A Novel GATA1 Variant in the C-Terminal Zinc Finger Compared with the Platelet Phenotype of Patients with A Likely Pathogenic Variant in the N-Terminal Zinc Finger
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Test
2.2.1. Cell Blood Count and Platelet Aggregometry Assays
2.2.2. Flow Cytometry Assays
2.2.3. Light and Immunofluorescence Microscopy
2.2.4. Transmission Electron Microscopy Assays
2.2.5. Molecular Genetic Analyses
3. Results
3.1. Blood Count, Blood Smear, Coagulation Parameters, and Bleeding Score
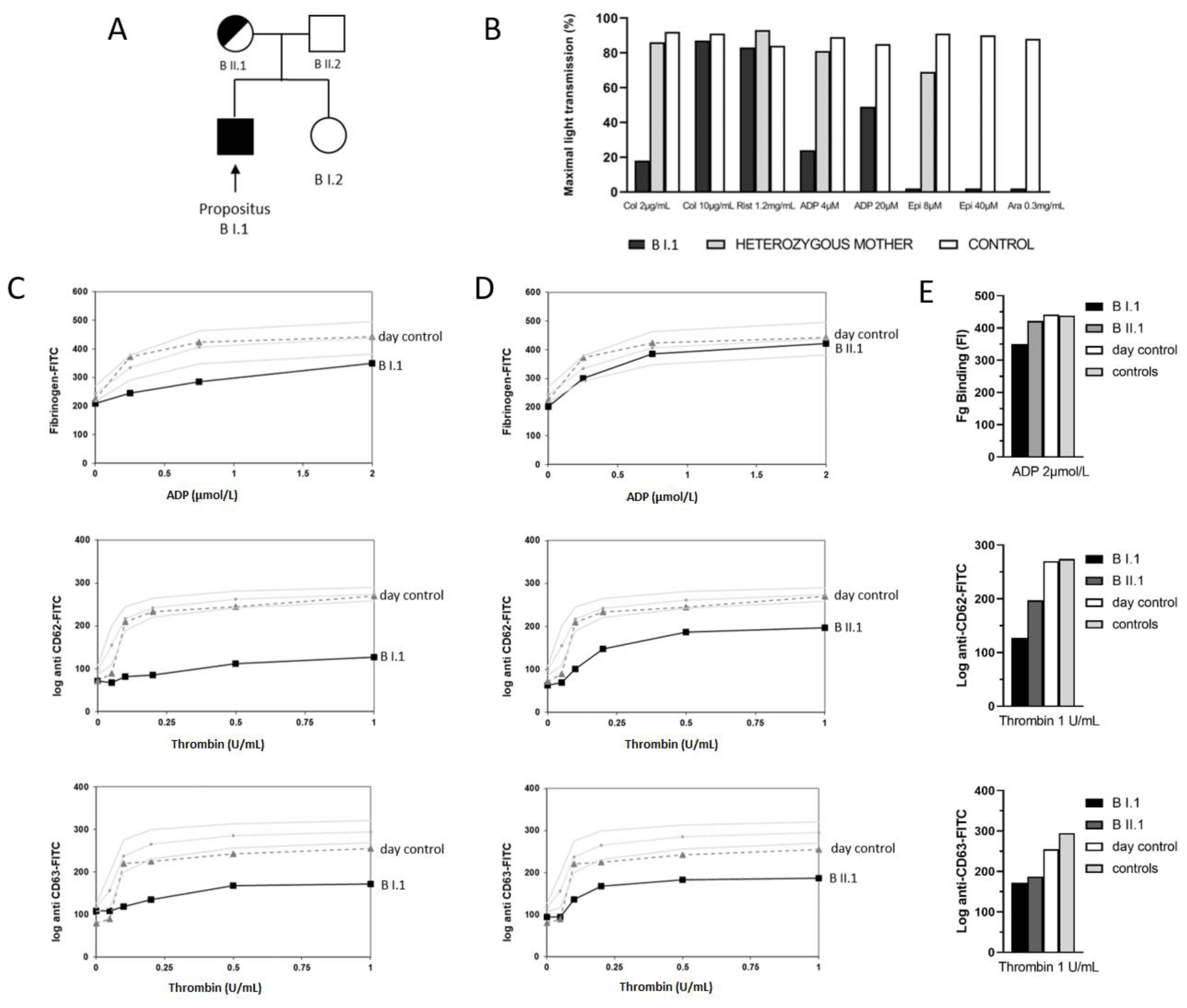
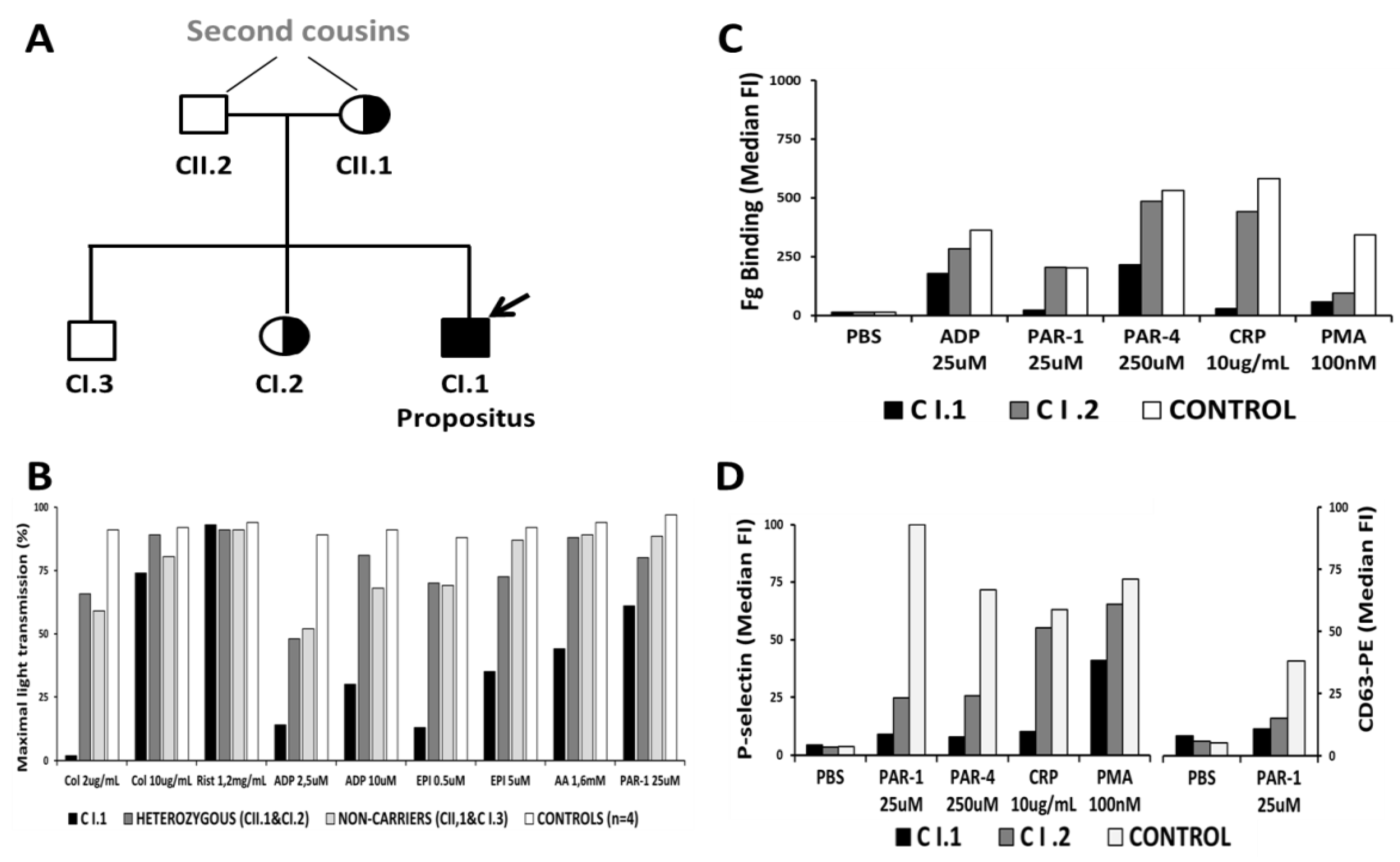
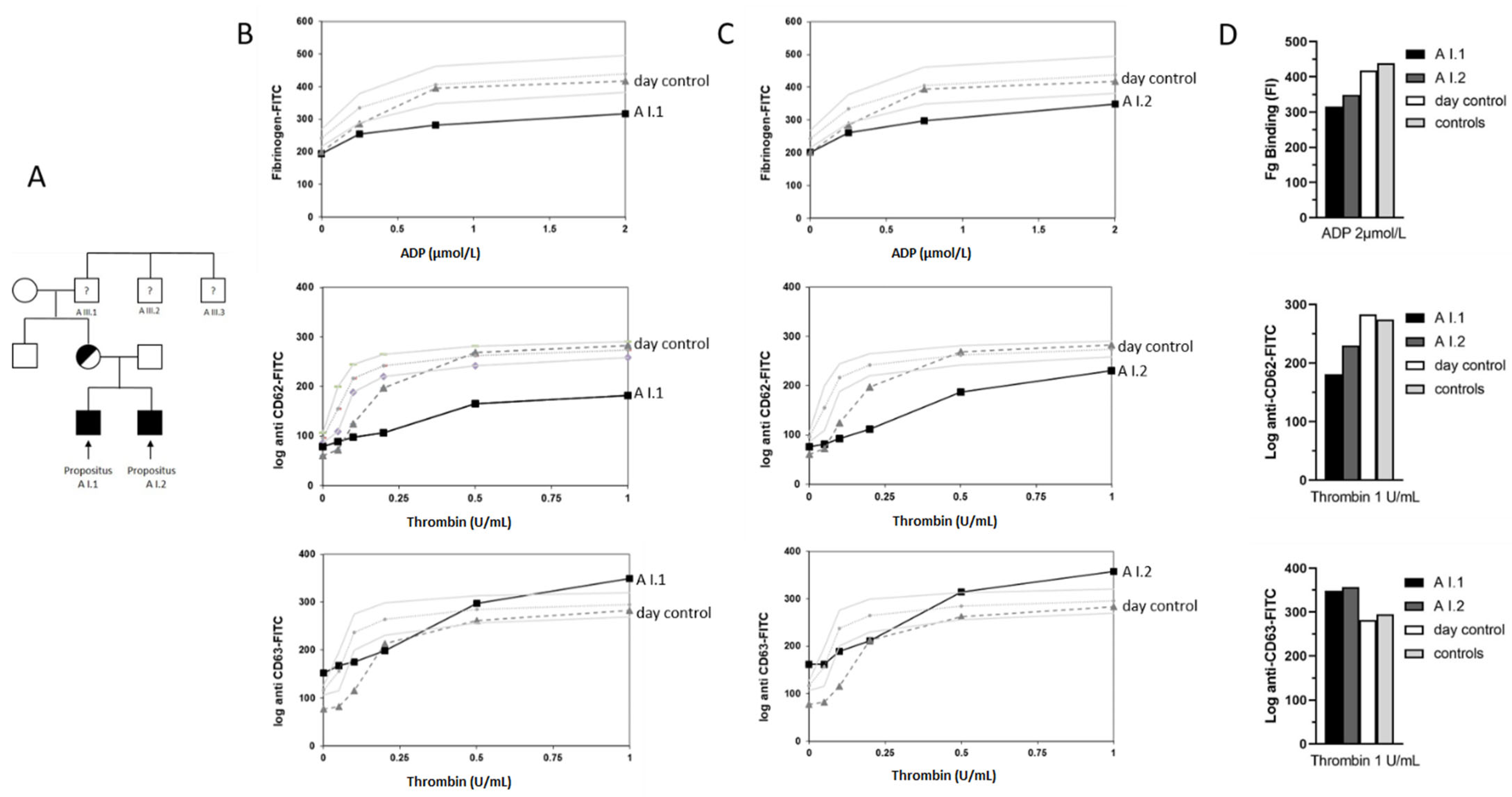
3.2. Light Transmission Aggregometry Indicated a Platelet Functional Defect
3.3. Platelet Flow Cytometric Analyses Indicated an α-Granule Defect
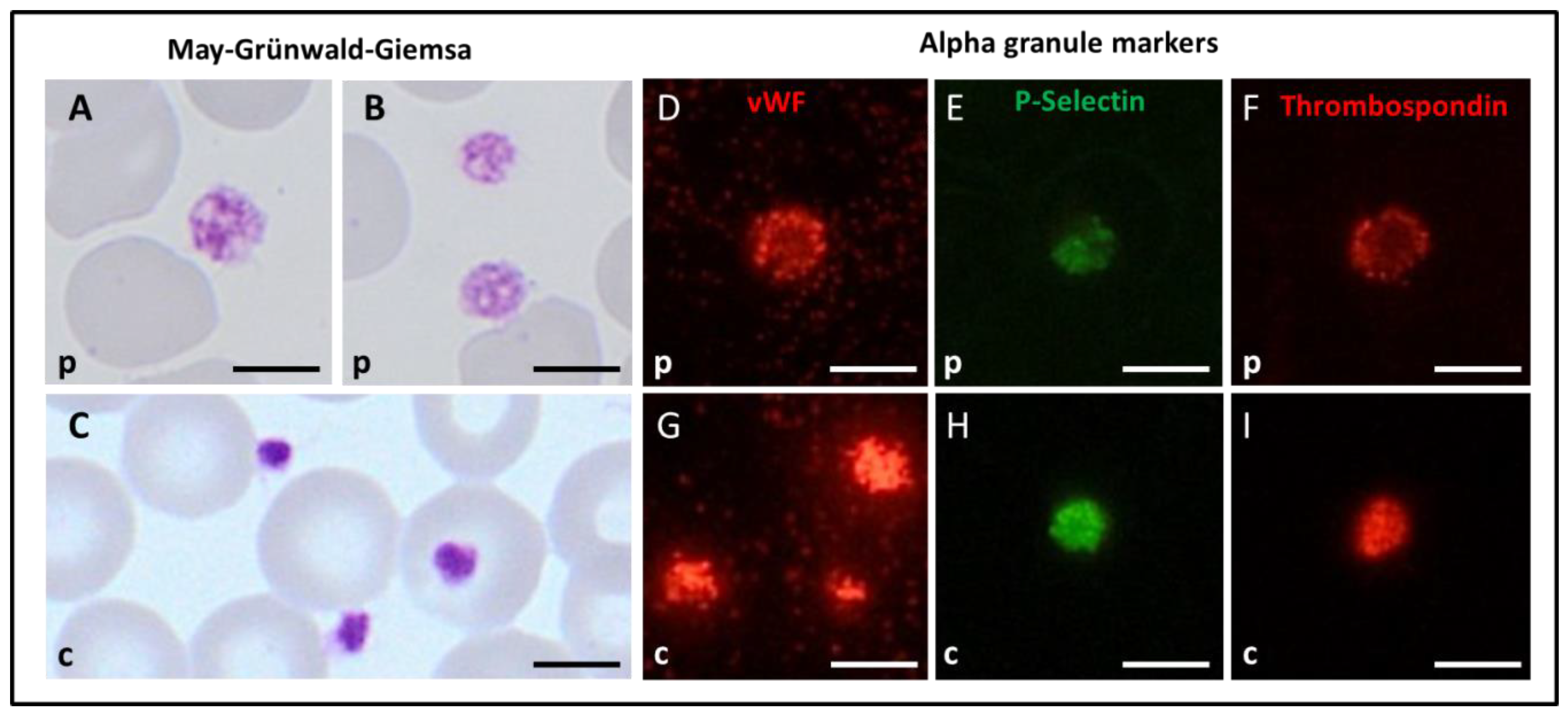
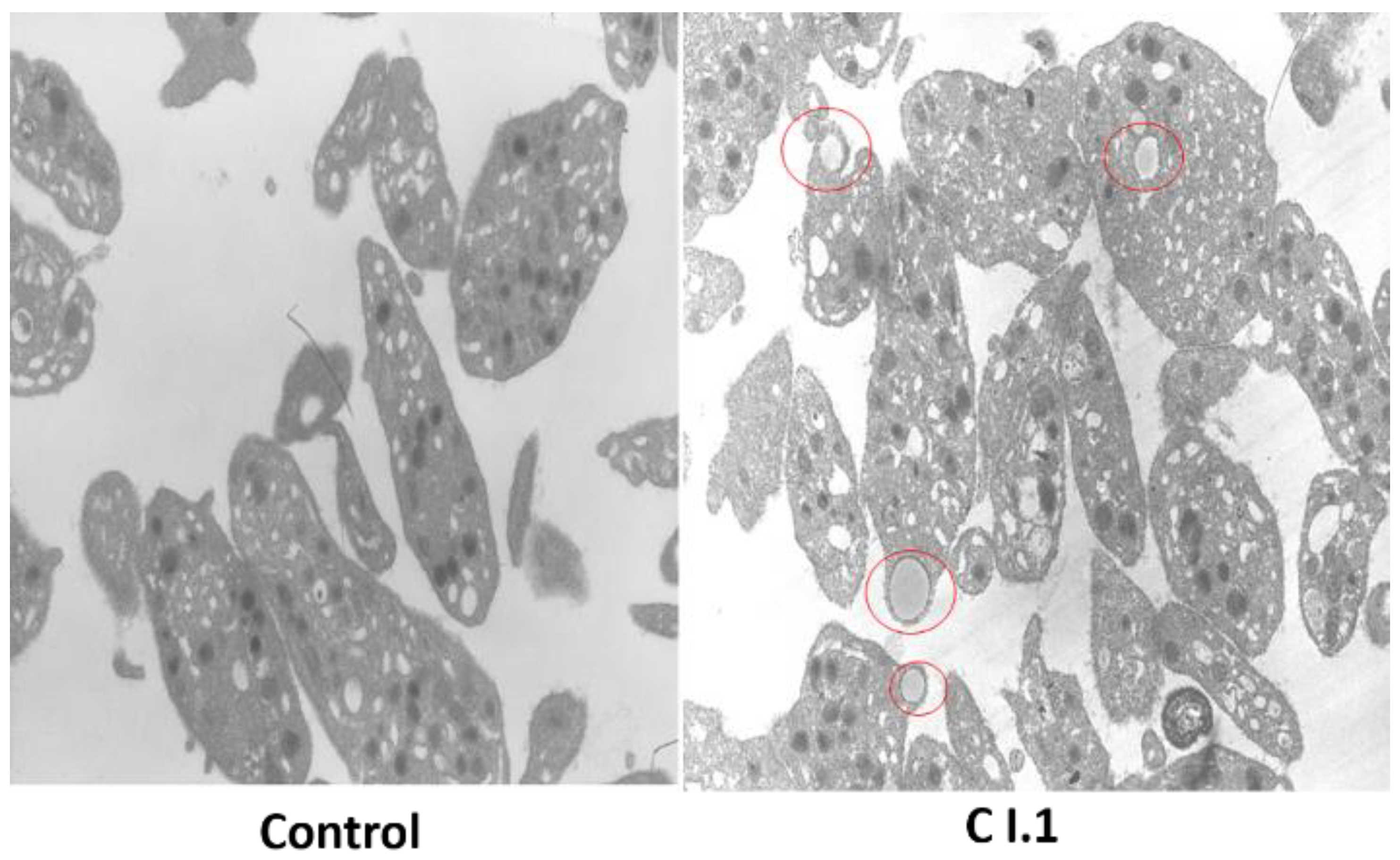
3.4. Platelet Immunofluorescence Microscopy, Morphology, and Ultrastructure for the Spanish Index Patient
3.5. Molecular Genetic Analyses Identified GATA1 Variants in the Two Zinc Finger Domains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nurden, P.; Stritt, S.; Favier, R.; Nurden, A.T. Inherited platelet diseases with normal platelet count: Phenotypes, genotypes and diagnostic strategy. Haematologica 2021, 106, 337–350. [Google Scholar] [CrossRef]
- Palma-Barqueros, V.; Revilla, N.; Sánchez, A.; Zamora Cánovas, A.; Rodriguez-Alén, A.; Marín-Quílez, A.; González-Porras, J.R.; Vicente, V.; Lozano, M.L.; Bastida, J.M.; et al. Inherited Platelet Disorders: An Updated Overview. Int. J. Mol. Sci. 2021, 22, 4521. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.T.; Di Paola, J. Genetics of inherited thrombocytopenias. Blood 2022, 139, 3264–3277. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.E. Transcription factor defects causing platelet disorders. Blood Rev. 2017, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ciovacco, W.A.; Raskind, W.H.; Kacena, M.A. Human phenotypes associated with GATA-1 mutations. Gene 2008, 427, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tijssen, M.R.; Cvejic, A.; Joshi, A.; Hannah, R.L.; Ferreira, R.; Forrai, A.; Bellissimo, D.C.; Oram, S.H.; Smethurst, P.A.; Wilson, N.K.; et al. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev. Cell 2011, 20, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Doré, L.C.; Crispino, J.D. Transcription factor networks in erythroid cell and megakaryocyte development. Blood 2011, 118, 231–239. [Google Scholar] [CrossRef]
- Songdej, N.; Rao, A.K. Inherited platelet dysfunction and hematopoietic transcription factor mutations. Platelets 2017, 28, 20–26. [Google Scholar] [CrossRef]
- Millikan, P.D.; Balamohan, S.M.; Raskind, W.H.; Kacena, M.A. Inherited thrombocytopenia due to GATA-1 mutations. Semin. Thromb. Hemost. 2011, 37, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.; Bonte, E.; Krijgsveld, J.; Kolodziej, K.E.; Guyot, B.; Heck, A.J.R.; Vyas, P.; de Boer, E.; Grosveld, F.; Strouboulis, J. GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J. 2005, 24, 2354–2366. [Google Scholar] [CrossRef]
- Eisbacher, M.; Holmes, M.L.; Newton, A.; Hogg, P.J.; Khachigian, L.M.; Crossley, M.; Chong, B.H. Protein-protein interaction between Fli-1 and GATA-1 mediates synergistic expression of megakaryocyte-specific genes through cooperative DNA binding. Mol. Cell. Biol. 2003, 23, 3427–3441. [Google Scholar] [CrossRef]
- Furihata, K.; Kunicki, T.J. Characterization of human glycoprotein VI gene 5’ regulatory and promoter regions. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, L.B.; Schick, B.P.; Budarf, M.L.; Driscoll, D.A.; Zackai, E.H.; Cohen, A.; Konkle, B.A. Identification of a mutation in a GATA binding site of the platelet glycoprotein Ibβ promoter resulting in the Bernard-Soulier syndrome. J. Biol. Chem. 1996, 271, 22076–22080. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Prandini, M.-H.; Thevenon, D.; Marguerie, G.; Uzan, G. The transcription factor GATA-1 regulates the promoter activity of the platelet glycoprotein IIb gene. J. Biol. Chem. 1993, 268, 21606–21612. [Google Scholar] [CrossRef]
- Ferreira, R.; Ohneda, K.; Yamamoto, M.; Philipsen, S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol. Cell. Biol. 2005, 25, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.P.; Visvader, J.E.; Turner, C.A.; Fujiwara, Y.; Yu, C.; Weiss, M.J.; Crossley, M.; Orkin, S.H. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell 1997, 90, 109–119. [Google Scholar] [CrossRef]
- Campbell, A.E.; Wilkinson-White, L.; Mackay, J.P.; Matthews, J.M.; Blobel, G.A. Analysis of disease-causing GATA1 mutations in murine gene complementation systems. Blood 2013, 121, 5218–5227. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-White, L.; Gamsjaeger, R.; Dastmalchi, S.; Wienert, B.; Stokes, P.H.; Crossley, M.; Mackay, J.P.; Matthews, J.M. Structural basis of simultaneous recruitment of the transcriptional regulators LMO2 and FOG1/ZFPM1 by the transcription factor GATA1. Proc. Natl. Acad. Sci. USA 2011, 108, 14443–14448. [Google Scholar] [CrossRef] [PubMed]
- Kadri, Z.; Shimizu, R.; Ohneda, O.; Maouche-Chretien, L.; Gisselbrecht, S.; Yamamoto, M.; Romeo, P.-H.; Leboulch, P.; Chretien, S. Direct binding of pRb/E2F-2 to GATA-1 regulates maturation and terminal cell division during erythropoiesis. PLoS Biol. 2009, 7, e1000123. [Google Scholar] [CrossRef] [PubMed]
- Rekhtman, N.; Radparvar, F.; Evans, T.; Skoultchi, A.I. Direct interaction of hematopoietic transcription factors PU.1 and GATA-1: Functional antagonism in erythroid cells. Genes Dev. 1999, 13, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Chlon, T.M.; McNulty, M.; Goldenson, B.; Rosinski, A.; Crispino, J.D. Global transcriptome and chromatin occupancy analysis reveal the short isoform of GATA1 is deficient for erythroid specification and gene expression. Haematologica 2015, 100, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Pevny, L.; Simon, M.C.; Robertson, E.; Klein, W.H.; Tsai, S.F.; D’Agati, V.; Orkin, S.H.; Costantini, F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 1991, 349, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.S.; Gazda, H.T.; Eng, J.C.; Eichhorn, S.W.; Thiru, P.; Ghazvinian, R.; George, T.I.; Gotlib, J.R.; Beggs, A.H.; Sieff, C.A.; et al. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat. Med. 2014, 20, 748–753. [Google Scholar] [CrossRef]
- Sankaran, V.G.; Ghazvinian, R.; Do, R.; Thiru, P.; Vergilio, J.-A.; Beggs, A.H.; Sieff, C.A.; Orkin, S.H.; Nathan, D.G.; Lander, E.S.; et al. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J. Clin. Investig. 2012, 122, 2439–2443. [Google Scholar] [CrossRef]
- Greene, M.E.; Mundschau, G.; Wechsler, J.; McDevitt, M.; Gamis, A.; Karp, J.; Gurbuxani, S.; Arceci, R.; Crispino, J.D. Mutations in GATA1 in both transient myeloproliferative disorder and acute megakaryoblastic leukemia of Down syndrome. Blood Cells Mol. Dis. 2003, 31, 351–356. [Google Scholar] [CrossRef]
- Wechsler, J.; Greene, M.; McDevitt, M.A.; Anastasi, J.; Karp, J.E.; Le Beau, M.M.; Crispino, J.D. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat. Genet. 2002, 32, 148–152. [Google Scholar] [CrossRef]
- Freson, K.; Wijgaerts, A.; Van Geet, C. GATA1 gene variants associated with thrombocytopenia and anemia. Platelets 2017, 28, 731–734. [Google Scholar] [CrossRef]
- Frisan, E.; Vandekerckhove, J.; de Thonel, A.; Pierre-Eugène, C.; Sternberg, A.; Arlet, J.-B.; Floquet, C.; Gyan, E.; Kosmider, O.; Dreyfus, F.; et al. Defective nuclear localization of Hsp70 is associated with dyserythropoiesis and GATA-1 cleavage in myelodysplastic syndromes. Blood 2012, 119, 1532–1542. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Caballero, N.; Fernández-Calleja, L.; Karkoulia, E.; Strouboulis, J. Regulation of GATA1 levels in erythropoiesis. IUBMB Life 2020, 72, 89–105. [Google Scholar] [CrossRef]
- Pereira, J.; Bento, C.; Manco, L.; Gonzalez, A.; Vagace, J.; Ribeiro, M.L. Congenital dyserythropoietic anemia associated to a GATA1 mutation aggravated by pyruvate kinase deficiency. Ann. Hematol. 2016, 95, 1551–1553. [Google Scholar] [CrossRef][Green Version]
- Tubman, V.N.; Levine, J.E.; Campagna, D.R.; Monahan-Earley, R.; Dvorak, A.M.; Neufeld, E.J.; Fleming, M.D. X-linked gray platelet syndrome due to a GATA1 Arg216Gln mutation. Blood 2007, 109, 3297–3299. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.E.; Crispino, J.D.; Poncz, M.; White, J.G.; Orkin, S.H.; Maris, J.M.; Weiss, M.J. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat. Genet. 2000, 24, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, G.C.; Giordani, L.; De Santis, A.; De Mattia, D. Dyserythropoietic anemia and thrombocytopenia due to a novel mutation in GATA-1. Acta Haematol. 2005, 114, 113–116. [Google Scholar] [CrossRef]
- Mehaffey, M.G.; Newton, A.L.; Gandhi, M.J.; Crossley, M.; Drachman, J.G. X-linked thrombocytopenia caused by a novel mutation of GATA-1. Blood 2001, 98, 2681–2688. [Google Scholar] [CrossRef]
- Freson, K.; Matthijs, G.; Thys, C.; Mariën, P.; Hoylaerts, M.F.; Vermylen, J.; Van Geet, C. Different substitutions at residue D218 of the X-linked transcription factor GATA1 lead to altered clinical severity of macrothrombocytopenia and anemia and are associated with variable skewed X inactivation. Hum. Mol. Genet. 2002, 11, 147–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freson, K.; Devriendt, K.; Matthijs, G.; Van Hoof, A.; De Vos, R.; Thys, C.; Minner, K.; Hoylaerts, M.F.; Vermylen, J.; Van Geet, C. Platelet characteristics in patients with X-linked macrothrombocytopenia because of a novel GATA1 mutation. Blood 2001, 98, 85–92. [Google Scholar] [CrossRef]
- Singleton, B.K.; Roxby, D.J.; Stirling, J.W.; Spring, F.A.; Wilson, C.; Poole, J.; Anstee, D.J. A novel GATA1 mutation (Stop414Arg) in a family with the rare X-linked blood group Lu(a-b-) phenotype and mild macrothrombocytic thrombocytopenia. Br. J. Haematol. 2013, 161, 139–142. [Google Scholar] [CrossRef]
- Phillips, J.D.; Steensma, D.P.; Pulsipher, M.A.; Spangrude, G.J.; Kushner, J.P. Congenital erythropoietic porphyria due to a mutation in GATA1: The first trans-acting mutation causative for a human porphyria. Blood 2007, 109, 2618–2621. [Google Scholar] [CrossRef]
- Yu, C.; Niakan, K.K.; Matsushita, M.; Stamatoyannopoulos, G.; Orkin, S.H.; Raskind, W.H. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood 2002, 100, 2040–2045. [Google Scholar] [CrossRef]
- Hermans, C.; De Waele, L.; Van Geet, C.; Freson, K. Novel GATA1 mutation in residue D218 leads to macrothrombocytopenia and clinical bleeding problems. Platelets 2014, 25, 305–307. [Google Scholar] [CrossRef][Green Version]
- Elbatarny, M.; Mollah, S.; Grabell, J.; Bae, S.; Deforest, M.; Tuttle, A.; Hopman, W.; Clark, D.S.; Mauer, A.C.; Bowman, M.; et al. Normal range of bleeding scores for the ISTH-BAT: Adult and pediatric data from the merging project. Haemophilia 2014, 20, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Tosetto, A.; Abshire, T.; Arnold, D.M.; Coller, B.; James, P.; Neunert, C.; Lillicrap, D.; ISTH/SSC joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group. ISTH/SSC bleeding assessment tool: A standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J. Thromb. Haemost. 2010, 8, 2063–2065. [Google Scholar] [CrossRef]
- Sánchez-Guiu, I.; Antón, A.I.; Padilla, J.; Velasco, F.; Lucia, J.F.; Lozano, M.; Cid, A.R.; Sevivas, T.; Lopez-Fernandez, M.F.; Vicente, V.; et al. Functional and molecular characterization of inherited platelet disorders in the Iberian Peninsula: Results from a collaborative study. Orphanet J. Rare Dis. 2014, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Lahav, J.; Jurk, K.; Hess, O.; Barnes, M.J.; Farndale, R.W.; Luboshitz, J.; Kehrel, B.E. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulfide exchange. Blood 2002, 100, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Palma-Barqueros, V.; Bury, L.; Kunishima, S.; Lozano, M.L.; Rodríguez-Alen, A.; Revilla, N.; Bohdan, N.; Padilla, J.; Fernandez-Perez, M.P.; de la Morena-Barrio, M.E.; et al. Expanding the genetic spectrum of TUBB1-related thrombocytopenia. Blood Adv. 2021, 5, 5453–5467. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Pecci, A.; Kunishima, S.; Althaus, K.; Nurden, P.; Balduini, C.L.; Bakchoul, T. Diagnosis of inherited platelet disorders on a blood smear: A tool to facilitate worldwide diagnosis of platelet disorders. J. Thromb. Haemost. 2017, 15, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Zaninetti, C.; Greinacher, A. Diagnosis of Inherited Platelet Disorders on a Blood Smear. J. Clin. Med. 2020, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Núñez, L.; Teruel, R.; Antón, A.I.; Nurden, P.; Martínez-Martínez, I.; Lozano, M.L.; Rivera, J.; Corral, J.; Mezzano, D.; Vicente, V.; et al. Rare homozygous status of P43 β1-tubulin polymorphism causes alterations in platelet ultrastructure. Thromb. Haemost. 2011, 105, 855–863. [Google Scholar] [CrossRef]
- Boeckelmann, D.; Wolter, M.; Neubauer, K.; Sobotta, F.; Lenz, A.; Glonnegger, H.; Käsmann-Kellner, B.; Mann, J.; Ehl, S.; Zieger, B. Hermansky-Pudlak Syndrome: Identification of Novel Variants in the Genes HPS3, HPS5, and DTNBP1 (HPS-7). Front. Pharmacol. 2021, 12, 786937. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.M.; Lozano, M.L.; Benito, R.; Janusz, K.; Palma-Barqueros, V.; Del Rey, M.; Hernandez-Sanchez, J.M.; Riesco, S.; Bermejo, N.; Gonzalez-Garcia, H.; et al. Introducing high-throughput sequencing into mainstream genetic diagnosis practice in inherited platelet disorders. Haematologica 2018, 103, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Saultier, P.; Cabantous, S.; Puceat, M.; Peiretti, F.; Bigot, T.; Saut, N.; Bordet, J.-C.; Canault, M.; van Agthoven, J.; Loosveld, M.; et al. GATA1 pathogenic variants disrupt MYH10 silencing during megakaryopoiesis. J. Thromb. Haemost. 2021, 19, 2287–2301. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Crossley, M.; Hill, J.; Orkin, S.H.; Adams, J.M. The C-terminal zinc finger of GATA-1 or GATA-2 is sufficient to induce megakaryocytic differentiation of an early myeloid cell line. Mol. Cell. Biol. 1995, 15, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Trainor, C.D.; Omichinski, J.G.; Vandergon, T.L.; Gronenborn, A.M.; Clore, G.M.; Felsenfeld, G. A palindromic regulatory site within vertebrate GATA-1 promoters requires both zinc fingers of the GATA-1 DNA-binding domain for high-affinity interaction. Mol. Cell. Biol. 1996, 16, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Wijgaerts, A.; Wittevrongel, C.; Thys, C.; Devos, T.; Peerlinck, K.; Tijssen, M.R.; Van Geet, C.; Freson, K. The transcription factor GATA1 regulates NBEAL2 expression through a long-distance enhancer. Haematologica 2017, 102, 695–706. [Google Scholar] [CrossRef]
- Svidnicki, M.C.C.M.; Filho, M.A.F.; Brandao, M.M.; Dos Santos, M.; de Oliveira Dias, R.; Tavares, R.S.; Assis-Mendonca, G.R.; Traina, F.; Saad, S.T.O. New germline GATA1 variant in females with anemia and thrombocytopenia. Blood Cells Mol. Dis. 2021, 88, 102545. [Google Scholar] [CrossRef] [PubMed]
- Hetzer, B.; Meryk, A.; Kropshofer, G.; Bargehr, C.; Jimenez-Heredia, R.; Boztug, K.; Mühlegger, B.E.; Dworzak, M.; Gruber, T.; Crazzolara, R. An R307H substitution in GATA1 that prevents Ser310 phosphorylation causes severe fetal anemia. Blood Adv. 2022, 6, 4330–4334. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.S.; Lareau, C.A.; Bao, E.L.; Liu, N.; Utsugisawa, T.; Tseng, A.M.; Myers, S.A.; Verboon, J.M.; Ulirsch, J.C.; Luo, W.; et al. Congenital anemia reveals distinct targeting mechanisms for master transcription factor GATA1. Blood 2022, 139, 2534–2546. [Google Scholar] [CrossRef]
| Individuals | GATA1 Status D218N | PLTs (×109/L) | MPV (fL) (7–12) | RBCs (×1012/L) | Hb (g/dL) | MCV (fL) | WBCs (×109/L) | Bleeding Time (Ivy) (2–6 min) | ISTH-SCC BAT Score (M < 4; F < 6) |
|---|---|---|---|---|---|---|---|---|---|
| A I.1 Propositus | Hemizygous | 37 (153–345) | 11.1 | 5.12 (4.38–5.92) | 15 (12.9–17.7) | 85.7 (78–95) | 7.2 (3.86–11.2) | >15 | 10 * |
| A I.2 Propositus | Hemizygous | 32 (148–358) | n.a. | 5.47 (4.31–5.86) | 13 (12.4–17.2) | 74.2 (77–93) | 5.7 (3.8–11.24) | 14.5 | 11 * |
| A II.1 Mother | Heterozygous | 151 (176–391) | 10.1 | 4.9 (4–5.2) | 13.2 (11.6–15.5) | 80.3 (80–95) | 6.1 (4–10.4) | 4.5 | 1 * |
| A II.2 Father | Wild type | 212 (146–328) | 11.1 | 4.7 (4.5–5.8) | 15.4 (13.5–17.6) | 93 (80–95.5) | 8.4 (3.9–9.8) | 3.0 | 0 |
| Individuals | GATA1 Status H289Y | PLTs (×109/L) | MPV (fL) (7–12) | RBCs (×1012/L) | Hb (g/dL) | MCV (fL) | WBCs (×109/L) | Bleeding Time (Ivy) (2–6 min) | ISTH-SCC BAT Score (M < 4; F < 6) |
|---|---|---|---|---|---|---|---|---|---|
| B I.1 Propositus | Hemizygous | 232 (146–328) | 9.9 | 3.99 (4.5–5.8) | 13.1 (13.5–17.6) | 101.5 | 6.2 (3.9–9.8) | >15 | 10 * |
| B II.1 Mother | Heterozygous | 251 (176–391) | 9.0 | 4.35 (4–5.2) | 13.5 (11.6–15.5) | 91.7 | 7.2 (4–10.4) | 4.5 | 2 * |
| B II.2 Father | Wildtype | 239 (146–328) | 11.0 | 5.25 (4.5–5.8) | 15.2 (13.5–17.6) | 83.2 | 5.2 (3.9–9.8) | n.a | 0 |
| B I.2 Sister | Wildtype | 199 (176–391) | 10.2 | 4.23 (4–5.2) | 13.3 (11.6–15.5) | 94.1 | 5.0 (4–10.4) | 6.5 | 1 * |
| Individuals | GATA1 Status H289Y | PLTs (×109/L) | MPV (fL) | RBCs (×1012/L) | Hb (g/dL) | MCV (fL) | WBCs (×109/L) | PFA-100 Col-Epi (s) | ISTH-SCC BAT Score (M < 4; F < 6) |
|---|---|---|---|---|---|---|---|---|---|
| CI.1 Propositus Year 2008 | Hemizygous | 123 | 8.2 | 3.72 | 12.4 | 97.0 | 6.0 | >300 s | 8 |
| Year 2022 | 182 | 11.8 | 4.26 | 13.5 | 103.1 | 12.1 | |||
| CI.2 Sister Year 2008 | Heterozygous | 186 | 8.4 | 4.13 | 13.5 | 94.0 | 8.2 | 141 | 0 |
| Year 2022 | 230 | 10.1 | 4.44 | 13.4 | 94.1 | 11.8 | |||
| CI.3 Brother | Wildtype | 209 | 8.8 | 4.75 | 14.4 | 84.5 | 8.9 | 85 | 0 |
| C2.1 Father | Wildtype | 149 | 9.2 | 4.56 | 14.0 | 89.9 | 5.5 | nr | 0 |
| C2.2 Mother | Heterozygous | 185 | 8.8 | 4.27 | 13.8 | 91.6 | 7.5 | 171 | 0 |
| c.DNA | Amino Acid | dbSNP(rs); ClinVar | Phenotype | Additional Information | Disease | References |
|---|---|---|---|---|---|---|
| Variants in the N-ZF and DNA-binding domain | ||||||
| 613G>A | V205M, Val205Met | rs104894815; pathogenic | Severe TP and dyserythropoietic anemia; heterozygous female: mild chronic TP | FOG-1 interaction impaired, UniProt: severe impairment of ZFPM1 binding and erythroid differentiation in vitro | XLT | Nichols et al. [32] |
| 617A>T | N206I, Asn206Ile | - | Severe TP without anemia, bone marrow: mild dyserythropoiesis | Disrupted MYH10 silencing during megakaryopoiesis | XLT | Saultier et al. [53] |
| 622G>A | G208R, Gly208Arg | rs587776454; pathogenic | TP and dyserythropoietic anemia | Decreased FOG1 binding, reduced transcriptional activation and repression, reduced megakaryocyte maturation (Campbell et al., [17]) | XLT | Del Vecchio et al. [33], further reports available |
| 622_623 delinsTC | G208S, Gly208Ser | rs137852312; pathogenic | MTP and severe bleeding, hypogranular macrothrombocytes, but most them contained some α-granules | FOG-1 interaction impaired, partially disrupts the interaction with ZFPM1 | XLT | Mehaffey et al. [34], further report available |
| 646C>T | R216W, Arg216Trp | rs387907207; pathogenic | Congenital erythropoietic porphyria, TP and thalassemia | Alters affinity of GATA1 either for FOG-1 or with GATA recognition sites | CEP | Phillips et al. [38] |
| 647G>A | R216Q, Arg216Gln | rs104894809; pathogenic | TP with thalassemia, absence or paucity of α-granules | Does not affect ZFPM1 binding; reduced affinity to palindromic GATA sites; supports erythroid maturation less efficiently than wild-type GATA1 | XLTT | Yu et al. [39], further reports available |
| 652G>A | D218N, Asp218Asn | rs104894808; ranges from VUS to pathogenic | TP, α-granule deficit | XLT | Hermans et al. [40]; this study | |
| 652G>T | D218Y, Asp218Tyr | rs104894808; pathogenic | MTP and marked anemia | FOG-1 interaction impaired | XLT | Freson et al. [35] |
| 653A>G | D218G, Asp218Gly | rs104894816; pathogenic | MTP and mild dyserythropoiesis without anemia | FOG-1 interaction impaired, partially disrupts the interaction with ZFPM1 | XLT | Freson et al. [36] |
| Variants in the C-ZF and DNA-binding domain | ||||||
| 788C>T | T263M, Thr263Met | - | Mild anemia, neutrophilia, thrombocytopenia, megakaryocyte proliferation with mild myelofibrosis in female carriers | Svidnicki et al. [57] | ||
| 802C>A | L268M | Normal platelet count at the beginning, then developing TP, TP with major δ-granule deficit, blood smear: red blood cell anisocytosis and poikilocytosis | Saultier et al. [53] | |||
| 865C>T | H289Y, His289Tyr | Two unrelated carriers: normal to variable platelet count (123 to 215 × 109/L), α- and δ-granule deficit | This study | |||
| 866A>G | H289R, His289Arg | Three hemizygous carriers: moderate decreased or normal platelet counts (129, 208, and 185 × 109/L, respectively) and mild macrocytic anemia | Pereira et al. [30] | |||
| 920G>A | R307H, Arg307His | - | Severe fetal anemia with sustained mild MTP (121 × 103/µL) and hyperchromic macrocytosis | Prevents Ser310 phosphorylation | HA | Hetzer et al. [58] |
| R307C, Arg307Cys | rs1057518396; ranges from VUS to pathogenic | Hemolytic anemia, mild TP, dyserythropoietic anemia | HA | Ludwig et al. [59] | ||
| Variants in C-terminal region of GATA1 | ||||||
| 1240T>C | Term414Argext*41 | rs587776456; pathogenic | Mild TP, and X-linked form of Lu(a-b-) blood group phenotype | MXLT | Singleton et al. [37] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastida, J.M.; Malvestiti, S.; Boeckelmann, D.; Palma-Barqueros, V.; Wolter, M.; Lozano, M.L.; Glonnegger, H.; Benito, R.; Zaninetti, C.; Sobotta, F.; et al. A Novel GATA1 Variant in the C-Terminal Zinc Finger Compared with the Platelet Phenotype of Patients with A Likely Pathogenic Variant in the N-Terminal Zinc Finger. Cells 2022, 11, 3223. https://doi.org/10.3390/cells11203223
Bastida JM, Malvestiti S, Boeckelmann D, Palma-Barqueros V, Wolter M, Lozano ML, Glonnegger H, Benito R, Zaninetti C, Sobotta F, et al. A Novel GATA1 Variant in the C-Terminal Zinc Finger Compared with the Platelet Phenotype of Patients with A Likely Pathogenic Variant in the N-Terminal Zinc Finger. Cells. 2022; 11(20):3223. https://doi.org/10.3390/cells11203223
Chicago/Turabian StyleBastida, José M., Stefano Malvestiti, Doris Boeckelmann, Verónica Palma-Barqueros, Mira Wolter, María L. Lozano, Hannah Glonnegger, Rocío Benito, Carlo Zaninetti, Felix Sobotta, and et al. 2022. "A Novel GATA1 Variant in the C-Terminal Zinc Finger Compared with the Platelet Phenotype of Patients with A Likely Pathogenic Variant in the N-Terminal Zinc Finger" Cells 11, no. 20: 3223. https://doi.org/10.3390/cells11203223
APA StyleBastida, J. M., Malvestiti, S., Boeckelmann, D., Palma-Barqueros, V., Wolter, M., Lozano, M. L., Glonnegger, H., Benito, R., Zaninetti, C., Sobotta, F., Schilling, F. H., Morgan, N. V., Freson, K., Rivera, J., & Zieger, B. (2022). A Novel GATA1 Variant in the C-Terminal Zinc Finger Compared with the Platelet Phenotype of Patients with A Likely Pathogenic Variant in the N-Terminal Zinc Finger. Cells, 11(20), 3223. https://doi.org/10.3390/cells11203223







