Fluorescence Molecular Targeting of Colon Cancer to Visualize the Invisible
Abstract
:1. Introduction
2. Impact of Margin Positivity on Colorectal Cancer and the Potential of Fluorescence Guidance
3. Principles of Fluorescence and Intraoperative Fluorescence Imaging
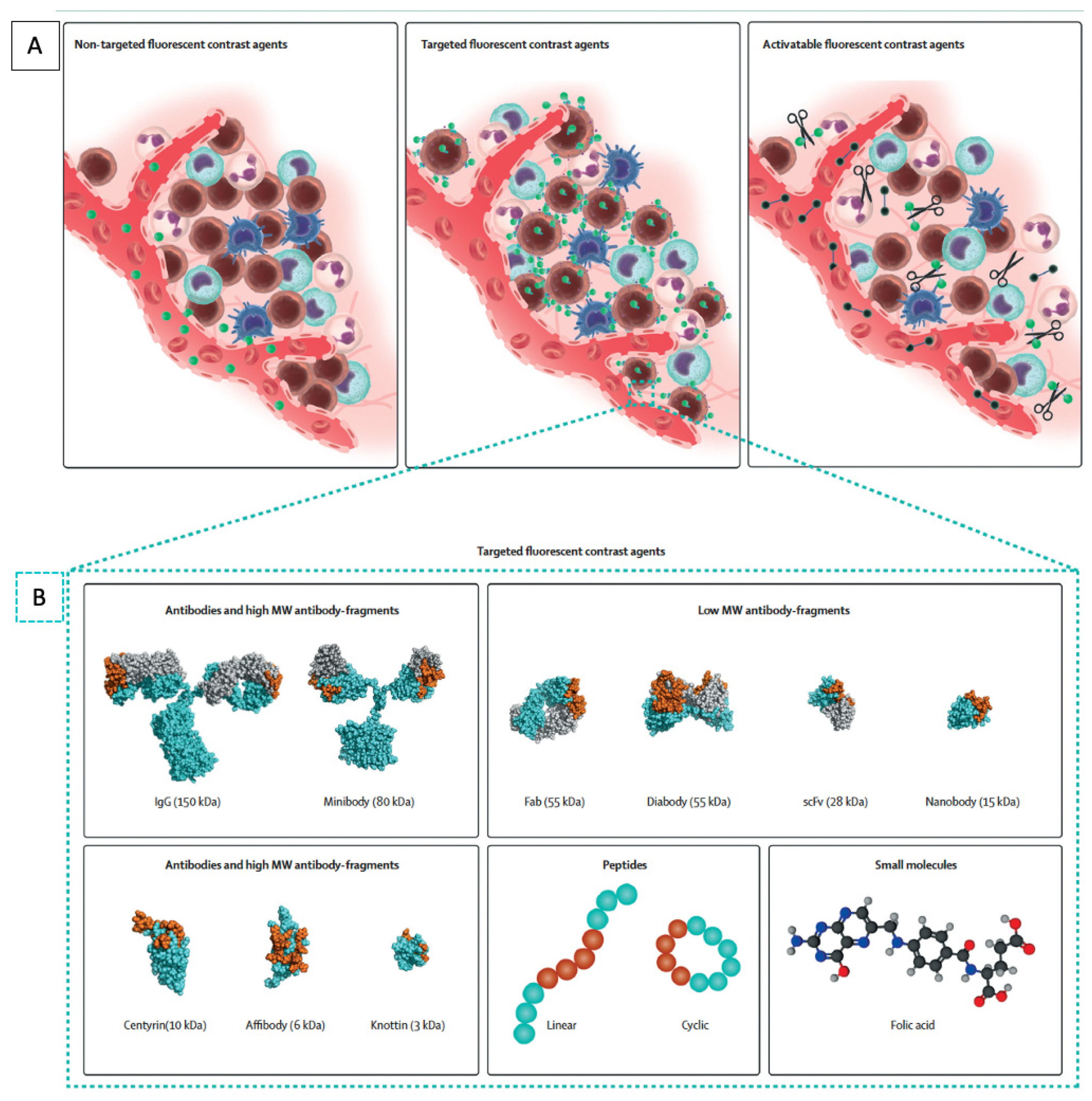
4. Non-Targeted Fluorescence Agents
5. Targeted Fluorescence Agents
6. Molecular Targets for CRC
7. Human Carcinoembryonic Antigen
8. CXCR4
9. EGFR
10. EpCAM
11. Matrix Metalloproteases
12. Mucins
13. Vascular Endothelial Growth Factor
14. Cathepsins
15. Tumor-Associated Glycoprotein-72
16. Folate Receptor Alpha
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R.; Garcia-Aguilar, J. Basic Principles of the Operative Treatment of Colorectal Cancer. In Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1981–1991. ISBN 978-0-323-40232-3. [Google Scholar]
- Orosco, R.K.; Tapia, V.J.; Califano, J.A.; Clary, B.; Cohen, E.E.W.; Kane, C.; Lippman, S.M.; Messer, K.; Molinolo, A.; Murphy, J.D.; et al. Positive Surgical Margins in the 10 Most Common Solid Cancers. Sci. Rep. 2018, 8, 5686. [Google Scholar] [CrossRef] [Green Version]
- MacFarlane, J.K.; Ryall, R.D.H.; Heald, R.J. Mesorectal Excision for Rectal Cancer. Lancet 1993, 341, 457–460. [Google Scholar] [CrossRef]
- Rickles, A.S.; Dietz, D.W.; Chang, G.J.; Wexner, S.D.; Berho, M.E.; Remzi, F.H.; Greene, F.L.; Fleshman, J.W.; Abbas, M.A.; Peters, W.; et al. High Rate of Positive Circumferential Resection Margins Following Rectal Cancer Surgery: A Call to Action. Ann. Surg. 2015, 262, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, K.C.M.J.; Marijnen, C.A.M.; Nagtegaal, I.D.; Kranenbarg, E.K.; Putter, H.; Wiggers, T.; Rutten, H.; Pahlman, L.; Glimelius, B.; Leer, J.W.; et al. The TME Trial After a Median Follow-up of 6 Years: Increased Local Control But No Survival Benefit in Irradiated Patients With Resectable Rectal Carcinoma. Ann. Surg. 2007, 246, 693–701. [Google Scholar] [CrossRef]
- Chu, Q.D.; Zhou, M.; Medeiros, K.; Peddi, P. Positive Surgical Margins Contribute to the Survival Paradox between Patients with Stage IIB/C (T4N0) and Stage IIIA (T1-2N1, T1N2a) Colon Cancer. Surgery 2016, 160, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Quirke, P. What Is the Role for the Circumferential Margin in the Modern Treatment of Rectal Cancer? JCO 2008, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Siebenhüner, A.R.; Güller, U.; Warschkow, R. Population-Based SEER Analysis of Survival in Colorectal Cancer Patients with or without Resection of Lung and Liver Metastases. BMC Cancer 2020, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; Neo, E.L.; Chen, J.W.C.; Maddern, G.J.; Wilson, T.G.; Padbury, R.T.A. Influence of Resection Margin on Survival in Hepatic Resections for Colorectal Liver Metastases. HPB 2009, 11, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rijn, J.C.; Reitsma, J.B.; Stoker, J.; Bossuyt, P.M.; van Deventer, S.J.; Dekker, E. Polyp Miss Rate Determined by Tandem Colonoscopy: A Systematic Review. Am. J. Gastroenterol. 2006, 101, 343–350. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. A Brief History of Fluorescence and Phosphorescence before the Emergence of Quantum Theory. J. Chem. Educ. 2011, 88, 731–738. [Google Scholar] [CrossRef]
- Monici, M. Cell and Tissue Autofluorescence Research and Diagnostic Applications. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 227–256. ISBN 978-0-444-51952-8. [Google Scholar]
- Benmiloud, F.; Godiris-Petit, G.; Gras, R.; Gillot, J.-C.; Turrin, N.; Penaranda, G.; Noullet, S.; Chéreau, N.; Gaudart, J.; Chiche, L.; et al. Association of Autofluorescence-Based Detection of the Parathyroid Glands During Total Thyroidectomy With Postoperative Hypocalcemia Risk: Results of the PARAFLUO Multicenter Randomized Clinical Trial. JAMA Surg. 2020, 155, 106. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.A.; Weissleder, R. Near-Infrared Fluorescence: Application to in Vivo Molecular Imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Maeda, H. Macromolecular Therapeutics in Cancer Treatment: The EPR Effect and Beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- Schupper, A.J.; Yong, R.L.; Hadjipanayis, C.G. The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection. J. Clin. Med. 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Murayama, Y.; Konishi, H.; Morimura, R.; Komatsu, S.; Shiozaki, A.; Kuriu, Y.; Ikoma, H.; Kubota, T.; Nakanishi, M.; et al. Fluorescent Detection of Peritoneal Metastasis in Human Colorectal Cancer Using 5-Aminolevulinic Acid. Int. J. Oncol. 2014, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Andrew, H.; Gossedge, G.; Croft, J.; Corrigan, N.; Brown, J.M.; West, N.; Quirke, P.; Tolan, D.; Cahill, R.; Jayne, D.G. Next Generation Intraoperative Lymph Node Staging for Stratified Colon Cancer Surgery (GLiSten): A Multicentre, Multinational Feasibility Study of Fluorescence in Predicting Lymph Node-Positive Disease. Efficacy Mech. Eval. 2016, 3, 1–122. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. Ann. Surg. 1994, 220, 391–398; discussion 398–401. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, F.P.R.; van der Vorst, J.R.; Schaafsma, B.E.; Swijnenburg, R.-J.; Gaarenstroom, K.N.; Elzevier, H.W.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L. Intraoperative near Infrared Fluorescence Guided Identification of the Ureters Using Low Dose Methylene Blue: A First in Human Experience. J. Urol. 2013, 190, 574–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, T.G.; Hompes, R.; Birks, J.; Mortensen, N.J.; Jones, O.; Lindsey, I.; Guy, R.; George, B.; Cunningham, C.; Yeung, T.M. Methylene Blue Fluorescence of the Ureter during Colorectal Surgery. Surg. Endosc. 2018, 32, 4036–4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Jiang, D.; Huang, B.; Wang, C.; Zhao, L.; Xie, X.; Zhang, Z.; Wang, K.; Tian, J.; Luo, Y. Methylene Blue–Based Near-Infrared Fluorescence Imaging for Breast Cancer Visualization in Resected Human Tissues. Technol. Cancer Res. Treat. 2019, 18, 153303381989433. [Google Scholar] [CrossRef]
- Yaroslavsky, A.N.; Feng, X.; Muzikansky, A.; Hamblin, M.R. Fluorescence Polarization of Methylene Blue as a Quantitative Marker of Breast Cancer at the Cellular Level. Sci. Rep. 2019, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-Guided Surgery. Front. Oncol. 2017, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, A.; Bonino, M.A.; Ris, F.; Boni, L.; Cassinotti, E.; Foo, D.C.C.; Shum, N.F.; Brolese, A.; Ciarleglio, F.; Keller, D.S.; et al. Intraoperative Use of Fluorescence with Indocyanine Green Reduces Anastomotic Leak Rates in Rectal Cancer Surgery: An Individual Participant Data Analysis. Surg. Endosc. 2020, 34, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Alius, C.; Tudor, C.; Badiu, C.D.; Dascalu, A.M.; Smarandache, C.G.; Sabau, A.D.; Tanasescu, C.; Balasescu, S.A.; Serban, D. Indocyanine Green-Enhanced Colorectal Surgery—between Being Superfluous and Being a Game-Changer. Diagnostics 2020, 10, 742. [Google Scholar] [CrossRef]
- Blanco-Colino, R.; Espin-Basany, E. Intraoperative Use of ICG Fluorescence Imaging to Reduce the Risk of Anastomotic Leakage in Colorectal Surgery: A Systematic Review and Meta-Analysis. Tech. Coloproctol. 2018, 22, 15–23. [Google Scholar] [CrossRef]
- Zako, T.; Ito, M.; Hyodo, H.; Yoshimoto, M.; Watanabe, M.; Takemura, H.; Kishimoto, H.; Kaneko, K.; Soga, K.; Maeda, M. Extra-Luminal Detection of Assumed Colonic Tumor Site by near-Infrared Laparoscopy. Surg. Endosc. 2016, 30, 4153–4159. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Murakami, M.; Ozawa, Y.; Yoshizawa, S.; Matsui, N.; Aoki, T. Intraoperative Identification of Colonic Tumor Sites Using a Near-Infrared Fluorescence Endoscopic Imaging System and Indocyanine Green. Dig. Surg. 2017, 34, 495–501. [Google Scholar] [CrossRef]
- Nagata, J.; Fukunaga, Y.; Akiyoshi, T.; Konishi, T.; Fujimoto, Y.; Nagayama, S.; Yamamoto, N.; Ueno, M. Colonic Marking With Near-Infrared, Light-Emitting, Diode-Activated Indocyanine Green for Laparoscopic Colorectal Surgery. Dis. Colon Rectum 2016, 59, e14–e18. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, P.; Wang, Z.; Zhang, W.; Lu, Q.; Butch, C.J.; Guissi, N.E.I.; You, Q.; Cai, H.; Ding, Y.; et al. A Pilot Study of Near-Infrared Fluorescence Guided Surgery for Primary Tumor Localization and Lymph Node Mapping in Colorectal Cancer. Ann. Transl. Med. 2021, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Liberale, G.; Vankerckhove, S.; Caldon, M.G.; Ahmed, B.; Moreau, M.; Nakadi, I.E.; Larsimont, D.; Donckier, V.; Bourgeois, P.; Group R&D for the Clinical Application of Fluorescence Imaging of the Jules Bordet’s Institute. Fluorescence Imaging After Indocyanine Green Injection for Detection of Peritoneal Metastases in Patients Undergoing Cytoreductive Surgery for Peritoneal Carcinomatosis From Colorectal Cancer: A Pilot Study. Ann. Surg. 2016, 264, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Basile, N.; Castellano, P.; Orditura, M.; Galizia, G. Fluorescence-Guided Surgery in the Combined Treatment of Peritoneal Carcinomatosis from Colorectal Cancer: Preliminary Results and Considerations. World J. Surg. 2018, 42, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.D.; Predina, J.D.; Shin, M.H.; Frenzel-Sulyok, L.G.; Vollmer, C.M.; Drebin, J.A.; Singhal, S.; Lee, M.K. Intraoperative Near-Infrared Imaging Can Identify Neoplasms and Aid in Real-Time Margin Assessment During Pancreatic Resection. Ann. Surg. 2019, 270, 12–20. [Google Scholar] [CrossRef]
- Predina, J.D.; Newton, A.D.; Corbett, C.; Shin, M.; Sulfyok, L.F.; Okusanya, O.T.; Delikatny, E.J.; Nie, S.; Gaughan, C.; Jarrar, D.; et al. Near-Infrared Intraoperative Imaging for Minimally Invasive Pulmonary Metastasectomy for Sarcomas. J. Thorac. Cardiovasc. Surg. 2019, 157, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Zeh, R.; Sheikh, S.; Xia, L.; Pierce, J.; Newton, A.; Predina, J.; Cho, S.; Nasrallah, M.; Singhal, S.; Dorsey, J.; et al. The Second Window ICG Technique Demonstrates a Broad Plateau Period for near Infrared Fluorescence Tumor Contrast in Glioblastoma. PLoS ONE 2017, 12, e0182034. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.K.; Pierce, J.T.; Zeh, R.; Cho, S.S.; Salinas, R.; Nie, S.; Singhal, S. Intraoperative Near-Infrared Optical Contrast Can Localize Brain Metastases. World Neurosurg. 2017, 106, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zeh, R.; Mizelle, J.; Newton, A.; Predina, J.; Nie, S.; Singhal, S.; Guzzo, T.J. Near-Infrared Intraoperative Molecular Imaging Can Identify Metastatic Lymph Nodes in Prostate Cancer. Urology 2017, 106, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.D.; Predina, J.D.; Frenzel-Sulyok, L.G.; Shin, M.H.; Wang, Y.; Singhal, S. Intraoperative Near-Infrared Imaging Can Identify Sub-Centimeter Colorectal Cancer Lung Metastases during Pulmonary Metastasectomy. J. Thorac. Dis. 2018, 10, E544–E548. [Google Scholar] [CrossRef]
- Debie, P.; Hernot, S. Emerging Fluorescent Molecular Tracers to Guide Intra-Operative Surgical Decision-Making. Front. Pharmacol. 2019, 10, 510. [Google Scholar] [CrossRef] [Green Version]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest Developments in Molecular Tracers for Fluorescence Image-Guided Cancer Surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Dennler, P.; Fischer, E.; Schibli, R. Antibody Conjugates: From Heterogeneous Populations to Defined Reagents. Antibodies 2015, 4, 197–224. [Google Scholar] [CrossRef]
- Olafsen, T.; Cheung, C.-W.; Yazaki, P.J.; Li, L.; Sundaresan, G.; Gambhir, S.S.; Sherman, M.A.; Williams, L.E.; Shively, J.E.; Raubitschek, A.A.; et al. Covalent Disulfide-Linked Anti-CEA Diabody Allows Site-Specific Conjugation and Radiolabeling for Tumor Targeting Applications. Protein Eng. Des. Sel. 2004, 17, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Sonn, G.A.; Behesnilian, A.S.; Jiang, Z.K.; Zettlitz, K.A.; Lepin, E.J.; Bentolila, L.A.; Knowles, S.M.; Lawrence, D.; Wu, A.M.; Reiter, R.E. Fluorescent Image-Guided Surgery with an Anti-Prostate Stem Cell Antigen (PSCA) Diabody Enables Targeted Resection of Mouse Prostate Cancer Xenografts in Real Time. Clin. Cancer Res. 2016, 22, 1403–1412. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Kobayashi, N.; Zettlitz, K.A.; Kono, E.A.; Yamashiro, J.M.; Tsai, W.-T.K.; Jiang, Z.K.; Tran, C.P.; Wang, C.; Guan, J.; et al. Near-Infrared Dye-Labeled Anti-Prostate Stem Cell Antigen Minibody Enables Real-Time Fluorescence Imaging and Targeted Surgery in Translational Mouse Models. Clin. Cancer Res. 2019, 25, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, C.; Muyldermans, S. Nanobody-Based Delivery Systems for Diagnosis and Targeted Tumor Therapy. Front. Immunol. 2017, 8, 1442. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, M.; Skerra, A. Engineered Protein Scaffolds as Next-Generation Antibody Therapeutics. Curr. Opin. Chem. Biol. 2009, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.A.; Case, B.A.; Hackel, B.J. Alternative Non-Antibody Protein Scaffolds for Molecular Imaging of Cancer. Curr. Opin. Chem. Eng. 2013, 2, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barozzi, A.; Lavoie, R.A.; Day, K.N.; Prodromou, R.; Menegatti, S. Affibody-Binding Ligands. Int. J. Mol. Sci. 2020, 21, 3769. [Google Scholar] [CrossRef] [PubMed]
- Frejd, F.Y.; Kim, K.-T. Affibody Molecules as Engineered Protein Drugs. Exp. Mol. Med. 2017, 49, e306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Bouvier-Müller, A.; Ducongé, F. Application of Aptamers for in Vivo Molecular Imaging and Theranostics. Adv. Drug Deliv. Rev. 2018, 134, 94–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacivita, E.; Leopoldo, M.; Berardi, F.A.; Colabufo, N.; Perrone, R. Activatable Fluorescent Probes: A New Concept in Optical Molecular Imaging. CMC 2012, 19, 4731–4741. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An Overview of Nanoparticles Commonly Used in Fluorescent Bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Oosten, M.; Crane, L.M.; Bart, J.; van Leeuwen, F.W.; van Dam, G.M. Selecting Potential Targetable Biomarkers for Imaging Purposes in Colorectal Cancer Using TArget Selection Criteria (TASC): A Novel Target Identification Tool. Transl. Oncol. 2011, 4, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammarström, S. The Carcinoembryonic Antigen (CEA) Family: Structures, Suggested Functions and Expression in Normal and Malignant Tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukarasu, P.; Sukumar, S.; Sathaiah, M.; Mahan, M.; Pragatheeshwar, K.D.; Pingpank, J.F.; Zeh, H.; Bartels, C.J.; Lee, K.K.W.; Bartlett, D.L. C-Stage in Colon Cancer: Implications of Carcinoembryonic Antigen Biomarker in Staging, Prognosis, and Management. JNCI J. Natl. Cancer Inst. 2011, 103, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moertel, C.G.; O’Fallon, J.R.; Go, V.L.W.; O’Connell, M.J.; Thynne, G.S. The Preoperative Carcinoembryonic Antigen Test in the Diagnosis, Staging, and Prognosis of Colorectal Cancer. Cancer 1986, 58, 603–610. [Google Scholar] [CrossRef]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef]
- Saito, S.; Yoshida, S.; Isayama, H.; Matsuzawa, T.; Kuwai, T.; Maetani, I.; Shimada, M.; Yamada, T.; Tomita, M.; Koizumi, K.; et al. A Prospective Multicenter Study on Self-Expandable Metallic Stents as a Bridge to Surgery for Malignant Colorectal Obstruction in Japan: Efficacy and Safety in 312 Patients. Surg. Endosc. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nazato, D.M.; de Matos, L.L.; Waisberg, D.R.; de Souza, J.R.M.; Martins, L.C.; Waisberg, J. Prognostic Value of Carcinoembryonic Antigen Distribution in Tumor Tissue of Colorectal Carcinoma. Arq. Gastroenterol. 2009, 46, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, J.; Tewari, H.B.; Bhatnagar, M.; Austin, G.E. Comparison of Carcinoembryonic Antigen in Tissue and Serum with Grade and Stage of Colon Cancer. Anticancer Res. 1999, 19, 2181–2187. [Google Scholar] [PubMed]
- Metildi, C.A.; Kaushal, S.; Luiken, G.A.; Talamini, M.A.; Hoffman, R.M.; Bouvet, M. Fluorescently Labeled Chimeric Anti-CEA Antibody Improves Detection and Resection of Human Colon Cancer in a Patient-Derived Orthotopic Xenograft (PDOX) Nude Mouse Model. J. Surg. Oncol. 2014, 109, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLong, J.C.; Murakami, T.; Yazaki, P.J.; Hoffman, R.M.; Bouvet, M. Near-Infrared-Conjugated Humanized Anti-Carcinoembryonic Antigen Antibody Targets Colon Cancer in an Orthotopic Nude-Mouse Model. J. Surg. Res. 2017, 218, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, M.; Framery, B.; Boonstra, M.C.; Garambois, V.; Quenet, F.; Dumas, K.; Scherninski, F.; Cailler, F.; Vahrmeijer, A.L.; Pèlegrin, A. SGM-101: An Innovative near-Infrared Dye-Antibody Conjugate That Targets CEA for Fluorescence-Guided Surgery. Surg. Oncol. 2017, 26, 153–162. [Google Scholar] [CrossRef]
- Sundaresan, G.; Yazaki, P.J.; Shively, J.E.; Finn, R.D.; Larson, S.M.; Raubitschek, A.A.; Williams, L.E.; Chatziioannou, A.F.; Gambhir, S.S.; Wu, A.M. 124I-Labeled Engineered Anti-CEA Minibodies and Diabodies Allow High-Contrast, Antigen-Specific Small-Animal PET Imaging of Xenografts in Athymic Mice. J. Nucl. Med. 2003, 44, 1962–1969. [Google Scholar] [PubMed]
- Boogerd, L.S.F.; Hoogstins, C.E.S.; Schaap, D.P.; Kusters, M.; Handgraaf, H.J.M.; van der Valk, M.J.M.; Hilling, D.E.; Holman, F.A.; Peeters, K.C.M.J.; Mieog, J.S.D.; et al. Safety and Effectiveness of SGM-101, a Fluorescent Antibody Targeting Carcinoembryonic Antigen, for Intraoperative Detection of Colorectal Cancer: A Dose-Escalation Pilot Study. Lancet Gastroenterol. Hepatol. 2018, 3, 181–191. [Google Scholar] [CrossRef]
- de Valk, K.S.; Deken, M.M.; Schaap, D.P.; Meijer, R.P.; Boogerd, L.S.; Hoogstins, C.E.; van der Valk, M.J.; Kamerling, I.M.; Bhairosingh, S.S.; Framery, B.; et al. Dose-Finding Study of a CEA-Targeting Agent, SGM-101, for Intraoperative Fluorescence Imaging of Colorectal Cancer. Ann. Surg. Oncol. 2021, 28, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Schaap, D.P.; de Valk, K.S.; Deken, M.M.; Meijer, R.P.J.; Burggraaf, J.; Vahrmeijer, A.L.; Kusters, M.; SGM-101 Study Group. Carcinoembryonic Antigen-Specific, Fluorescent Image-Guided Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Metastatic Colorectal Cancer. Br. J. Surg. 2020, 107, 334–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debie, P.; Devoogdt, N.; Hernot, S. Targeted Nanobody-Based Molecular Tracers for Nuclear Imaging and Image-Guided Surgery. Antibodies 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lwin, T.M.; Turner, M.A.; Amirfakhri, S.; Nishino, H.; Debie, P.; Cosman, B.C.; Hoffman, R.M.; Hernot, S.; Bouvet, M. Rapid Tumor-Labeling Kinetics with a Site-Specific near-Infrared Anti-CEA Nanobody in a Patient-Derived Orthotopic Xenograft Mouse Model of Colon Cancer. J. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Altınoğlu, E.İ.; Adair, J.H. Near Infrared Imaging with Nanoparticles. WIREs Nanomed. Nanobiotechnol. 2010, 2, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Tiernan, J.P.; Ingram, N.; Marston, G.; Perry, S.L.; Rushworth, J.V.; Coletta, P.L.; Millner, P.A.; Jayne, D.G.; Hughes, T.A. CEA-Targeted Nanoparticles Allow Specific in Vivo Fluorescent Imaging of Colorectal Cancer Models. Nanomedicine 2015, 10, 1223–1231. [Google Scholar] [CrossRef]
- Hollandsworth, H.M.; Amirfakhri, S.; Filemoni, F.; Schmitt, V.; Wennemuth, G.; Schmidt, A.; Hoffman, R.M.; Singer, B.B.; Bouvet, M. Anti-Carcinoembryonic Antigen-Related Cell Adhesion Molecule Antibody for Fluorescence Visualization of Primary Colon Cancer and Metastases in Patient-Derived Orthotopic Xenograft Mouse Models. Oncotarget 2020, 11, 429–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, H.; Hollandsworth, H.M.; Amirfakhri, S.; Tashiro, Y.; Yamamoto, J.; Turner, M.A.; Lwin, T.M.; Singer, B.B.; Hoffman, R.M.; Bouvet, M. A Novel Color-Coded Liver Metastasis Mouse Model to Distinguish Tumor and Adjacent Liver Segment. J. Surg. Res. 2021, 264, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.C.; Tolner, B.; Schaafsma, B.E.; Boogerd, L.S.F.; Prevoo, H.A.J.M.; Bhavsar, G.; Kuppen, P.J.K.; Sier, C.F.M.; Bonsing, B.A.; Frangioni, J.V.; et al. Preclinical Evaluation of a Novel CEA-Targeting near-Infrared Fluorescent Tracer Delineating Colorectal and Pancreatic Tumors. Int. J. Cancer 2015, 137, 1910–1920. [Google Scholar] [CrossRef] [Green Version]
- Lwin, T.M.; Murakami, T.; Miyake, K.; Yazaki, P.J.; Shivley, J.E.; Hoffman, R.M.; Bouvet, M. Tumor-Specific Labeling of Pancreatic Cancer Using a Humanized Anti-CEA Antibody Conjugated to a Near-Infrared Fluorophore. Ann. Surg. Oncol. 2018, 25, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Lwin, T.M.; Hernot, S.; Hollandsworth, H.; Amirfakhri, S.; Filemoni, F.; Debie, P.; Hoffman, R.M.; Bouvet, M. Tumor-Specific near-Infrared Nanobody Probe Rapidly Labels Tumors in an Orthotopic Mouse Model of Pancreatic Cancer. Surgery 2020, 168, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C. CXCR4: Chemokine Receptor Extraordinaire. Immunol. Rev. 2000, 177, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jin, M.; Xu, H.; Shimin, Z.; He, S.; Wang, L.; Zhang, Y. Clinicopathologic Significance of HIF-1α, CXCR4, and VEGF Expression in Colon Cancer. Clin. Dev. Immunol. 2010, 2010, 537531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkwill, F. The Significance of Cancer Cell Expression of the Chemokine Receptor CXCR4. Semin. Cancer Biol. 2004, 14, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Rupertus, K.; Sinistra, J.; Scheuer, C.; Nickels, R.M.; Schilling, M.K.; Menger, M.D.; Kollmar, O. Interaction of the Chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the Regulation of Tumor Angiogenesis of Colorectal Cancer. Clin. Exp. Metastasis 2014, 31, 447–459. [Google Scholar] [CrossRef]
- Rubie, C.; Kollmar, O.; Frick, V.O.; Wagner, M.; Brittner, B.; Gräber, S.; Schilling, M.K. Differential CXC Receptor Expression in Colorectal Carcinomas. Scand. J. Immunol. 2008, 68, 635–644. [Google Scholar] [CrossRef]
- Meincke, M.; Tiwari, S.; Hattermann, K.; Kalthoff, H.; Mentlein, R. Near-Infrared Molecular Imaging of Tumors via Chemokine Receptors CXCR4 and CXCR7. Clin. Exp. Metastasis 2011, 28, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishizawa, K.; Nishiyama, H.; Oishi, S.; Tanahara, N.; Kotani, H.; Mikami, Y.; Toda, Y.; Evans, B.J.; Peiper, S.C.; Saito, R.; et al. Fluorescent Imaging of High-Grade Bladder Cancer Using a Specific Antagonist for Chemokine Receptor CXCR4. Int. J. Cancer 2010, 127, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Kondo, K.; Yamaguchi, M.; Richmond, G.; Hutchison, M.; Wakeling, A.; Averbuch, S.; Wadsworth, P. Distribution and Function of EGFR in Human Tissue and the Effect of EGFR Tyrosine Kinase Inhibition. Anticancer Res. 2003, 23, 3639–3650. [Google Scholar] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon Cancer and the Epidermal Growth Factor Receptor: Current Treatment Paradigms, the Importance of Diet, and the Role of Chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, S.; Varese, M.; Sánchez-Navarro, M.; Giralt, E. A Third Shot at EGFR: New Opportunities in Cancer Therapy. Trends Pharmacol. Sci. 2019, 40, 941–955. [Google Scholar] [CrossRef]
- Yamaoka, T.; Ohba, M.; Ohmori, T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int. J. Mol. Sci. 2017, 18, 2420. [Google Scholar] [CrossRef] [Green Version]
- Sihver, W.; Pietzsch, J.; Krause, M.; Baumann, M.; Steinbach, J.; Pietzsch, H.-J. Radiolabeled Cetuximab Conjugates for EGFR Targeted Cancer Diagnostics and Therapy. Pharmaceuticals 2014, 7, 311–338. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Shen, B.; Sun, X. Analysis of Progress and Challenges of EGFR-Targeted Molecular Imaging in Cancer With a Focus on Affibody Molecules. Mol. Imaging 2019, 18, 1536012118823473. [Google Scholar] [CrossRef] [Green Version]
- Marston, J.C.; Kennedy, G.D.; Lapi, S.E.; Hartman, Y.E.; Richardson, M.T.; Modi, H.M.; Warram, J.M. Panitumumab-IRDye800CW for Fluorescence-Guided Surgical Resection of Colorectal Cancer. J. Surg. Res. 2019, 239, 44–51. [Google Scholar] [CrossRef]
- Heath, C.H.; Deep, N.L.; Beck, L.N.; Day, K.E.; Sweeny, L.; Zinn, K.R.; Huang, C.C.; Rosenthal, E.L. Use of Panitumumab-IRDye800 to Image Cutaneous Head and Neck Cancer in Mice. Otolaryngol. Head Neck Surg. 2013, 148, 982–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, C.H.; Deep, N.L.; Sweeny, L.; Zinn, K.R.; Rosenthal, E.L. Use of Panitumumab-IRDye800 to Image Microscopic Head and Neck Cancer in an Orthotopic Surgical Model. Ann. Surg. Oncol. 2012, 19, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Warram, J.M.; de Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.W.; Teraphongphom, N.; de Boer, E.; van den Berg, N.S.; Divi, V.; Kaplan, M.J.; Oberhelman, N.J.; Hong, S.S.; Capes, E.; Colevas, A.D.; et al. Safety of Panitumumab-IRDye800CW and Cetuximab-IRDye800CW for Fluorescence-Guided Surgical Navigation in Head and Neck Cancers. Theranostics 2018, 8, 2488–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; van den Berg, N.S.; Hasan, A.; Longacre, T.A.; Fisher, G.A.; Bonsing, B.A.; Vahrmeijer, A.L.; Gambhir, S.S.; et al. Detection of Visually Occult Metastatic Lymph Nodes Using Molecularly Targeted Fluorescent Imaging during Surgical Resection of Pancreatic Cancer. HPB 2019, 21, 883–890. [Google Scholar] [CrossRef]
- Tummers, W.S.; Miller, S.E.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.M.; Hong, S.; Kothapalli, S.-R.; Hasan, A.; Ertsey, R.; et al. Intraoperative Pancreatic Cancer Detection Using Tumor-Specific Multimodality Molecular Imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef]
- Lu, G.; van den Berg, N.S.; Martin, B.A.; Nishio, N.; Hart, Z.P.; van Keulen, S.; Fakurnejad, S.; Chirita, S.U.; Raymundo, R.C.; Yi, G.; et al. Tumour-Specific Fluorescence-Guided Surgery for Pancreatic Cancer Using Panitumumab-IRDye800CW: A Phase 1 Single-Centre, Open-Label, Single-Arm, Dose-Escalation Study. Lancet Gastroenterol. Hepatol. 2020, 5, 753–764. [Google Scholar] [CrossRef]
- Zhou, Q.; Vega Leonel, J.C.M.; Santoso, M.R.; Wilson, C.; van den Berg, N.S.; Chan, C.T.; Aryal, M.; Vogel, H.; Cayrol, R.; Mandella, M.J.; et al. Molecular Imaging of a Fluorescent Antibody against Epidermal Growth Factor Receptor Detects High-Grade Glioma. Sci. Rep. 2021, 11, 5710. [Google Scholar] [CrossRef]
- Zhou, Q.; van den Berg, N.S.; Rosenthal, E.L.; Iv, M.; Zhang, M.; Vega Leonel, J.C.M.; Walters, S.; Nishio, N.; Granucci, M.; Raymundo, R.; et al. EGFR-Targeted Intraoperative Fluorescence Imaging Detects High-Grade Glioma with Panitumumab-IRDye800 in a Phase 1 Clinical Trial. Theranostics 2021, 11, 7130–7143. [Google Scholar] [CrossRef]
- Bernhard, W.; El-Sayed, A.; Barreto, K.; Gonzalez, C.; Hill, W.; Parada, A.C.; Fonge, H.; Geyer, C.R. Near Infrared Fluorescence Imaging of EGFR Expression in Vivo Using IRDye800CW-Nimotuzumab. Oncotarget 2018, 9, 6213–6227. [Google Scholar] [CrossRef] [Green Version]
- Samkoe, K.S.; Gunn, J.R.; Marra, K.; Hull, S.M.; Moodie, K.L.; Feldwisch, J.; Strong, T.V.; Draney, D.R.; Hoopes, P.J.; Roberts, D.W.; et al. Toxicity and Pharmacokinetic Profile for Single-Dose Injection of ABY-029: A Fluorescent Anti-EGFR Synthetic Affibody Molecule for Human Use. Mol. Imaging Biol. 2017, 19, 512–521. [Google Scholar] [CrossRef]
- Oliveira, S.; van Dongen, G.A.M.S.; Stigter-van Walsum, M.; Roovers, R.C.; Stam, J.C.; Mali, W.; van Diest, P.J.; van Bergen en Henegouwen, P.M.P. Rapid Visualization of Human Tumor Xenografts through Optical Imaging with a Near-Infrared Fluorescent Anti-Epidermal Growth Factor Receptor Nanobody. Mol. Imaging 2012, 11, 33–46. [Google Scholar] [CrossRef]
- Sharifi, J.; Khirehgesh, M.R.; Safari, F.; Akbari, B. EGFR and Anti-EGFR Nanobodies: Review and Update. J. Drug Target. 2021, 29, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM Expression in Primary Tumour Tissues and Metastases: An Immunohistochemical Analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, M.J.; Nagtegaal, I.D.; van Krieken, J.H.J.M.; Litvinov, S.V. The Epithelial Cell Adhesion Molecule (Ep-CAM) as a Morphoregulatory Molecule Is a Tool in Surgical Pathology. Am. J. Pathol. 2003, 163, 2139–2148. [Google Scholar] [CrossRef] [Green Version]
- Gires, O.; Pan, M.; Schinke, H.; Canis, M.; Baeuerle, P.A. Expression and Function of Epithelial Cell Adhesion Molecule EpCAM: Where Are We after 40 Years? Cancer Metastasis Rev. 2020, 39, 969–987. [Google Scholar] [CrossRef]
- van Driel, P.B.A.A.; Boonstra, M.C.; Prevoo, H.A.J.M.; van de Giessen, M.; Snoeks, T.J.A.; Tummers, Q.R.J.G.; Keereweer, S.; Cordfunke, R.A.; Fish, A.; van Eendenburg, J.D.H.; et al. EpCAM as Multi-Tumour Target for near-Infrared Fluorescence Guided Surgery. BMC Cancer 2016, 16, 884. [Google Scholar] [CrossRef] [Green Version]
- Boogerd, L.S.F.; Boonstra, M.C.; Prevoo, H.A.J.M.; Handgraaf, H.J.M.; Kuppen, P.J.K.; van de Velde, C.J.H.; Fish, A.; Cordfunke, R.A.; Valentijn, A.R.P.M.; Terwisscha van Scheltinga, A.G.; et al. Fluorescence-Guided Tumor Detection with a Novel Anti-EpCAM Targeted Antibody Fragment: Preclinical Validation. Surg. Oncol. 2019, 28, 1–8. [Google Scholar] [CrossRef]
- Mysliwiec, A.G.; Ornstein, D.L. Matrix Metalloproteinases in Colorectal Cancer. Clin. Colorectal Cancer 2002, 1, 208–219. [Google Scholar] [CrossRef]
- Zucker, S.; Vacirca, J. Role of Matrix Metalloproteinases (MMPs) in Colorectal Cancer. Cancer Metastasis Rev. 2004, 23, 101–117. [Google Scholar] [CrossRef]
- McKerrow, J.H.; Bhargava, V.; Hansell, E.; Huling, S.; Kuwahara, T.; Matley, M.; Coussens, L.; Warren, R. A Functional Proteomics Screen of Proteases In Colorectal Carcinoma. Mol. Med. 2000, 6, 450–460. [Google Scholar] [CrossRef]
- Jiang, T.; Olson, E.S.; Nguyen, Q.T.; Roy, M.; Jennings, P.A.; Tsien, R.Y. Tumor Imaging by Means of Proteolytic Activation of Cell-Penetrating Peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 17867–17872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissleder, R.; Tung, C.-H.; Mahmood, U.; Bogdanov, A. In Vivo Imaging of Tumors with Protease-Activated near-Infrared Fluorescent Probes. Nat. Biotechnol. 1999, 17, 375–378. [Google Scholar] [CrossRef]
- Clapper, M.L.; Hensley, H.H.; Chang, W.-C.L.; Devarajan, K.; Nguyen, M.T.; Cooper, H.S. Detection of Colorectal Adenomas Using a Bioactivatable Probe Specific for Matrix Metalloproteinase Activity. Neoplasia 2011, 13, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, Q.T.; Olson, E.S.; Aguilera, T.A.; Jiang, T.; Scadeng, M.; Ellies, L.G.; Tsien, R.Y. Surgery with Molecular Fluorescence Imaging Using Activatable Cell-Penetrating Peptides Decreases Residual Cancer and Improves Survival. Proc. Natl. Acad. Sci. USA 2010, 107, 4317–4322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miampamba, M.; Liu, J.; Harootunian, A.; Gale, A.J.; Baird, S.; Chen, S.L.; Nguyen, Q.T.; Tsien, R.Y.; González, J.E. Sensitive in Vivo Visualization of Breast Cancer Using Ratiometric Protease-Activatable Fluorescent Imaging Agent, AVB-620. Theranostics 2017, 7, 3369–3386. [Google Scholar] [CrossRef]
- Unkart, J.T.; Chen, S.L.; Wapnir, I.L.; González, J.E.; Harootunian, A.; Wallace, A.M. Intraoperative Tumor Detection Using a Ratiometric Activatable Fluorescent Peptide: A First-in-Human Phase 1 Study. Ann. Surg. Oncol. 2017, 24, 3167–3173. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, J.; Luo, S.; Dong, J.; Hu, H.; Yang, Z.; Feng, X.; Liu, Y.; Liu, B.; Pan, G.; et al. Targeting and Imaging Colorectal Cancer by Activatable Cell-Penetrating Peptides. Am. J. Transl. Res. 2020, 12, 1754–1766. [Google Scholar] [PubMed]
- Veiseh, M.; Gabikian, P.; Bahrami, S.-B.; Veiseh, O.; Zhang, M.; Hackman, R.C.; Ravanpay, A.C.; Stroud, M.R.; Kusuma, Y.; Hansen, S.J.; et al. Tumor Paint: A Chlorotoxin:Cy5.5 Bioconjugate for Intraoperative Visualization of Cancer Foci. Cancer Res. 2007, 67, 6882–6888. [Google Scholar] [CrossRef] [Green Version]
- Baik, F.M.; Hansen, S.; Knoblaugh, S.E.; Sahetya, D.; Mitchell, R.M.; Xu, C.; Olson, J.M.; Parrish-Novak, J.; Méndez, E. Fluorescence Identification of Head and Neck Squamous Cell Carcinoma and High-Risk Oral Dysplasia With BLZ-100, a Chlorotoxin-Indocyanine Green Conjugate. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults With Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E649. [Google Scholar] [CrossRef]
- Yamada, M.; Miller, D.M.; Lowe, M.; Rowe, C.; Wood, D.; Soyer, H.P.; Byrnes-Blake, K.; Parrish-Novak, J.; Ishak, L.; Olson, J.M.; et al. A First-in-Human Study of BLZ-100 (Tozuleristide) Demonstrates Tolerability and Safety in Skin Cancer Patients. Contemp. Clin. Trials Commun. 2021, 23, 100830. [Google Scholar] [CrossRef]
- Dintzis, S.M.; Hansen, S.; Harrington, K.M.; Tan, L.C.; Miller, D.M.; Ishak, L.; Parrish-Novak, J.; Kittle, D.; Perry, J.; Gombotz, C.; et al. Real-Time Visualization of Breast Carcinoma in Pathology Specimens From Patients Receiving Fluorescent Tumor-Marking Agent Tozuleristide. Arch. Pathol. Lab. Med. 2019, 143, 1076–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, S.; Mukherjee, P. MUC1: A Multifaceted Oncoprotein with a Key Role in Cancer Progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz Del Arco, C.; Garré, P.; Molina Roldán, E.; Lorca, V.; Cerón Nieto, M.Á.; Fernández Aceñero, M.J. MUC1 Expression in Colorectal Carcinoma: Clinicopathological Correlation and Prognostic Significance. Rev. Esp. Patol. 2018, 51, 204–209. [Google Scholar] [CrossRef]
- Suzuki, H.; Shoda, J.; Kawamoto, T.; Shinozaki, E.; Miyahara, N.; Hotta, S.; Iizuka, Y.; Nakahara, A.; Tanaka, N.; Yanaka, A.; et al. Expression of MUC1 Recognized by Monoclonal Antibody MY.1E12 Is a Useful Biomarker for Tumor Aggressiveness of Advanced Colon Carcinoma. Clin. Exp. Metastasis 2004, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Pham, W.; Medarova, Z.; Moore, A. Synthesis and Application of a Water-Soluble Near-Infrared Dye for Cancer Detection Using Optical Imaging. Bioconjug. Chem. 2005, 16, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Hiroshima, Y.; Lee, J.Y.; Maawy, A.A.; Hoffman, R.M.; Bouvet, M. MUC1 Selectively Targets Human Pancreatic Cancer in Orthotopic Nude Mouse Models. PLoS ONE 2015, 10, e0122100. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, J.; Zhang, M.; Yang, H.; Ma, Y.; Gu, Y. MUC1 Aptamer-Based Near-Infrared Fluorescence Probes for Tumor Imaging. Mol. Imaging Biol. 2015, 17, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, Y.; Cui, S.; Cao, J.; Achilefu, S.; Gu, Y. MUC1 Aptamer Based near Infrared Fluorescence Probes for Tumor Diagnosis. In Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications V; International Society for Optics and Photonics: Bellingham, WA, USA, 2013; Volume 8596, p. 859614. [Google Scholar]
- Ferrara, N. Role of Vascular Endothelial Growth Factor in Physiologic and Pathologic Angiogenesis: Therapeutic Implications. Semin. Oncol. 2002, 29, 10–14. [Google Scholar] [CrossRef] [PubMed]
- George, M.L.; Tutton, M.G.; Janssen, F.; Arnaout, A.; Abulafi, A.M.; Eccles, S.A.; Swift, R.I. VEGF-A, VEGF-C, and VEGF-D in Colorectal Cancer Progression. Neoplasia 2001, 3, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Nieves, B.J.; D’Amore, P.A.; Bryan, B.A. The Function of Vascular Endothelial Growth Factor. Biofactors 2009, 35, 332–337. [Google Scholar] [CrossRef]
- Bendardaf, R.; Buhmeida, A.; Hilska, M.; Laato, M.; Syrjänen, S.; Syrjänen, K.; Collan, Y.; Pyrhönen, S. VEGF-1 Expression in Colorectal Cancer Is Associated with Disease Localization, Stage, and Long-Term Disease-Specific Survival. Anticancer Res. 2008, 28, 3865–3870. [Google Scholar]
- Guba, M.; Seeliger, H.; Kleespies, A.; Jauch, K.-W.; Bruns, C. Vascular Endothelial Growth Factor in Colorectal Cancer. Int. J. Colorectal Dis. 2004, 19, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Terwisscha van Scheltinga, A.G.T.; van Dam, G.M.; Nagengast, W.B.; Ntziachristos, V.; Hollema, H.; Herek, J.L.; Schröder, C.P.; Kosterink, J.G.W.; Lub-de Hoog, M.N.; de Vries, E.G.E. Intraoperative Near-Infrared Fluorescence Tumor Imaging with Vascular Endothelial Growth Factor and Human Epidermal Growth Factor Receptor 2 Targeting Antibodies. J. Nucl. Med. 2011, 52, 1778–1785. [Google Scholar] [CrossRef] [Green Version]
- Harlaar, N.J.; Koller, M.; de Jongh, S.J.; van Leeuwen, B.L.; Hemmer, P.H.; Kruijff, S.; van Ginkel, R.J.; Been, L.B.; de Jong, J.S.; Kats-Ugurlu, G.; et al. Molecular Fluorescence-Guided Surgery of Peritoneal Carcinomatosis of Colorectal Origin: A Single-Centre Feasibility Study. Lancet Gastroenterol. Hepatol. 2016, 1, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Lamberts, L.E.; Koch, M.; de Jong, J.S.; Adams, A.L.L.; Glatz, J.; Kranendonk, M.E.G.; Terwisscha van Scheltinga, A.G.T.; Jansen, L.; de Vries, J.; Lub-de Hooge, M.N.; et al. Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin. Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jongh, S.J.; Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Vonk, J.; Dobosz, M.; Jorritsma-Smit, A.; Kleibeuker, J.H.; Hospers, G.A.P.; Havenga, K.; et al. Back-Table Fluorescence-Guided Imaging for Circumferential Resection Margin Evaluation Using Bevacizumab-800CW in Patients with Locally Advanced Rectal Cancer. J. Nucl. Med. 2020, 61, 655–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjalma, J.J.; Garcia-Allende, P.B.; Hartmans, E.; Terwisscha van Scheltinga, A.G.; Boersma-van Ek, W.; Glatz, J.; Koch, M.; van Herwaarden, Y.J.; Bisseling, T.M.; Nagtegaal, I.D.; et al. Molecular Fluorescence Endoscopy Targeting Vascular Endothelial Growth Factor A for Improved Colorectal Polyp Detection. J. Nucl. Med. 2016, 57, 480–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjalma, J.J.J.; Koller, M.; Linssen, M.D.; Hartmans, E.; de Jongh, S.J.; Jorritsma-Smit, A.; Karrenbeld, A.; de Vries, E.G.; Kleibeuker, J.H.; Pennings, J.P.; et al. Quantitative Fluorescence Endoscopy: An Innovative Endoscopy Approach to Evaluate Neoadjuvant Treatment Response in Locally Advanced Rectal Cancer. Gut 2020, 69, 406–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuester, D.; Lippert, H.; Roessner, A.; Krueger, S. The Cathepsin Family and Their Role in Colorectal Cancer. Pathol.-Res. Pract. 2008, 204, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Adenis, A.; Huet, G.; Zerimech, F.; Hecquet, B.; Balduyck, M.; Peyrat, J.P. Cathepsin B, L, and D Activities in Colorectal Carcinomas: Relationship with Clinico-Pathological Parameters. Cancer Lett. 1995, 96, 267–275. [Google Scholar] [CrossRef]
- Arao, J.; Fukui, H.; Ono, Y.; Ueda, Y.; Chiba, T.; Fujimori, T. Immunohistochemical Localization of Cathepsin D in Colorectal Tumors. Dis. Colon Rectum 2000, 43, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Baba, Y.; Shima, K.; Nosho, K.; Chung, D.C.; Hung, K.E.; Mahmood, U.; Madden, K.; Poss, K.; Ranieri, A.; et al. Cathepsin B Expression and Survival in Colon Cancer: Implications for Molecular Detection of Neoplasia. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2777–2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herszényi, L.; Farinati, F.; Cardin, R.; István, G.; Molnár, L.D.; Hritz, I.; De Paoli, M.; Plebani, M.; Tulassay, Z. Tumor Marker Utility and Prognostic Relevance of Cathepsin B, Cathepsin L, Urokinase-Type Plasminogen Activator, Plasminogen Activator Inhibitor Type-1, CEA and CA 19-9 in Colorectal Cancer. BMC Cancer 2008, 8, 194. [Google Scholar] [CrossRef]
- Esfahani, S.A.; Heidari, P.; Kucherlapati, M.H.; Ferrer, J.M.; Kucherlapati, R.S.; Mahmood, U. Optical Imaging with a Novel Cathepsin-Activatable Probe for Enhanced Detection of Colorectal Cancer. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 230–242. [Google Scholar] [PubMed]
- Whitley, M.J.; Cardona, D.M.; Lazarides, A.L.; Spasojevic, I.; Ferrer, J.M.; Cahill, J.; Lee, C.-L.; Snuderl, M.; Blazer, D.G.; Hwang, E.S.; et al. A Mouse-Human Phase 1 Co-Clinical Trial of a Protease-Activated Fluorescent Probe for Imaging Cancer. Sci. Transl. Med. 2016, 8, 320ra4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanahan, C.R.; Kelly, B.N.; Gadd, M.A.; Specht, M.C.; Brown, C.L.; Hughes, K.S.; Tang, R.; Rai, U.; Brachtel, E.F.; Rice-Stitt, T.; et al. Performance of a Novel Protease-Activated Fluorescent Imaging System for Intraoperative Detection of Residual Breast Cancer during Breast Conserving Surgery. Breast Cancer Res. Treat. 2021, 187, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.L.; Lanahan, C.R.; Specht, M.C.; Kelly, B.N.; Brown, C.; Strasfeld, D.B.; Ferrer, J.M.; Rai, U.; Tang, R.; Rice-Stitt, T.; et al. Feasibility Study of a Novel Protease-Activated Fluorescent Imaging System for Real-Time, Intraoperative Detection of Residual Breast Cancer in Breast Conserving Surgery. Ann. Surg. Oncol. 2020, 27, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Prestwood, T.R.; van der Linden, W.A.; Carmi, Y.; Bhattacharya, N.; Withana, N.; Verdoes, M.; Habtezion, A.; Engleman, E.G.; Bogyo, M. Detection of Intestinal Cancer by Local, Topical Application of a Quenched Fluorescence Probe for Cysteine Cathepsins. Chem. Biol. 2015, 22, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Kim, K.-M.; Kim, H.C.; Lee, W.Y.; Kang, W.K.; Park, Y.S.; Ha, S.Y. The Prognostic Role of Tumor Associated Glycoprotein 72 (TAG-72) in Stage II and III Colorectal Adenocarcinoma. Pathol. Res. Pract. 2019, 215, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, F.; Roselli, M.; Cosimelli, M.; Ferroni, P.; Spila, A.; Cavaliere, F.; Arcuri, R.; Carlini, S.; Mariotti, S.; Gandolfo, G.M.; et al. TAG-72 Expression and Its Role in the Biological Evaluation of Human Colorectal Cancer. Anticancer Res. 1996, 16, 2141–2148. [Google Scholar]
- Hollandsworth, H.M.; Amirfakhri, S.; Filemoni, F.; Hoffman, R.M.; Molnar, J.; Yazaki, P.J.; Bouvet, M. Humanized Anti-Tumor-Associated Glycoprotein-72 for Submillimeter Near-Infrared Detection of Colon Cancer in Metastatic Mouse Models. J. Surg. Res. 2020, 252, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Ding, H.; Long, N.E.; Sullivan, B.J.; Martin, E.W.; Magliery, T.J.; Tweedle, M.F. A 3E8.ScFv.Cys-IR800 Conjugate Targeting TAG-72 in an Orthotopic Colorectal Cancer Model. Mol. Imaging Biol. 2018, 20, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Malleo, G.; Maggino, L.; Capelli, P.; Gulino, F.; Segattini, S.; Scarpa, A.; Bassi, C.; Butturini, G.; Salvia, R. Reappraisal of Nodal Staging and Study of Lymph Node Station Involvement in Pancreaticoduodenectomy with the Standard International Study Group of Pancreatic Surgery Definition of Lymphadenectomy for Cancer. J. Am. Coll. Surg. 2015, 221, 367–379.e4. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Low, P.S. Folate-Targeted Therapies for Cancer. J. Med. Chem. 2010, 53, 6811–6824. [Google Scholar] [CrossRef] [PubMed]
- Shia, J.; Klimstra, D.S.; Nitzkorski, J.R.; Low, P.S.; Gonen, M.; Landmann, R.; Weiser, M.R.; Franklin, W.A.; Prendergast, F.G.; Murphy, L.; et al. Immunohistochemical Expression of Folate Receptor Alpha in Colorectal Carcinoma: Patterns and Biological Significance. Hum. Pathol. 2008, 39, 498–505. [Google Scholar] [CrossRef] [PubMed]
- D’Angelica, M.; Ammori, J.; Gonen, M.; Klimstra, D.S.; Low, P.S.; Murphy, L.; Weiser, M.R.; Paty, P.B.; Fong, Y.; DeMatteo, R.P.; et al. Folate Receptor-α Expression in Resectable Hepatic Colorectal Cancer Metastases: Patterns and Significance. Mod. Pathol. 2011, 24, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and Development of Folic-Acid-Based Receptor Targeting for Imaging and Therapy of Cancer and Inflammatory Diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef] [PubMed]
- van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative Tumor-Specific Fluorescence Imaging in Ovarian Cancer by Folate Receptor-α Targeting: First in-Human Results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Ham, N.S.; Myung, S.-J. Endoscopic Molecular Imaging in Inflammatory Bowel Disease. Intest. Res. 2021, 19, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogue, B.W. Perspective Review of What Is Needed for Molecular-Specific Fluorescence-Guided Surgery. J. Biomed. Opt. 2018, 23, 100601. [Google Scholar] [CrossRef] [Green Version]
- Lwin, T.M.; Hoffman, R.M.; Bouvet, M. Advantages of Patient-Derived Orthotopic Mouse Models and Genetic Reporters for Developing Fluorescence-Guided Surgery. J. Surg. Oncol. 2018, 118, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.D. The Cost of Developing Imaging Agents for Routine Clinical Use. Investig. Radiol. 2006, 41, 206–212. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lwin, T.M.; Turner, M.A.; Amirfakhri, S.; Nishino, H.; Hoffman, R.M.; Bouvet, M. Fluorescence Molecular Targeting of Colon Cancer to Visualize the Invisible. Cells 2022, 11, 249. https://doi.org/10.3390/cells11020249
Lwin TM, Turner MA, Amirfakhri S, Nishino H, Hoffman RM, Bouvet M. Fluorescence Molecular Targeting of Colon Cancer to Visualize the Invisible. Cells. 2022; 11(2):249. https://doi.org/10.3390/cells11020249
Chicago/Turabian StyleLwin, Thinzar M., Michael A. Turner, Siamak Amirfakhri, Hiroto Nishino, Robert M. Hoffman, and Michael Bouvet. 2022. "Fluorescence Molecular Targeting of Colon Cancer to Visualize the Invisible" Cells 11, no. 2: 249. https://doi.org/10.3390/cells11020249
APA StyleLwin, T. M., Turner, M. A., Amirfakhri, S., Nishino, H., Hoffman, R. M., & Bouvet, M. (2022). Fluorescence Molecular Targeting of Colon Cancer to Visualize the Invisible. Cells, 11(2), 249. https://doi.org/10.3390/cells11020249






