.E.; Tang, Z.; Okamoto, T.; Li, S.; Chafel, M.; Chu, C.; Kohtz, D.S.; Lisanti, M.P. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 1996, 271, 15160–15165. [Google Scholar] [CrossRef]
- McClure, M.J.; Clark, N.M.; Hyzy, S.L.; Chalfant, C.E.; Olivares-Navarrete, R.; Boyan, B.D.; Schwartz, Z. Role of integrin α7β1 signaling in myoblast differentiation on aligned polydioxanone scaffolds. Acta Biomater. 2016, 39, 44–54. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.H.; Ahmad, K.; Chun, H.J.; Hwang, Y.C.; Qadri, A.F.; Ali, S.; Ahmad, S.S.; Shaikh, S.; Choi, J.; Kim, J.; et al. IgLON4 Regulates Myogenesis via Promoting Cell Adhesion and Maintaining Myotube Orientation. Cells 2022, 11, 3265. https://doi.org/10.3390/cells11203265
Lim JH, Ahmad K, Chun HJ, Hwang YC, Qadri AF, Ali S, Ahmad SS, Shaikh S, Choi J, Kim J, et al. IgLON4 Regulates Myogenesis via Promoting Cell Adhesion and Maintaining Myotube Orientation. Cells. 2022; 11(20):3265. https://doi.org/10.3390/cells11203265
Chicago/Turabian StyleLim, Jeong Ho, Khurshid Ahmad, Hee Jin Chun, Ye Chan Hwang, Afsha Fatima Qadri, Shahid Ali, Syed Sayeed Ahmad, Sibhghatulla Shaikh, Jungseok Choi, Jihoe Kim, and et al. 2022. "IgLON4 Regulates Myogenesis via Promoting Cell Adhesion and Maintaining Myotube Orientation" Cells 11, no. 20: 3265. https://doi.org/10.3390/cells11203265
APA StyleLim, J. H., Ahmad, K., Chun, H. J., Hwang, Y. C., Qadri, A. F., Ali, S., Ahmad, S. S., Shaikh, S., Choi, J., Kim, J., Jin, J.-O., Kim, M., Han, S. S., Choi, I., & Lee, E. J. (2022). IgLON4 Regulates Myogenesis via Promoting Cell Adhesion and Maintaining Myotube Orientation. Cells, 11(20), 3265. https://doi.org/10.3390/cells11203265








