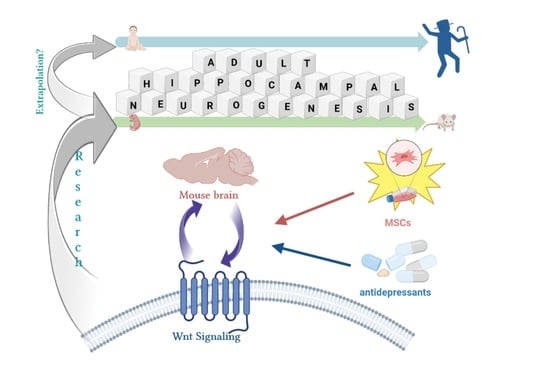
Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells
Abstract
1. Introduction
2. Strategies for Influencing Neurogenesis in the Adult Hippocampus Based on the Wnt Signaling Pathway Modulation
2.1. Regeneration of Hippocampal Neurons by the Modulation of Wnt Signaling Pathway
2.2. Decreasing Neurogenesis in the Adult Hippocampus by the Modulation of Wnt Signaling Pathway
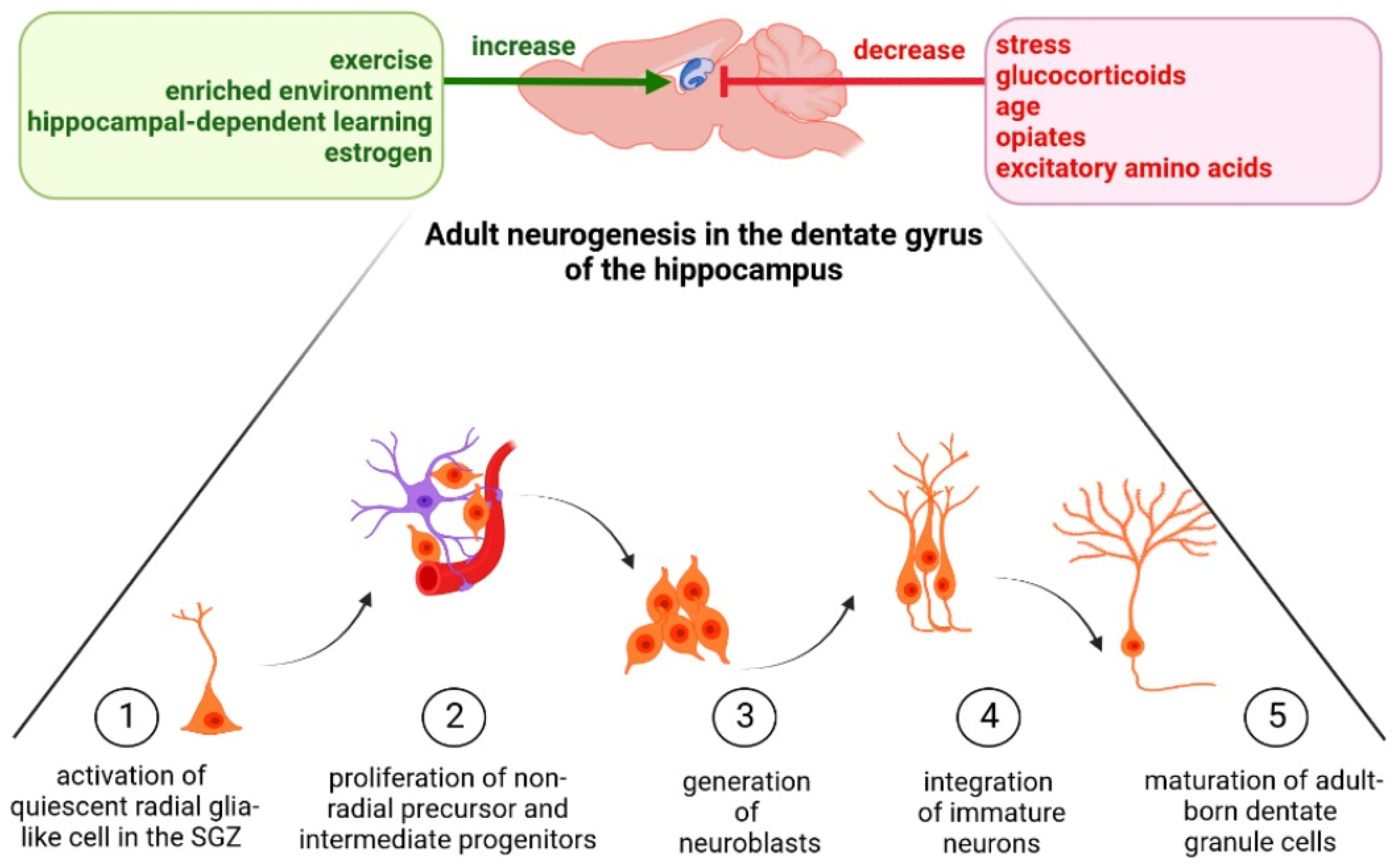
3. Stress as an Environmental Factor Regulating Hippocampal Neurogenesis
4. The Influence of Wnt Signaling Pathway on the Interaction between Hippocampal Neurogenesis and Learning
5. Boosting Hippocampal Neurogenesis by Mood Stabilizers and Antidepressant Treatment
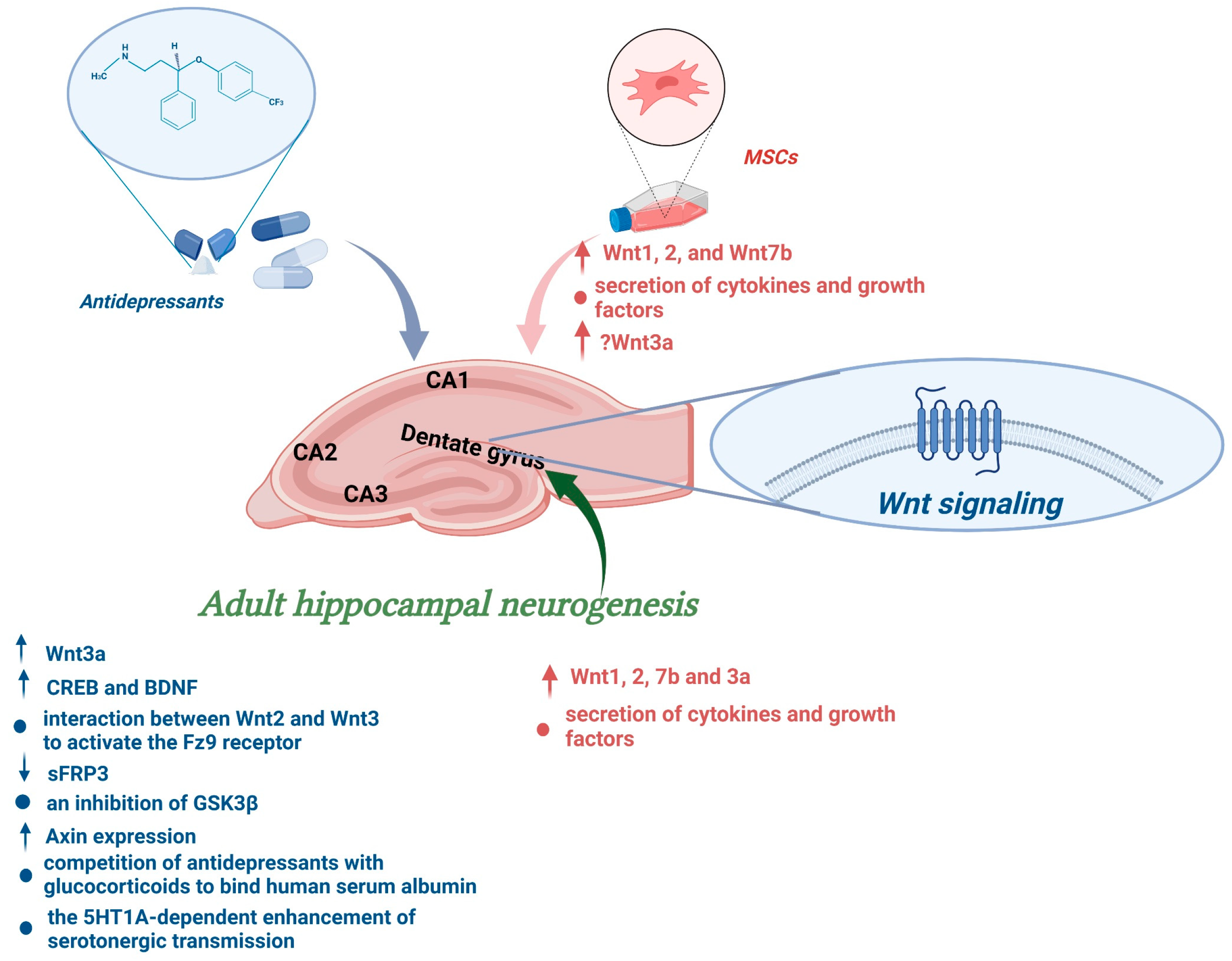
6. Influence of Chronic Antidepressant Administration on Adult Hippocampal Neurogenesis via the Wnt Signalling Pathway
7. Mesenchymal Stem Cells Can Open the Plasticity Window in Personalized Therapy
8. Possible In Vitro and In Vivo Models of Human Hippocampal Neurogenesis—Implications of Interspecies Differences
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altman, J.; Das, G.D. Autoradiographic and Histological Evidence of Postnatal Hippocampal Neurogenesis in Rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the Adult Human Hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional Neurogenesis in the Adult Hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.-H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-Coupled Astrocytic Differentiation and Age-Related Depletion of Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Seri, B.; García-Verdugo, J.M.; McEwen, B.S.; Alvarez-Buylla, A. Astrocytes Give Rise to New Neurons in the Adult Mammalian Hippocampus. J. Neurosci. 2001, 21, 7153–7160. [Google Scholar] [CrossRef]
- Suh, H.; Consiglio, A.; Ray, J.; Sawai, T.; D’Amour, K.A.; Gage, F.H. In Vivo Fate Analysis Reveals the Multipotent and Self-Renewal Capacities of Sox2+ Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell 2007, 1, 515–528. [Google Scholar] [CrossRef]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Götz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and Active Hippocampal Neural Stem Cells with Distinct Morphologies Respond Selectively to Physiological and Pathological Stimuli and Aging. Cell Stem Cell 2010, 6, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Battista, D.; Ferrari, C.C.; Gage, F.H.; Pitossi, F.J. Neurogenic Niche Modulation by Activated Microglia: Transforming Growth Factor β Increases Neurogenesis in the Adult Dentate Gyrus. Eur. J. Neurosci. 2006, 23, 83–93. [Google Scholar] [CrossRef]
- Duman, R.S.; Nakagawa, S.; Malberg, J. Regulation of Adult Neurogenesis by Antidepressant Treatment. Neuropsychopharmacology 2001, 25, 836–844. [Google Scholar] [CrossRef]
- van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise Enhances Learning and Hippocampal Neurogenesis in Aged Mice. J. Neurosci. 2005, 25, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.A.; Gould, E. Adult Neurogenesis Is Regulated by Adrenal Steroids in the Dentate Gyrus. Neuroscience 1994, 61, 203–209. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gast, D.; Gage, F.H. Neuroplasticity in Old Age: Sustained Fivefold Induction of Hippocampal Neurogenesis by Long-Term Environmental Enrichment. Ann. Neurol. 2002, 52, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Montaron, M.F.; Petry, K.G.; Rodriguez, J.J.; Marinelli, M.; Aurousseau, C.; Rougon, G.; Le Moal, M.; Abrous, D.N. Adrenalectomy Increases Neurogenesis but Not PSA-NCAM Expression in Aged Dentate Gyrus. Eur. J. Neurosci. 1999, 11, 1479–1485. [Google Scholar] [CrossRef]
- Ormerod, B.K.; Hanft, S.J.; Asokan, A.; Haditsch, U.; Lee, S.W.; Palmer, T.D. PPARγ Activation Prevents Impairments in Spatial Memory and Neurogenesis Following Transient Illness. Brain Behav. Immun. 2013, 29, 28–38. [Google Scholar] [CrossRef]
- Speisman, R.B.; Kumar, A.; Rani, A.; Pastoriza, J.M.; Severance, J.E.; Foster, T.C.; Ormerod, B.K. Environmental Enrichment Restores Neurogenesis and Rapid Acquisition in Aged Rats. Neurobiol. Aging 2013, 34, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C. The Effects of Stressful Life Events on Depression. Annu. Rev. Psychol. 1997, 48, 191–214. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, G.; Frodl, T. The Hippocampus in Major Depression: Evidence for the Convergence of the Bench and Bedside in Psychiatric Research? Mol. Psychiatry 2011, 16, 252–264. [Google Scholar] [CrossRef]
- Woolley, C.S.; Gould, E.; McEwen, B.S. Exposure to Excess Glucocorticoids Alters Dendritic Morphology of Adult Hippocampal Pyramidal Neurons. Brain Res. 1990, 531, 225–231. [Google Scholar] [CrossRef]
- Watanabe, Y.; Gould, E.; McEwen, B.S. Stress Induces Atrophy of Apical Dendrites of Hippocampal CA3 Pyramidal Neurons. Brain Res. 1992, 588, 341–345. [Google Scholar] [CrossRef]
- McEwen, B.S.; Magarinos, A.M. Stress and Hippocampal Plasticity: Implications for the Pathophysiology of Affective Disorders. Hum. Psychopharmacol. Clin. Exp. 2001, 16, S7–S19. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; McCausland, H.C.; Morris, H.D.; Padmanaban, V.; Cameron, H.A. Stress and Loss of Adult Neurogenesis Differentially Reduce Hippocampal Volume. Biol. Psychiatry 2017, 82, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Tanapat, P.; Rydel, T.; Hastings, N. Regulation of Hippocampal Neurogenesis in Adulthood. Biol. Psychiatry 2000, 48, 715–720. [Google Scholar] [CrossRef]
- Drevets, W.C. Neuroplasticity in Mood Disorders. Dialogues Clin. Neurosci. 2004, 6, 199–216. [Google Scholar] [CrossRef]
- Boldrini, M.; Santiago, A.N.; Hen, R.; Dwork, A.J.; Rosoklija, G.B.; Tamir, H.; Arango, V.; John Mann, J. Hippocampal Granule Neuron Number and Dentate Gyrus Volume in Antidepressant-Treated and Untreated Major Depression. Neuropsychopharmacology 2013, 38, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Lie, D.-C.; Colamarino, S.A.; Song, H.-J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt Signalling Regulates Adult Hippocampal Neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, S.B.; Valenzuela-Bezanilla, D.; Mardones, M.D.; Varela-Nallar, L. Role of Wnt Signaling in Adult Hippocampal Neurogenesis in Health and Disease. Front. Cell Dev. Biol. 2020, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Hsieh, J.; Muotri, A.; Yeo, G.; Warashina, M.; Lie, D.C.; Moore, L.; Nakashima, K.; Asashima, M.; Gage, F.H. Wnt-Mediated Activation of NeuroD1 and Retro-Elements during Adult Neurogenesis. Nat. Neurosci. 2009, 12, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.D.; Nusse, R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Zeng, X.; Liu, C.; Zhang, X.; Harada, Y.; Chang, Z.; He, X. A Mechanism for Wnt Coreceptor Activation. Mol. Cell 2004, 13, 149–156. [Google Scholar] [CrossRef]
- Brennan, K.; Gonzalez-Sancho, J.M.; Castelo-Soccio, L.A.; Howe, L.R.; Brown, A.M.C. Truncated Mutants of the Putative Wnt Receptor LRP6/Arrow Can Stabilize Beta-Catenin Independently of Frizzled Proteins. Oncogene 2004, 23, 4873–4884. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M.; et al. Wnt/Beta-Catenin Signaling Controls Development of the Blood-Brain Barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef]
- Alafuzoff, I.; Adolfsson, R.; Bucht, G.; Winblad, B. Albumin and Immunoglobulin in Plasma and Cerebrospinal Fluid, and Blood-Cerebrospinal Fluid Barrier Function in Patients with Dementia of Alzheimer Type and Multi-Infarct Dementia. J. Neurol. Sci. 1983, 60, 465–472. [Google Scholar] [CrossRef]
- Belayev, L.; Liu, Y.; Zhao, W.; Busto, R.; Ginsberg, M.D. Human Albumin Therapy of Acute Ischemic Stroke: Marked Neuroprotective Efficacy at Moderate Doses and with a Broad Therapeutic Window. Stroke 2001, 32, 553–560. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, J.; Zhang, F.; Yang, F.-Y.; Liu, M. Human Serum Albumin Attenuates Global Cerebral Ischemia/Reperfusion-Induced Brain Injury in a Wnt/β-Catenin/ROS Signaling-Dependent Manner in Rats. Biomed. Pharmacother. 2019, 115, 108871. [Google Scholar] [CrossRef] [PubMed]
- Daujat-Chavanieu, M.; Kot, M. Albumin Is a Secret Factor Involved in Multidirectional Interactions among the Serotoninergic, Immune and Endocrine Systems That Supervises the Mechanism of CYP1A and CYP3A Regulation in the Liver. Pharmacol. Ther. 2020, 215, 107616. [Google Scholar] [CrossRef]
- Arredondo, S.B.; Valenzuela-Bezanilla, D.; Santibanez, S.H.; Varela-Nallar, L. Wnt Signaling in the Adult Hippocampal Neurogenic Niche. Stem Cells 2022, 40, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Angers, S.; Moon, R.T. Proximal Events in Wnt Signal Transduction. Nat. Rev. Mol. Cell Biol. 2009, 10, 468–477. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Mikels, A.; Nusse, R. Alternative Wnt Signaling Is Initiated by Distinct Receptors. Sci. Signal. 2008, 1, re9. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Varela-Nallar, L. Wnt Signalling in Neuronal Differentiation and Development. Cell Tissue Res. 2015, 359, 215–223. [Google Scholar] [CrossRef]
- Varela-Nallar, L.; Ramirez, V.T.; Gonzalez-Billault, C.; Inestrosa, N.C. Frizzled Receptors in Neurons: From Growth Cones to the Synapse. Cytoskeleton 2012, 69, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Mardones, M.D.; Andaur, G.A.; Varas-Godoy, M.; Henriquez, J.F.; Salech, F.; Behrens, M.I.; Couve, A.; Inestrosa, N.C.; Varela-Nallar, L. Frizzled-1 Receptor Regulates Adult Hippocampal Neurogenesis. Mol. Brain 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Sahores, M.; Gibb, A.; Salinas, P.C. Frizzled-5, a Receptor for the Synaptic Organizer Wnt7a, Regulates Activity-Mediated Synaptogenesis. Development 2010, 137, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Slater, P.G.; Ramirez, V.T.; Gonzalez-Billault, C.; Varela-Nallar, L.; Inestrosa, N.C. Frizzled-5 Receptor Is Involved in Neuronal Polarity and Morphogenesis of Hippocampal Neurons. PLoS ONE 2013, 8, e78892. [Google Scholar] [CrossRef]
- Zhao, C.; Avilés, C.; Abel, R.A.; Almli, C.R.; McQuillen, P.; Pleasure, S.J. Hippocampal and Visuospatial Learning Defects in Mice with a Deletion of Frizzled 9, a Gene in the Williams Syndrome Deletion Interval. Development 2005, 132, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Chailangkarn, T.; Muotri, A.R. Modeling Williams Syndrome with Induced Pluripotent Stem Cells. Neurogenesis 2017, 4, e1283187. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.; Yin, D.; Malbon, C.C. Abundance, Complexation, and Trafficking of Wnt/β-Catenin Signaling Elements in Response to Wnt3a. J. Mol. Signal. 2007, 2, 11. [Google Scholar] [CrossRef][Green Version]
- Taelman, V.F.; Dobrowolski, R.; Plouhinec, J.-L.; Fuentealba, L.C.; Vorwald, P.P.; Gumper, I.; Sabatini, D.D.; De Robertis, E.M. Wnt Signaling Requires Sequestration of Glycogen Synthase Kinase 3 inside Multivesicular Endosomes. Cell 2010, 143, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Castro-Piedras, I.; Simmons, G.E.; Pruitt, K. Dishevelled: A Masterful Conductor of Complex Wnt Signals. Cell Signal. 2018, 47, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Matsumoto, S.; Sada, R. Dickkopf Signaling, beyond Wnt-Mediated Biology. Semin. Cell Dev. Biol. 2022, 125, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, J.; Yang, G.-Y.; Wang, Q.-J.; Qian, L.; Chen, Y.-M.; Chen, F.; Tao, Y.; Hu, H.-S.; Wang, T.; et al. Dishevelled Promotes Axon Differentiation by Regulating Atypical Protein Kinase C. Nat. Cell Biol. 2007, 9, 743–754. [Google Scholar] [CrossRef]
- Hapak, S.M.; Rothlin, C.V.; Ghosh, S. PAR3-PAR6-Atypical PKC Polarity Complex Proteins in Neuronal Polarization. Cell Mol. Life Sci. 2018, 75, 2735–2761. [Google Scholar] [CrossRef]
- Qu, Q.; Sun, G.; Murai, K.; Ye, P.; Li, W.; Asuelime, G.; Cheung, Y.-T.; Shi, Y. Wnt7a Regulates Multiple Steps of Neurogenesis. Mol. Cell Biol. 2013, 33, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Cerpa, W.; Godoy, J.A.; Alfaro, I.; Farías, G.G.; Metcalfe, M.J.; Fuentealba, R.; Bonansco, C.; Inestrosa, N.C. Wnt-7a Modulates the Synaptic Vesicle Cycle and Synaptic Transmission in Hippocampal Neurons. J. Biol. Chem. 2008, 283, 5918–5927. [Google Scholar] [CrossRef]
- McLeod, F.; Salinas, P.C. Wnt Proteins as Modulators of Synaptic Plasticity. Curr. Opin. Neurobiol. 2018, 53, 90–95. [Google Scholar] [CrossRef]
- Davis, E.K.; Zou, Y.; Ghosh, A. Wnts Acting through Canonical and Noncanonical Signaling Pathways Exert Opposite Effects on Hippocampal Synapse Formation. Neural Dev. 2008, 3, 32. [Google Scholar] [CrossRef]
- Winn, R.A.; Marek, L.; Han, S.-Y.; Rodriguez, K.; Rodriguez, N.; Hammond, M.; Van Scoyk, M.; Acosta, H.; Mirus, J.; Barry, N.; et al. Restoration of Wnt-7a Expression Reverses Non-Small Cell Lung Cancer Cellular Transformation through Frizzled-9-Mediated Growth Inhibition and Promotion of Cell Differentiation. J. Biol. Chem. 2005, 280, 19625–19634. [Google Scholar] [CrossRef]
- Valenta, T.; Degirmenci, B.; Moor, A.E.; Herr, P.; Zimmerli, D.; Moor, M.B.; Hausmann, G.; Cantù, C.; Aguet, M.; Basler, K. Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell Rep. 2016, 15, 911–918. [Google Scholar] [CrossRef]
- Rosso, S.B.; Sussman, D.; Wynshaw-Boris, A.; Salinas, P.C. Wnt Signaling through Dishevelled, Rac and JNK Regulates Dendritic Development. Nat. Neurosci. 2005, 8, 34–42. [Google Scholar] [CrossRef]
- Heppt, J.; Wittmann, M.; Schäffner, I.; Billmann, C.; Zhang, J.; Vogt-Weisenhorn, D.; Prakash, N.; Wurst, W.; Taketo, M.M.; Lie, D.C. β-Catenin Signaling Modulates the Tempo of Dendritic Growth of Adult-Born Hippocampal Neurons. EMBO J. 2020, 39, e104472. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, F.; Busceti, C.L.; Biagioni, F.; Moyanova, S.G.; Meisler, M.H.; Battaglia, G.; Caricasole, A.; Bruno, V.; Nicoletti, F. Induction of the Wnt Antagonist, Dickkopf-1, Contributes to the Development of Neuronal Death in Models of Brain Focal Ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Shruster, A.; Ben-Zur, T.; Melamed, E.; Offen, D. Wnt Signaling Enhances Neurogenesis and Improves Neurological Function after Focal Ischemic Injury. PLoS ONE 2012, 7, e40843. [Google Scholar] [CrossRef]
- Semënov, M.V.; Tamai, K.; Brott, B.K.; Kühl, M.; Sokol, S.; He, X. Head Inducer Dickkopf-1 Is a Ligand for Wnt Coreceptor LRP6. Curr. Biol. 2001, 11, 951–961. [Google Scholar] [CrossRef]
- Seib, D.R.M.M.; Corsini, N.S.; Ellwanger, K.; Plaas, C.; Mateos, A.; Pitzer, C.; Niehrs, C.; Celikel, T.; Martin-Villalba, A. Loss of Dickkopf-1 Restores Neurogenesis in Old Age and Counteracts Cognitive Decline. Cell Stem Cell 2013, 12, 204–214. [Google Scholar] [CrossRef]
- Caricasole, A.; Copani, A.; Caraci, F.; Aronica, E.; Rozemuller, A.J.; Caruso, A.; Storto, M.; Gaviraghi, G.; Terstappen, G.C.; Nicoletti, F. Induction of Dickkopf-1, a Negative Modulator of the Wnt Pathway, Is Associated with Neuronal Degeneration in Alzheimer’s Brain. J. Neurosci. 2004, 24, 6021–6027. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Chacón, M.A.; Barría, M.I.; Garrido, J.L.; Godoy, J.A.; Olivares, G.; Reyes, A.E.; Alvarez, A.; Bronfman, M.; Inestrosa, N.C. Activation of Wnt Signaling Rescues Neurodegeneration and Behavioral Impairments Induced by Beta-Amyloid Fibrils. Mol. Psychiatry 2003, 8, 195–208. [Google Scholar] [CrossRef]
- Shi, L.; Winchester, L.M.; Liu, B.Y.; Killick, R.; Ribe, E.M.; Westwood, S.; Baird, A.L.; Buckley, N.J.; Hong, S.; Dobricic, V.; et al. Dickkopf-1 Overexpression in Vitro Nominates Candidate Blood Biomarkers Relating to Alzheimer’s Disease Pathology. J. Alzheimers Dis. 2020, 77, 1353–1368. [Google Scholar] [CrossRef]
- Kot, M.; Daujat-Chavanieu, M. The Impact of Serotonergic System Dysfunction on the Regulation of P4501A Isoforms during Liver Insufficiency and Consequences for Thyroid Hormone Homeostasis. Food Chem. Toxicol. 2016, 97, 70–81. [Google Scholar] [CrossRef]
- Kot, M.; Daujat-Chavanieu, M. Altered Cytokine Profile under Control of the Serotonergic System Determines the Regulation of CYP2C11 and CYP3A Isoforms. Food Chem. Toxicol. 2018, 116, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Yanaga, F.; Shiraishi, F.; Hirata, M.; Miwa, Y.; Morimoto, S.; Sasaguri, T. Glycogen Synthase Kinase-3beta Is Tyrosine-Phosphorylated by MEK1 in Human Skin Fibroblasts. Biochem. Biophys. Res. Commun. 2004, 316, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, W.; Roh, M.-S.; Friedman, A.B.; Rosborough, K.; Jope, R.S. In Vivo Regulation of Glycogen Synthase Kinase-3β (GSK3β) by Serotonergic Activity in Mouse Brain. Neuropsychopharmacology 2004, 29, 1426–1431. [Google Scholar] [CrossRef]
- Omata, N.; Chiu, C.-T.; Moya, P.R.; Leng, Y.; Wang, Z.; Hunsberger, J.G.; Leeds, P.; Chuang, D.-M. Lentivirally Mediated GSK-3β Silencing in the Hippocampal Dentate Gyrus Induces Antidepressant-like Effects in Stressed Mice. Int. J. Neuropsychopharmacol. 2011, 14, 711–717. [Google Scholar] [CrossRef]
- Prickaerts, J.; Moechars, D.; Cryns, K.; Lenaerts, I.; van Craenendonck, H.; Goris, I.; Daneels, G.; Bouwknecht, J.A.; Steckler, T. Transgenic Mice Overexpressing Glycogen Synthase Kinase 3beta: A Putative Model of Hyperactivity and Mania. J. Neurosci. 2006, 26, 9022–9029. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased Neurogenesis and the Ectopic Granule Cells after Intrahippocampal BDNF Infusion in Adult Rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef]
- Nibuya, M.; Nestler, E.J.; Duman, R.S. Chronic Antidepressant Administration Increases the Expression of CAMP Response Element Binding Protein (CREB) in Rat Hippocampus. J. Neurosci. 1996, 16, 2365–2372. [Google Scholar] [CrossRef]
- Yim, I.S.; Tanner Stapleton, L.R.; Guardino, C.M.; Hahn-Holbrook, J.; Dunkel Schetter, C. Biological and Psychosocial Predictors of Postpartum Depression: Systematic Review and Call for Integration. Annu. Rev. Clin. Psychol. 2015, 11, 99–137. [Google Scholar] [CrossRef]
- Gould, E.; Tanapat, P.; McEwen, B.S.; Flügge, G.; Fuchs, E. Proliferation of Granule Cell Precursors in the Dentate Gyrus of Adult Monkeys Is Diminished by Stress. Proc. Natl. Acad. Sci. USA 1998, 95, 3168–3171. [Google Scholar] [CrossRef]
- Fujita, M.; Charney, D.S.; Innis, R.B. Imaging Serotonergic Neurotransmission in Depression: Hippocampal Pathophysiology May Mirror Global Brain Alterations. Biol. Psychiatry 2000, 48, 801–812. [Google Scholar] [CrossRef]
- Matrisciano, F.; Busceti, C.L.; Bucci, D.; Orlando, R.; Caruso, A.; Molinaro, G.; Cappuccio, I.; Riozzi, B.; Gradini, R.; Motolese, M.; et al. Induction of the Wnt Antagonist Dickkopf-1 Is Involved in Stress-Induced Hippocampal Damage. PLoS ONE 2011, 6, e16447. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Xu, N.; Kong, L.; Sun, S.-C.; Xu, X.-F.; Jia, M.-Z.; Wang, Y.; Chen, Z.-Y. The Antidepressant Roles of Wnt2 and Wnt3 in Stress-Induced Depression-like Behaviors. Transl. Psychiatry 2016, 6, e892. [Google Scholar] [CrossRef] [PubMed]
- Marzo, A.; Galli, S.; Lopes, D.; McLeod, F.; Podpolny, M.; Segovia-Roldan, M.; Ciani, L.; Purro, S.; Cacucci, F.; Gibb, A.; et al. Reversal of Synapse Degeneration by Restoring Wnt Signaling in the Adult Hippocampus. Curr. Biol. 2016, 26, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; De Kock, S.; Schild, V.; Veldhuis, H.D. Antiglucocorticoid RU 38486 Attenuates Retention of a Behaviour and Disinhibits the Hypothalamic-Pituitary Adrenal Axis at Different Brain Sites. Neuroendocrinology 1988, 47, 109–115. [Google Scholar] [CrossRef]
- Nestler, E.J.; Rainbow, T.C.; McEwen, B.S.; Greengard, P. Corticosterone Increases the Amount of Protein 1, a Neuron-Specific Phosphoprotein, in Rat Hippocampus. Science 1981, 212, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-M.; Han, H.; Wang, Q.-N.; Hou, H.-L.; Tong, H.; Yan, X.-B.; Zhou, J.-N. Mifepristone Repairs Region-Dependent Alteration of Synapsin I in Hippocampus in Rat Model of Depression. Neuropsychopharmacology 2007, 32, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of Stress in the Brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef]
- Farooq, R.K.; Asghar, K.; Kanwal, S.; Zulqernain, A. Role of Inflammatory Cytokines in Depression: Focus on Interleukin-1β. Biomed. Rep. 2017, 6, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Spokoini, R.; Kfir-Erenfeld, S.; Yefenof, E.; Sionov, R.V. Glycogen Synthase Kinase-3 Plays a Central Role in Mediating Glucocorticoid-Induced Apoptosis. Mol. Endocrinol. 2010, 24, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Pan, W. GSK3: A Multifaceted Kinase in Wnt Signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Abrous, D.N.; Wojtowicz, J.M. Interaction between Neurogenesis and Hippocampal Memory System: New Vistas. Cold Spring Harb. Perspect. Biol. 2015, 7, a018952. [Google Scholar] [CrossRef]
- Olton, D.S.; Branch, M.; Best, P.J. Spatial Correlates of Hippocampal Unit Activity. Exp. Neurol. 1978, 58, 387–409. [Google Scholar] [CrossRef]
- Abrous, N.; Koehl, M.; Lemaire, V.; Le Moal, M. Stress Prénatals: Effets Délétères à Long Terme Sur La Plasticité Hippocampique et Les Fonctions Cognitives. Med. Sci. 2001, 17, 119. [Google Scholar] [CrossRef]
- Flores-Ramirez, F.J.; Parise, L.F.; Alipio, J.B.; Garcia-Carachure, I.; Castillo, S.A.; Rodriguez, M.; Themman, A.; Lira, O.; Preciado-Piña, J.; Iñiguez, S.D. Adolescent Fluoxetine History Impairs Spatial Memory in Adult Male, but Not Female, C57BL/6 Mice. J. Affect. Disord. 2019, 249, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Beylin, A.; Tanapat, P.; Reeves, A.; Shors, T.J. Learning Enhances Adult Neurogenesis in the Hippocampal Formation. Nat. Neurosci. 1999, 2, 260–265. [Google Scholar] [CrossRef]
- Tabatadze, N.; Tomas, C.; McGonigal, R.; Lin, B.; Schook, A.; Routtenberg, A. Wnt Transmembrane Signaling and Long-Term Spatial Memory. Hippocampus 2012, 22, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Clark, R.E.; Broadbent, N.J.; Clemenson, G.D.; Consiglio, A.; Lie, D.C.; Squire, L.R.; Gage, F.H. Dentate Gyrus-Specific Knockdown of Adult Neurogenesis Impairs Spatial and Object Recognition Memory in Adult Rats. Learn. Mem. 2009, 16, 147–154. [Google Scholar] [CrossRef]
- Kuwabara, T.; Kagalwala, M.N.; Onuma, Y.; Ito, Y.; Warashina, M.; Terashima, K.; Sanosaka, T.; Nakashima, K.; Gage, F.H.; Asashima, M. Insulin Biosynthesis in Neuronal Progenitors Derived from Adult Hippocampus and the Olfactory Bulb. EMBO Mol. Med. 2011, 3, 742–754. [Google Scholar] [CrossRef]
- Soto, M.; Cai, W.; Konishi, M.; Kahn, C.R. Insulin Signaling in the Hippocampus and Amygdala Regulates Metabolism and Neurobehavior. Proc. Natl. Acad. Sci. USA 2019, 116, 6379–6384. [Google Scholar] [CrossRef]
- Liu, W.; Ye, P.; O’Kusky, J.R.; D’Ercole, A.J. Type 1 Insulin-like Growth Factor Receptor Signaling Is Essential for the Development of the Hippocampal Formation and Dentate Gyrus. J. Neurosci. Res. 2009, 87, 2821–2832. [Google Scholar] [CrossRef] [PubMed]
- Aberg, M.A.; Aberg, N.D.; Hedbäcker, H.; Oscarsson, J.; Eriksson, P.S. Peripheral Infusion of IGF-I Selectively Induces Neurogenesis in the Adult Rat Hippocampus. J. Neurosci. 2000, 20, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The Role of Insulin-Like Growth Factor 1 (IGF-1) in Brain Development, Maturation and Neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Conchillo, M.; de Knegt, R.J.; Payeras, M.; Quiroga, J.; Sangro, B.; Herrero, J.-I.; Castilla-Cortazar, I.; Frystyk, J.; Flyvbjerg, A.; Yoshizawa, C.; et al. Insulin-like Growth Factor I (IGF-I) Replacement Therapy Increases Albumin Concentration in Liver Cirrhosis: Results of a Pilot Randomized Controlled Clinical Trial. J. Hepatol. 2005, 43, 630–636. [Google Scholar] [CrossRef]
- Paslakis, G.; Blum, W.F.; Deuschle, M. Intranasal Insulin-like Growth Factor I (IGF-I) as a Plausible Future Treatment of Depression. Med. Hypotheses 2012, 79, 222–225. [Google Scholar] [CrossRef]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef]
- Encinas, J.M.; Vaahtokari, A.; Enikolopov, G. Fluoxetine Targets Early Progenitor Cells in the Adult Brain. Proc. Natl. Acad. Sci. USA 2006, 103, 8233–8238. [Google Scholar] [CrossRef]
- Wang, J.-W.; David, D.J.; Monckton, J.E.; Battaglia, F.; Hen, R. Chronic Fluoxetine Stimulates Maturation and Synaptic Plasticity of Adult-Born Hippocampal Granule Cells. J. Neurosci. 2008, 28, 1374–1384. [Google Scholar] [CrossRef]
- Chen, G.; Rajkowska, G.; Du, F.; Seraji-Bozorgzad, N.; Manji, H.K. Enhancement of Hippocampal Neurogenesis by Lithium. J. Neurochem. 2002, 75, 1729–1734. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liang, Y.; Chen, H.; Xu, B.; Chai, C.; Xing, P. The Role of Fluoxetine in Activating Wnt/β-Catenin Signaling and Repressing β-Amyloid Production in an Alzheimer Mouse Model. Front. Aging Neurosci. 2018, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, X.; Xu, Y.; Li, X.; Liu, P.; Sun, N.; Zhao, X.; Liu, Z.; Xie, Z.; Peng, J. Continuous GSK-3β Overexpression in the Hippocampal Dentate Gyrus Induces Prodepressant-like Effects and Increases Sensitivity to Chronic Mild Stress in Mice. J. Affect. Disord. 2013, 146, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pinnock, S.B.; Blake, A.M.; Platt, N.J.; Herbert, J. The Roles of BDNF, PCREB and Wnt3a in the Latent Period Preceding Activation of Progenitor Cell Mitosis in the Adult Dentate Gyrus by Fluoxetine. PLoS ONE 2010, 5, e13652. [Google Scholar] [CrossRef]
- Lonze, B.E.; Ginty, D.D. Function and Regulation of CREB Family Transcription Factors in the Nervous System. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef]
- Adachi, M.; Barrot, M.; Autry, A.E.; Theobald, D.; Monteggia, L.M. Selective Loss of Brain-Derived Neurotrophic Factor in the Dentate Gyrus Attenuates Antidepressant Efficacy. Biol. Psychiatry 2008, 63, 642–649. [Google Scholar] [CrossRef]
- Takano, K.; Yamasaki, H.; Kawabe, K.; Moriyama, M.; Nakamura, Y. Imipramine Induces Brain-Derived Neurotrophic Factor MRNA Expression in Cultured Astrocytes. J. Pharmacol. Sci. 2012, 120, 176–186. [Google Scholar] [CrossRef]
- Schloesser, R.J.; Orvoen, S.; Jimenez, D.V.; Hardy, N.F.; Maynard, K.R.; Sukumar, M.; Manji, H.K.; Gardier, A.M.; David, D.J.; Martinowich, K. Antidepressant-like Effects of Electroconvulsive Seizures Require Adult Neurogenesis in a Neuroendocrine Model of Depression. Brain Stimul. 2015, 8, 862–867. [Google Scholar] [CrossRef]
- Jang, M.-H.; Bonaguidi, M.A.; Kitabatake, Y.; Sun, J.; Song, J.; Kang, E.; Jun, H.; Zhong, C.; Su, Y.; Guo, J.U.; et al. Secreted Frizzled-Related Protein 3 Regulates Activity-Dependent Adult Hippocampal Neurogenesis. Cell Stem Cell 2013, 12, 215–223. [Google Scholar] [CrossRef]
- Jang, M.-H.; Kitabatake, Y.; Kang, E.; Jun, H.; Pletnikov, M.V.; Christian, K.M.; Hen, R.; Lucae, S.; Binder, E.B.; Song, H.; et al. Secreted Frizzled-Related Protein 3 (SFRP3) Regulates Antidepressant Responses in Mice and Humans. Mol. Psychiatry 2013, 18, 957–958. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The Tail Suspension Test as a Model for Assessing Antidepressant Activity: Review of Pharmacological and Genetic Studies in Mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 2015, 52587. [Google Scholar] [CrossRef]
- Manev, H.; Uz, T.; Smalheiser, N.R.; Manev, R. Antidepressants Alter Cell Proliferation in the Adult Brain in Vivo and in Neural Cultures in Vitro. Eur. J. Pharmacol. 2001, 411, 67–70. [Google Scholar] [CrossRef]
- Huang, G.-J.; Herbert, J. Stimulation of Neurogenesis in the Hippocampus of the Adult Rat by Fluoxetine Requires Rhythmic Change in Corticosterone. Biol. Psychiatry 2006, 59, 619–624. [Google Scholar] [CrossRef]
- Bessa, J.M.; Ferreira, D.; Melo, I.; Marques, F.; Cerqueira, J.J.; Palha, J.A.; Almeida, O.F.X.; Sousa, N. The Mood-Improving Actions of Antidepressants Do Not Depend on Neurogenesis but Are Associated with Neuronal Remodeling. Mol. Psychiatry 2009, 14, 764–773. [Google Scholar] [CrossRef]
- Cowen, D.S.; Takase, L.F.; Fornal, C.A.; Jacobs, B.L. Age-Dependent Decline in Hippocampal Neurogenesis Is Not Altered by Chronic Treatment with Fluoxetine. Brain Res. 2008, 1228, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Jedynak, P.; Kos, T.; Sandi, C.; Kaczmarek, L.; Filipkowski, R.K. Mice with Ablated Adult Brain Neurogenesis Are Not Impaired in Antidepressant Response to Chronic Fluoxetine. J. Psychiatr. Res. 2014, 56, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Ceccarelli, M.; D’Andrea, G.; Costanzi, M.; Giacovazzo, G.; Coccurello, R.; Caruso, C.; Tirone, F. Fluoxetine or Sox2 Reactivate Proliferation-Defective Stem and Progenitor Cells of the Adult and Aged Dentate Gyrus. Neuropharmacology 2018, 141, 316–330. [Google Scholar] [CrossRef]
- Huang, W.; Chang, H.Y.; Fei, T.; Wu, H.; Chen, Y.-G. GSK3 Beta Mediates Suppression of Cyclin D2 Expression by Tumor Suppressor PTEN. Oncogene 2007, 26, 2471–2482. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Filipkowski, R.K.; Rylski, M.; Wilczynski, G.M.; Konopacki, F.A.; Jaworski, J.; Ciemerych, M.A.; Sicinski, P.; Kaczmarek, L. The Critical Role of Cyclin D2 in Adult Neurogenesis. J. Cell Biol. 2004, 167, 209–213. [Google Scholar] [CrossRef]
- Palmos, A.B.; Duarte, R.R.R.; Smeeth, D.M.; Hedges, E.C.; Nixon, D.F.; Thuret, S.; Powell, T.R. Lithium Treatment and Human Hippocampal Neurogenesis. Transl. Psychiatry 2021, 11, 555. [Google Scholar] [CrossRef]
- Klein, P.S.; Melton, D.A. A Molecular Mechanism for the Effect of Lithium on Development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef]
- Toledo, E.M.; Inestrosa, N.C. Activation of Wnt Signaling by Lithium and Rosiglitazone Reduced Spatial Memory Impairment and Neurodegeneration in Brains of an APPswe/PSEN1DeltaE9 Mouse Model of Alzheimer’s Disease. Mol. Psychiatry 2010, 15, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Godavarthi, S.K.; Dey, P.; Sharma, A.; Jana, N.R. Impaired Adult Hippocampal Neurogenesis and Its Partial Reversal by Chronic Treatment of Fluoxetine in a Mouse Model of Angelman Syndrome. Biochem. Biophys. Res. Commun. 2015, 464, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Saaltink, D.-J.; Vreugdenhil, E. Stress, Glucocorticoid Receptors, and Adult Neurogenesis: A Balance between Excitation and Inhibition? Cell Mol. Life Sci. 2014, 71, 2499–2515. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Cameron, H.A.; Daniels, D.C.; Woolley, C.S.; McEwen, B.S. Adrenal Hormones Suppress Cell Division in the Adult Rat Dentate Gyrus. J. Neurosci. 1992, 12, 3642–3650. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. The Neuroendocrinology of Stress and Aging: The Glucocorticoid Cascade Hypothesis. Endocr. Rev. 1986, 7, 284–301. [Google Scholar] [CrossRef]
- Neumaier, J.F.; Sexton, T.J.; Hamblin, M.W.; Beck, S.G. Corticosteroids Regulate 5-HT(1A) but Not 5-HT(1B) Receptor MRNA in Rat Hippocampus. Mol. Brain Res. 2000, 82, 65–73. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sakai, R.R.; McEwen, B.S.; Mendelson, S. Stress and Antidepressant Effects on Hippocampal and Cortical 5-HT1A and 5-HT2 Receptors and Transport Sites for Serotonin. Brain Res. 1993, 615, 87–94. [Google Scholar] [CrossRef]
- Rezaei-Tavirani, M.; Tadayon, R.; Mortazavi, S.A.; Medhet, A.; Namaki, S.; Kalantari, S.; Noshinfar, E. Fluoxetine Competes with Cortisol for Binding to Human Serum Albumin. Iran. J. Pharm. Res. IJPR 2012, 11, 325–330. [Google Scholar] [CrossRef]
- Yoo, M.J.; Smith, Q.R.; Hage, D.S. Studies of Imipramine Binding to Human Serum Albumin by High-Performance Affinity Chromatography. J. Chromatogr. B 2009, 877, 1149–1154. [Google Scholar] [CrossRef]
- Rub, M.A.; Khan, J.M.; Yaseen, Z.; Khan, R.H. Conformational Changes of Serum Albumin upon Complexation with Amphiphilic Drug Imipramine Hydrochloride. J. Proteins Proteom. 2012, 3, 207–215. [Google Scholar]
- Brezun, J.; Daszuta, A. Depletion in Serotonin Decreases Neurogenesis in the Dentate Gyrus and the Subventricular Zone of Adult Rats. Neuroscience 1999, 89, 999–1002. [Google Scholar] [CrossRef]
- Belayev, L.; Alonso, O.F.; Huh, P.W.; Zhao, W.; Busto, R.; Ginsberg, M.D. Posttreatment with High-Dose Albumin Reduces Histopathological Damage and Improves Neurological Deficit Following Fluid Percussion Brain Injury in Rats. J. Neurotrauma 1999, 16, 445–453. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Michalski, S.; Zhao, S.; Chen, J. Traumatic Brain Injury Severity Affects Neurogenesis in Adult Mouse Hippocampus. J. Neurotrauma 2016, 33, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Saul, I.; Huh, P.W.; Finotti, N.; Zhao, W.; Busto, R.; Ginsberg, M.D. Neuroprotective Effect of High-Dose Albumin Therapy against Global Ischemic Brain Injury in Rats. Brain Res. 1999, 845, 107–111. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z. Neurogenesis Following Stroke Affecting the Adult Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a019034. [Google Scholar] [CrossRef]
- Darsalia, V.; Heldmann, U.; Lindvall, O.; Kokaia, Z. Stroke-Induced Neurogenesis in Aged Brain. Stroke 2005, 36, 1790–1795. [Google Scholar] [CrossRef]
- Chen, W.-W.; Fu, W.-Y.; Su, Y.-T.; Fang, W.-Q.; Fu, A.K.Y.; Ip, N.Y. Increased Axin Expression Enhances Adult Hippocampal Neurogenesis and Exerts an Antidepressant Effect. Sci. Rep. 2019, 9, 1190. [Google Scholar] [CrossRef]
- Okamoto, H.; Voleti, B.; Banasr, M.; Sarhan, M.; Duric, V.; Girgenti, M.J.; DiLeone, R.J.; Newton, S.S.; Duman, R.S. Wnt2 Expression and Signaling Is Increased by Different Classes of Antidepressant Treatments. Biol. Psychiatry 2010, 68, 521–527. [Google Scholar] [CrossRef]
- Madsen, T.M.; Newton, S.S.; Eaton, M.E.; Russell, D.S.; Duman, R.S. Chronic Electroconvulsive Seizure Up-Regulates β-Catenin Expression in Rat Hippocampus: Role in Adult Neurogenesis. Biol. Psychiatry 2003, 54, 1006–1014. [Google Scholar] [CrossRef]
- Galceran, J.; Miyashita-Lin, E.M.; Devaney, E.; Rubenstein, J.L.; Grosschedl, R. Hippocampus Development and Generation of Dentate Gyrus Granule Cells Is Regulated by LEF1. Development 2000, 127, 469–482. [Google Scholar] [CrossRef]
- Karasawa, T.; Yokokura, H.; Kitajewski, J.; Lombroso, P.J. Frizzled-9 Is Activated by Wnt-2 and Functions in Wnt/Beta -Catenin Signaling. J. Biol. Chem. 2002, 277, 37479–37486. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, Y.; Kagawa, T.; Shimizu, T.; Inoue, T.; Takada, S.; Kuratsu, J.; Taga, T. Wnt3a Promotes Hippocampal Neurogenesis by Shortening Cell Cycle Duration of Neural Progenitor Cells. Cell Mol. Neurobiol. 2010, 30, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Wayman, G.A.; Impey, S.; Marks, D.; Saneyoshi, T.; Grant, W.F.; Derkach, V.; Soderling, T.R. Activity-Dependent Dendritic Arborization Mediated by CaM-Kinase I Activation and Enhanced CREB-Dependent Transcription of Wnt-2. Neuron 2006, 50, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Katakowski, M.; Li, Y.; Lu, D.; Wang, L.; Zhang, L.; Chen, J.; Xu, Y.; Gautam, S.; Mahmood, A.; et al. Human Bone Marrow Stromal Cell Cultures Conditioned by Traumatic Brain Tissue Extracts: Growth Factor Production. J. Neurosci. Res. 2002, 69, 687–691. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Wang, L.; Katakowski, M.; Zhang, L.; Chen, J.; Xu, Y.; Gautam, S.C.; Chopp, M. Ischemic Rat Brain Extracts Induce Human Marrow Stromal Cell Growth Factor Production. Neuropathology 2002, 22, 275–279. [Google Scholar] [CrossRef]
- Wei, X.; Du, Z.; Zhao, L.; Feng, D.; Wei, G.; He, Y.; Tan, J.; Lee, W.-H.; Hampel, H.; Dodel, R.; et al. IFATS Collection: The Conditioned Media of Adipose Stromal Cells Protect Against Hypoxia-Ischemia-Induced Brain Damage in Neonatal Rats. Stem Cells 2009, 27, 478–488. [Google Scholar] [CrossRef]
- Xiao, N.; Le, Q.-T. Neurotrophic Factors and Their Potential Applications in Tissue Regeneration. Arch. Immunol. Ther. Exp. 2016, 64, 89–99. [Google Scholar] [CrossRef]
- Cozene, B.; Sadanandan, N.; Farooq, J.; Kingsbury, C.; Park, Y.J.; Wang, Z.-J.; Moscatello, A.; Saft, M.; Cho, J.; Gonzales-Portillo, B.; et al. Mesenchymal Stem Cell-Induced Anti-Neuroinflammation Against Traumatic Brain Injury. Cell Transplant. 2021, 30, 9636897211035715. [Google Scholar] [CrossRef]
- Pati, S.; Khakoo, A.Y.; Zhao, J.; Jimenez, F.; Gerber, M.H.; Harting, M.; Redell, J.B.; Grill, R.; Matsuo, Y.; Guha, S.; et al. Human Mesenchymal Stem Cells Inhibit Vascular Permeability by Modulating Vascular Endothelial Cadherin/β-Catenin Signaling. Stem Cells Dev. 2011, 20, 89–101. [Google Scholar] [CrossRef]
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.-C.; Wu, C.-C.; Chuang, D.-M.; Chiang, Y.-H.; Chen, K.-Y. Transplantation of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 Facilitates Cognitive Recovery and Enhances Neurogenesis in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 14–26. [Google Scholar] [CrossRef]
- Huang, P.; Freeman, W.D.; Edenfield, B.H.; Brott, T.G.; Meschia, J.F.; Zubair, A.C. Safety and Efficacy of Intraventricular Delivery of Bone Marrow-Derived Mesenchymal Stem Cells in Hemorrhagic Stroke Model. Sci. Rep. 2019, 9, 5674. [Google Scholar] [CrossRef] [PubMed]
- Coquery, N.; Blesch, A.; Stroh, A.; Fernández-Klett, F.; Klein, J.; Winter, C.; Priller, J. Intrahippocampal Transplantation of Mesenchymal Stromal Cells Promotes Neuroplasticity. Cytotherapy 2012, 14, 1041–1053. [Google Scholar] [CrossRef]
- Bao, X.; Wei, J.; Feng, M.; Lu, S.; Li, G.; Dou, W.; Ma, W.; Ma, S.; An, Y.; Qin, C.; et al. Transplantation of Human Bone Marrow-Derived Mesenchymal Stem Cells Promotes Behavioral Recovery and Endogenous Neurogenesis after Cerebral Ischemia in Rats. Brain Res. 2011, 1367, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, M.; Gemma, C.; Pennypacker, K.R.; Bickford, P.C.; Davis Sanberg, C.; Sanberg, P.R.; Willing, A.E. Cord Blood Rescues Stroke-Induced Changes in Splenocyte Phenotype and Function. Exp. Neurol. 2006, 199, 191–200. [Google Scholar] [CrossRef]
- Acosta, S.A.; Tajiri, N.; Hoover, J.; Kaneko, Y.; Borlongan, C.V. Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke. Stroke 2015, 46, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult Bone Marrow Stromal Cells Differentiate into Neural Cells in Vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic Administration of Cell-Free Exosomes Generated by Human Bone Marrow Derived Mesenchymal Stem Cells Cultured under 2D and 3D Conditions Improves Functional Recovery in Rats after Traumatic Brain Injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Lech, W.; Sarnowska, A.; Kuczynska, Z.; Dabrowski, F.; Figiel-Dabrowska, A.; Domanska-Janik, K.; Buzanska, L.; Zychowicz, M. Biomimetic Microenvironmental Preconditioning Enhance Neuroprotective Properties of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly (WJ-MSCs). Sci. Rep. 2020, 10, 16946. [Google Scholar] [CrossRef]
- Tomecka, E.; Lech, W.; Zychowicz, M.; Sarnowska, A.; Murzyn, M.; Oldak, T.; Domanska-Janik, K.; Buzanska, L.; Rozwadowska, N. Assessment of the Neuroprotective and Stemness Properties of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells under Variable (5% vs. 21%) Aerobic Conditions. Cells 2021, 10, 717. [Google Scholar] [CrossRef]
- Chang, C.P.; Chio, C.C.; Cheong, C.U.; Chao, C.M.; Cheng, B.C.; Lin, M.T. Hypoxic Preconditioning Enhances the Therapeutic Potential of the Secretome from Cultured Human Mesenchymal Stem Cells in Experimental Traumatic Brain Injury. Clin. Sci. 2013, 124, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Wedzinska, A.; Kot, M.; Sarnowska, A. Effect of Long-Term 3D Spheroid Culture on WJ-MSC. Cells 2021, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Roch, M.; Altschüler, J.; Winter, C.; Schwerk, A.; Kurtz, A.; Steiner, B. Human Adipose-Derived Mesenchymal Stem Cells Improve Motor Functions and Are Neuroprotective in the 6-Hydroxydopamine-Rat Model for Parkinson’s Disease When Cultured in Monolayer Cultures but Suppress Hippocampal Neurogenesis and Hippocampal Memory Function when Cultured in Spheroids. Stem Cell Rev. Rep. 2015, 11, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.R.; Stoutenger, B.R.; Robinson, A.P.; Spees, J.L.; Prockop, D.J. Human Stem/Progenitor Cells from Bone Marrow Promote Neurogenesis of Endogenous Neural Stem Cells in the Hippocampus of Mice. Proc. Natl. Acad. Sci. USA 2005, 102, 18171–18176. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, K.; Xie, L.; Childs, J.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. VEGF-Induced Neuroprotection, Neurogenesis, and Angiogenesis after Focal Cerebral Ischemia. J. Clin. Investig. 2003, 111, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular Endothelial Growth Factor (VEGF) Stimulates Neurogenesis in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D. Secretion of Immunoregulatory Cytokines by Mesenchymal Stem Cells. World J. Stem Cells 2014, 6, 552. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, S.L.; Spencer, G.J.; Heath, D.J.; Genever, P.G. Expression Profiling and Functional Analysis of Wnt Signaling Mechanisms in Mesenchymal Stem Cells. Stem Cells 2004, 22, 849–860. [Google Scholar] [CrossRef]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of Proliferative and Multilineage Differentiation Potential of Human Mesenchymal Stem Cells Derived from Umbilical Cord and Bone Marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Markov, V.; Kusumi, K.; Tadesse, M.G.; William, D.A.; Hall, D.M.; Lounev, V.; Carlton, A.; Leonard, J.; Cohen, R.I.; Rappaport, E.F.; et al. Identification of Cord Blood-Derived Mesenchymal Stem/Stromal Cell Populations with Distinct Growth Kinetics, Differentiation Potentials, and Gene Expression Profiles. Stem Cells Dev. 2007, 16, 53–73. [Google Scholar] [CrossRef]
- Salazar, K.D.; Lankford, S.M.; Brody, A.R. Mesenchymal Stem Cells Produce Wnt Isoforms and TGF-Beta1 That Mediate Proliferation and Procollagen Expression by Lung Fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L1002–L1011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gibb, S.L.; Zhao, J.; Moore, A.N.; Hylin, M.J.; Menge, T.; Xue, H.; Baimukanova, G.; Potter, D.; Johnson, E.M.; et al. Wnt3a, a Protein Secreted by Mesenchymal Stem Cells Is Neuroprotective and Promotes Neurocognitive Recovery Following Traumatic Brain Injury. Stem Cells 2016, 34, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, H.N.; Park, H.-J.; Shin, J.Y.; Lee, P.H. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer’s Disease Model. Cell Transplant. 2015, 24, 1097–1109. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, L.; Hu, W.; Shang, X.; He, Y.; Zhang, W. Zeb2/Axin2-Enriched BMSC-Derived Exosomes Promote Post-Stroke Functional Recovery by Enhancing Neurogenesis and Neural Plasticity. J. Mol. Neurosci. 2022, 72, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Liao, T.; Liu, J.; Fan, Z.; Zeng, Q.; Zhou, J.; Pei, H.; Xi, J.; He, L.; Chen, L.; et al. Clinical-Grade Human Umbilical Cord-Derived Mesenchymal Stem Cells Reverse Cognitive Aging via Improving Synaptic Plasticity and Endogenous Neurogenesis. Cell Death Dis. 2017, 8, e2996. [Google Scholar] [CrossRef]
- Hoshaw, B.A.; Hill, T.I.; Crowley, J.J.; Malberg, J.E.; Khawaja, X.; Rosenzweig-Lipson, S.; Schechter, L.E.; Lucki, I. Antidepressant-like Behavioral Effects of IGF-I Produced by Enhanced Serotonin Transmission. Eur. J. Pharmacol. 2008, 594, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Shirayama, Y.; Chen, A.C.H.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-Derived Neurotrophic Factor Produces Antidepressant Effects in Behavioral Models of Depression. J. Neurosci. 2002, 22, 3251–3261. [Google Scholar] [CrossRef] [PubMed]
- Tfilin, M.; Sudai, E.; Merenlender, A.; Gispan, I.; Yadid, G.; Turgeman, G. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Counteract Depressive-like Behavior. Mol. Psychiatry 2010, 15, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Kushnir-Sukhov, N.M.; Brown, J.M.; Wu, Y.; Kirshenbaum, A.; Metcalfe, D.D. Human Mast Cells Are Capable of Serotonin Synthesis and Release. J. Allergy Clin. Immunol. 2007, 119, 498–499. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Li, N.; Zhang, S.; Sun, J.; Zhang, S.; Qian, Y. Activated Brain Mast Cells Contribute to Postoperative Cognitive Dysfunction by Evoking Microglia Activation and Neuronal Apoptosis. J. Neuroinflamm. 2016, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, S.; Pejler, G. Mast Cell Secretory Granules: Armed for Battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulou, A.; Lyras, G.A.; Grigoriadis, N. Long-Term Effects of Autoimmune CNS Inflammation on Adult Hippocampal Neurogenesis. J. Neurosci. Res. 2017, 95, 1446–1458. [Google Scholar] [CrossRef]
- Doshmanziari, M.; Shirian, S.; Kouchakian, M.-R.; Moniri, S.F.; Jangnoo, S.; Mohammadi, N.; Zafari, F. Mesenchymal Stem Cells Act as Stimulators of Neurogenesis and Synaptic Function in a Rat Model of Alzheimer’s Disease. Heliyon 2021, 7, e07996. [Google Scholar] [CrossRef] [PubMed]
- Menge, T.; Zhao, Y.; Zhao, J.; Wataha, K.; Gerber, M.; Zhang, J.; Letourneau, P.; Redell, J.; Shen, L.; Wang, J.; et al. Mesenchymal Stem Cells Regulate Blood-Brain Barrier Integrity Through TIMP3 Release After Traumatic Brain Injury. Sci. Transl. Med. 2012, 4, 161ra150. [Google Scholar] [CrossRef] [PubMed]
- Lipp, H.-P.; Bonfanti, L. Adult Neurogenesis in Mammals: Variations and Confusions. Brain. Behav. Evol. 2016, 87, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Dong, H.-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zelikowsky, M.; Bissiere, S.; Hast, T.A.; Bennett, R.Z.; Abdipranoto, A.; Vissel, B.; Fanselow, M.S. Prefrontal Microcircuit Underlies Contextual Learning after Hippocampal Loss. Proc. Natl. Acad. Sci. USA 2013, 110, 9938–9943. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Geroldi, C.; Nobili, F.; Barnden, L.R.; Guerra, U.P.; Bonetti, M.; Frisoni, G.B. Functional Compensation in Incipient Alzheimer’s Disease. Neurobiol. Aging 2010, 31, 387–397. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human Hippocampal Neurogenesis Drops Sharply in Children to Undetectable Levels in Adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Boström, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of Hippocampal Neurogenesis in Adult Humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine Features of Neurogenesis in the Human Hippocampus across the Lifespan from 0 to 100 Years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.V.; Suh, L.S.; Rodriguez, M.L.; Kril, J.J.; Sutherland, G.T. Human Adult Neurogenesis across the Ages: An Immunohistochemical Study. Neuropathol. Appl. Neurobiol. 2016, 42, 621–638. [Google Scholar] [CrossRef]
- Mathews, K.J.; Allen, K.M.; Boerrigter, D.; Ball, H.; Shannon Weickert, C.; Double, K.L. Evidence for Reduced Neurogenesis in the Aging Human Hippocampus despite Stable Stem Cell Markers. Aging Cell 2017, 16, 1195–1199. [Google Scholar] [CrossRef]
- Seki, T.; Hori, T.; Miyata, H.; Maehara, M.; Namba, T. Analysis of Proliferating Neuronal Progenitors and Immature Neurons in the Human Hippocampus Surgically Removed from Control and Epileptic Patients. Sci. Rep. 2019, 9, 18194. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult Hippocampal Neurogenesis Is Abundant in Neurologically Healthy Subjects and Drops Sharply in Patients with Alzheimer’s Disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982.e3. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Terreros-Roncal, J.; Flor-García, M.; Rábano, A.; Llorens-Martín, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the Dentate Gyrus of the Adult Rat: Age-Related Decrease of Neuronal Progenitor Proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Cameron, H.A.; McKay, R.D.G. Restoring Production of Hippocampal Neurons in Old Age. Nat. Neurosci. 1999, 2, 894–897. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.Y.; Wojtowicz, J.M. Dynamics of Neurogenesis in the Dentate Gyrus of Adult Rats. Neurosci. Lett. 2005, 385, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, N.M.B.; Slomianka, L.; Vyssotski, A.L.; Lipp, H.-P. Early Age-Related Changes in Adult Hippocampal Neurogenesis in C57 Mice. Neurobiol. Aging 2010, 31, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Patzke, N.; Spocter, M.A.; Karlsson, K.Æ.; Bertelsen, M.F.; Haagensen, M.; Chawana, R.; Streicher, S.; Kaswera, C.; Gilissen, E.; Alagaili, A.N.; et al. In Contrast to Many Other Mammals, Cetaceans Have Relatively Small Hippocampi That Appear to Lack Adult Neurogenesis. Brain Struct. Funct. 2015, 220, 361–383. [Google Scholar] [CrossRef]
- Gould, E.; Vail, N.; Wagers, M.; Gross, C.G. Adult-Generated Hippocampal and Neocortical Neurons in Macaques Have a Transient Existence. Proc. Natl. Acad. Sci. USA 2001, 98, 10910–10917. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, M.; Yang, M.; Zeng, B.; Qiu, W.; Ma, Q.; Jing, X.; Zhang, Q.; Wang, B.; Yin, C.; et al. Transcriptome Dynamics of Hippocampal Neurogenesis in Macaques across the Lifespan and Aged Humans. Cell Res. 2022, 32, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Flor-García, M.; Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Ávila, J.; Rábano, A.; Llorens-Martín, M. Unraveling Human Adult Hippocampal Neurogenesis. Nat. Protoc. 2020, 15, 668–693. [Google Scholar] [CrossRef] [PubMed]
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef]
- Yu, D.X.; Di Giorgio, F.P.; Yao, J.; Marchetto, M.C.; Brennand, K.; Wright, R.; Mei, A.; McHenry, L.; Lisuk, D.; Grasmick, J.M.; et al. Modeling Hippocampal Neurogenesis Using Human Pluripotent Stem Cells. Stem Cell Rep. 2014, 2, 295–310. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Kadoshima, T.; Soen, M.; Narii, N.; Ishida, Y.; Ohgushi, M.; Takahashi, J.; Eiraku, M.; Sasai, Y. Generation of Functional Hippocampal Neurons from Self-Organizing Human Embryonic Stem Cell-Derived Dorsomedial Telencephalic Tissue. Nat. Commun. 2015, 6, 8896. [Google Scholar] [CrossRef]
- Del Dosso, A.; Urenda, J.-P.; Nguyen, T.; Quadrato, G. Upgrading the Physiological Relevance of Human Brain Organoids. Neuron 2020, 107, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Susaimanickam, P.J.; Kiral, F.R.; Park, I.-H. Region Specific Brain Organoids to Study Neurodevelopmental Disorders. Int. J. Stem Cells 2022, 15, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, T.J.; Salama, S.R. Cerebral Organoids as an Experimental Platform for Human Neurogenomics. Cells 2022, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.F.; Sorrells, S.F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Brain Size and Limits to Adult Neurogenesis. J. Comp. Neurol. 2016, 524, 646–664. [Google Scholar] [CrossRef]
- La Rosa, C.; Parolisi, R.; Bonfanti, L. Brain Structural Plasticity: From Adult Neurogenesis to Immature Neurons. Front. Neurosci. 2020, 14, 75. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kot, M.; Neglur, P.K.; Pietraszewska, A.; Buzanska, L. Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells. Cells 2022, 11, 3234. https://doi.org/10.3390/cells11203234
Kot M, Neglur PK, Pietraszewska A, Buzanska L. Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells. Cells. 2022; 11(20):3234. https://doi.org/10.3390/cells11203234
Chicago/Turabian StyleKot, Marta, Pawan Kumar Neglur, Anna Pietraszewska, and Leonora Buzanska. 2022. "Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells" Cells 11, no. 20: 3234. https://doi.org/10.3390/cells11203234
APA StyleKot, M., Neglur, P. K., Pietraszewska, A., & Buzanska, L. (2022). Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells. Cells, 11(20), 3234. https://doi.org/10.3390/cells11203234