High-Dose Mycobacterium tuberculosis H37rv Infection in IL-17A- and IL-17A/F-Deficient Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Bacteria and Infection
2.3. In Vivo Depletion of Neutrophils
2.4. Colony Enumeration Assay
2.5. Histology
2.6. Preparation of Single-Cell Suspensions from Infected Lungs
2.7. Flow Cytometry
2.8. Quantitative Real-Time PCR
2.9. Statistics
3. Results
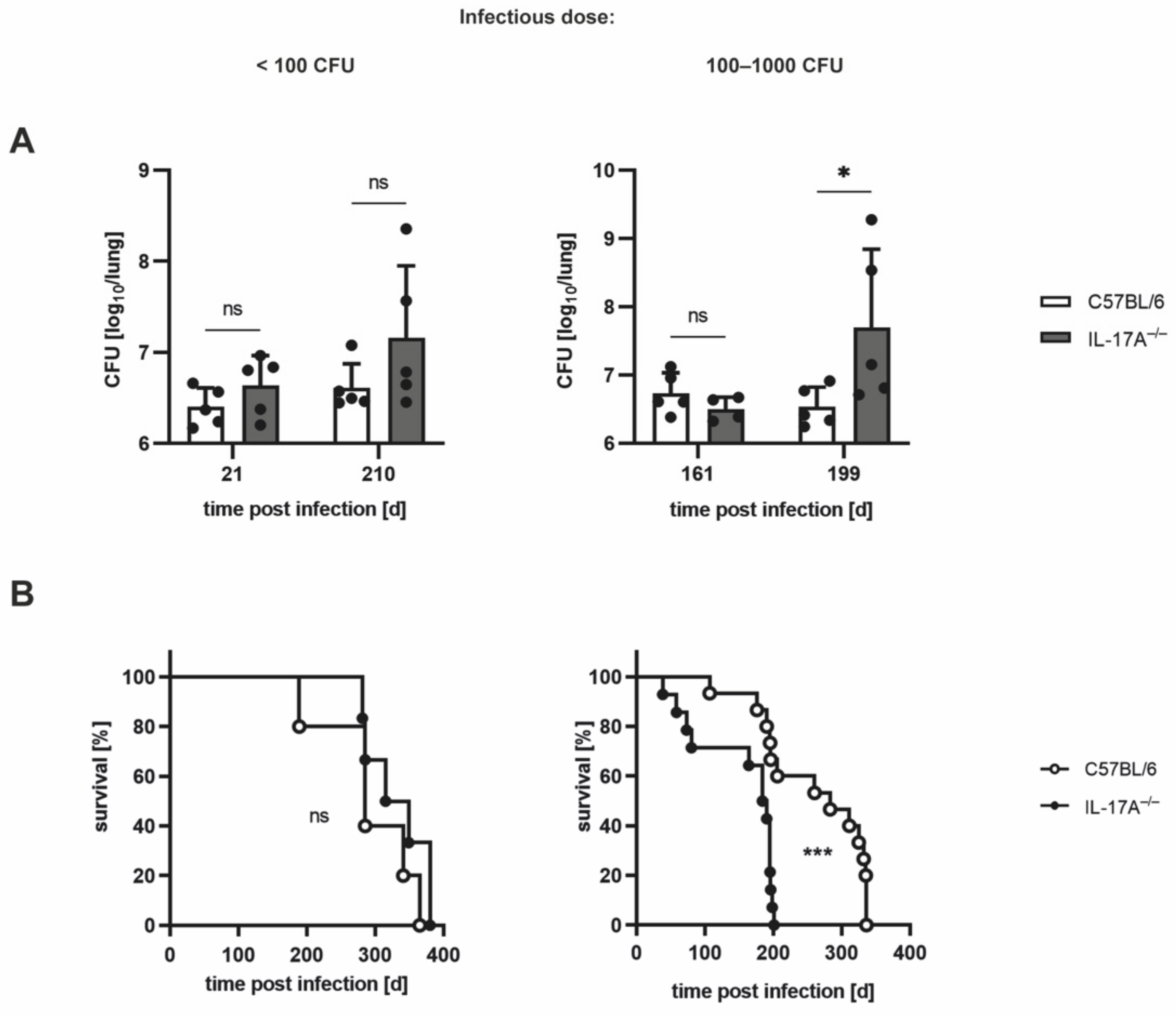
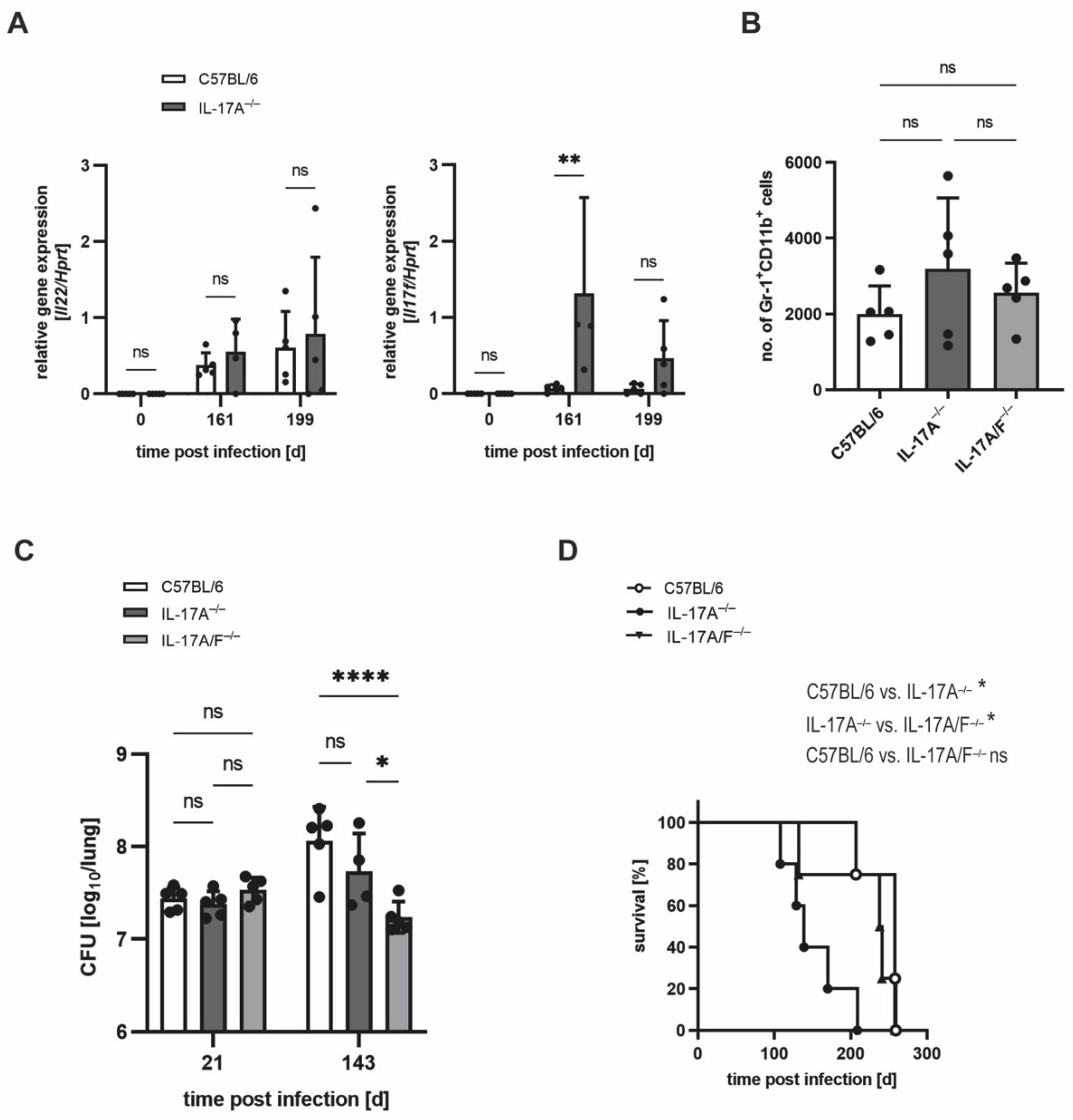
3.1. The Requirement of IL-17A for Controlling Bacterial Growth Depends on the Dose of Infection with Mtb H37rv
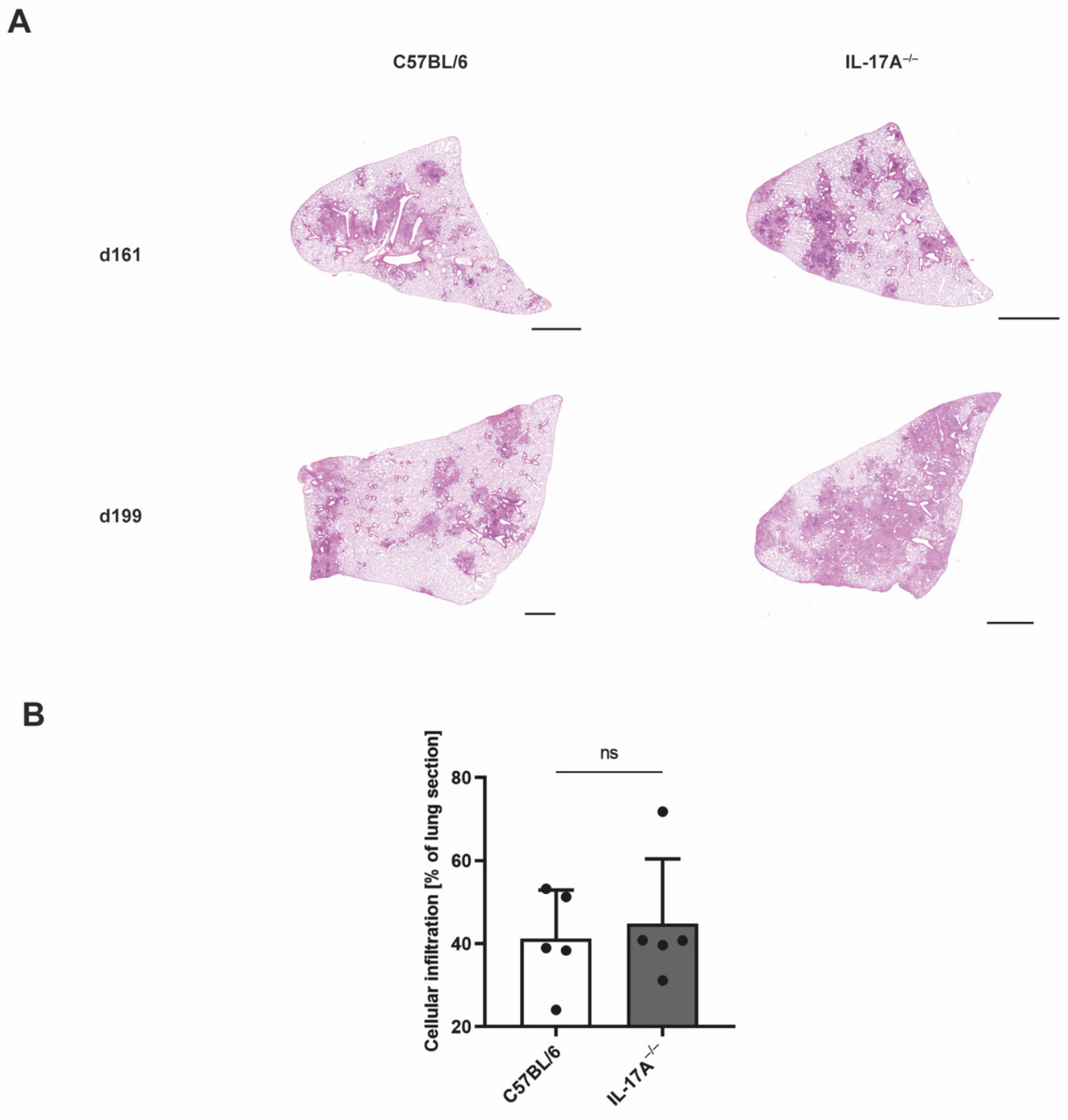
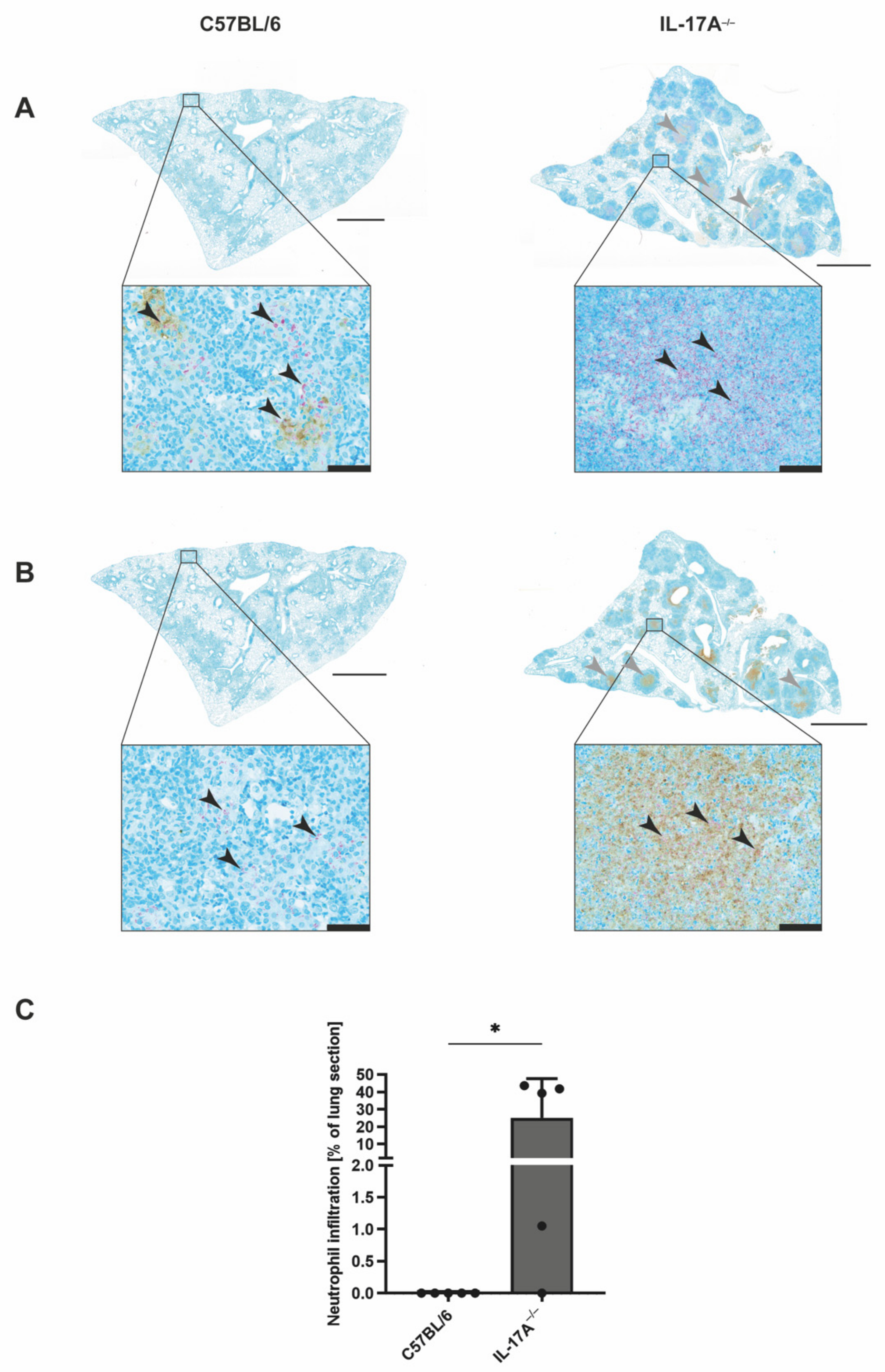
3.2. Mtb-Containing Lesions in IL-17A−/− Mice after Infection with an Elevated Dose of Mtb Are Partly Associated with Enhanced Numbers of Neutrophils
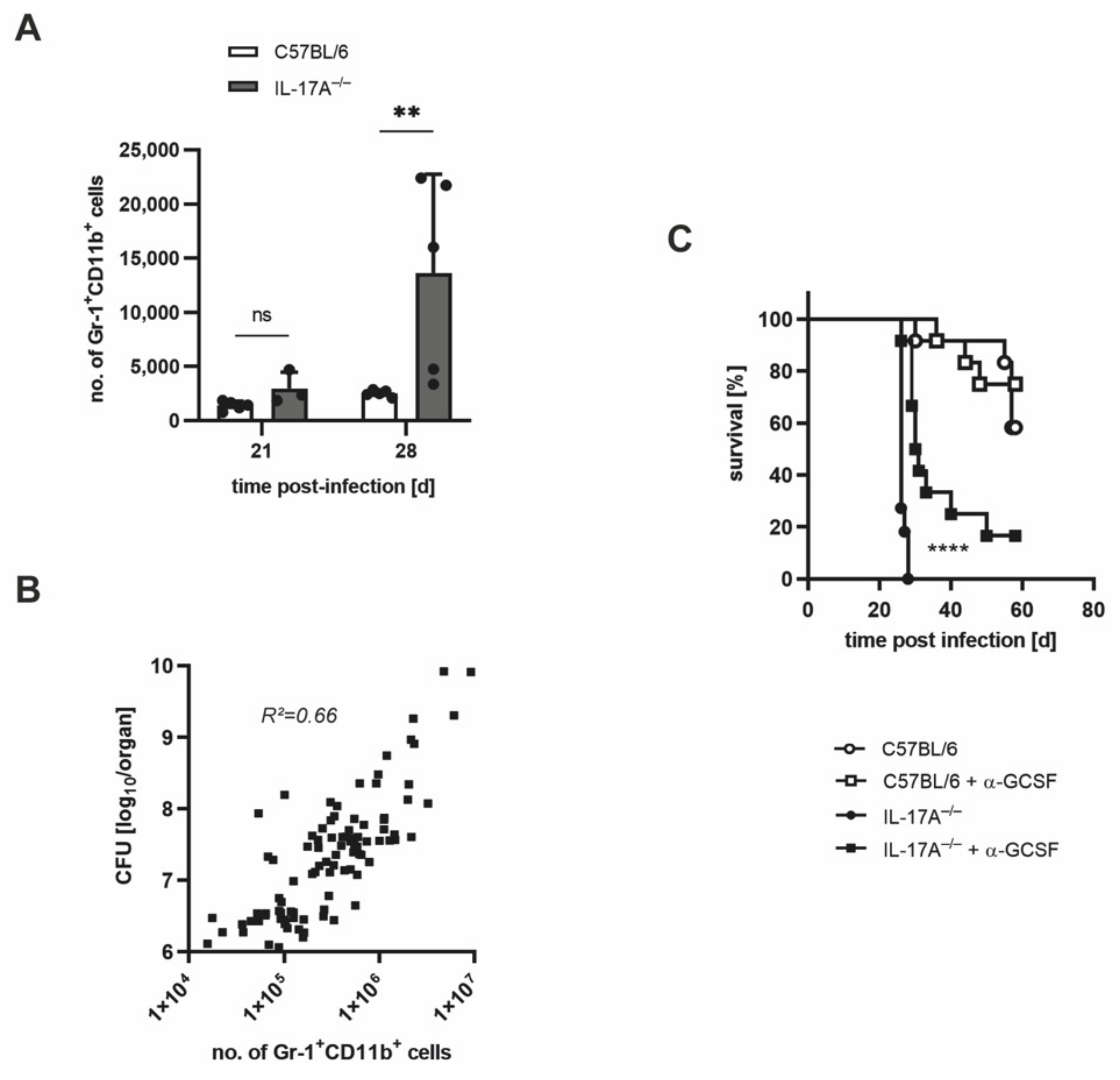
3.3. The Increased Infiltration of Neutrophils Is Involved in the Strongly Enhanced Susceptibility of IL-17A−/− Mice after Infection with a High Dose of Mtb H37rv
3.4. In IL-17A−/− Mice, IL-17F Mediates Susceptibility to Elevated Infectious Doses of Mtb H37rv
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Mayer-Barber, K.D.; Barber, D.L. Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection. Cold Spring Harb. Perspect. Med. 2015, 5, a018424. [Google Scholar] [CrossRef]
- Cooper, A.M.; Dalton, D.K.; Stewart, T.A.; Griffin, J.P.; Russell, D.G.; Orme, I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993, 178, 2243–2247. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J.; Schreiber, R.; Mak, T.W.; Bloom, B.R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef]
- Korbel, D.S.; Schneider, B.E.; Schaible, U.E. Innate immunity in tuberculosis: Myths and truth. Microbes Infect. 2008, 10, 995–1004. [Google Scholar] [CrossRef]
- Erdmann, H.; Behrends, J.; Ritter, K.; Hölscher, A.; Volz, J.; Rosenkrands, I.; Hölscher, C. The increased protection and pathology in Mycobacterium tuberculosis-infected IL-27R-alpha-deficient mice is supported by IL-17A and is associated with the IL-17A-induced expansion of multifunctional T cells. Mucosal. Immunol. 2018, 11, 1168–1180. [Google Scholar] [CrossRef]
- Gopal, R.; Monin, L.; Slight, S.; Uche, U.; Blanchard, E.; Fallert Junecko, B.A.; Ramos-Payan, R.; Stallings, C.L.; Reinhart, T.A.; Kolls, J.K.; et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014, 10, e1004099. [Google Scholar] [CrossRef]
- Khader, S.A.; Bell, G.K.; Pearl, J.E.; Fountain, J.J.; Rangel-Moreno, J.; Cilley, G.E.; Shen, F.; Eaton, S.M.; Gaffen, S.L.; Swain, S.L.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007, 8, 369–377. [Google Scholar] [CrossRef]
- Khader, S.A.; Pearl, J.E.; Sakamoto, K.; Gilmartin, L.; Bell, G.K.; Jelley-Gibbs, D.M.; Ghilardi, N.; de Sauvage, F.; Cooper, A.M. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 2005, 175, 788–795. [Google Scholar] [CrossRef]
- Hölscher, C.; Atkinson, R.A.; Arendse, B.; Brown, N.; Myburgh, E.; Alber, G.; Brombacher, F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J. Immunol. 2001, 167, 6957–6966. [Google Scholar] [CrossRef]
- Ritter, K.; Sodenkamp, J.C.; Hölscher, A.; Behrends, J.; Hölscher, C. IL-6 is not Absolutely Essential for the Development of a TH17 Immune Response after an Aerosol Infection with Mycobacterium Tuberculosis H37rv. Cells 2020, 10. [Google Scholar] [CrossRef]
- Jin, W.; Dong, C. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2013, 2, e60. [Google Scholar] [CrossRef]
- Scriba, T.J.; Kalsdorf, B.; Abrahams, D.A.; Isaacs, F.; Hofmeister, J.; Black, G.; Hassan, H.Y.; Wilkinson, R.J.; Walzl, G.; Gelderbloem, S.J.; et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 2008, 180, 1962–1970. [Google Scholar] [CrossRef]
- Okamoto Yoshida, Y.; Umemura, M.; Yahagi, A.; O‘Brien, R.L.; Ikuta, K.; Kishihara, K.; Hara, H.; Nakae, S.; Iwakura, Y.; Matsuzaki, G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 2010, 184, 4414–4422. [Google Scholar] [CrossRef]
- Umemura, M.; Yahagi, A.; Hamada, S.; Begum, M.D.; Watanabe, H.; Kawakami, K.; Suda, T.; Sudo, K.; Nakae, S.; Iwakura, Y.; et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007, 178, 3786–3796. [Google Scholar] [CrossRef]
- Ritter, K.; Behrends, J.; Erdmann, H.; Rousseau, J.; Hölscher, A.; Volz, J.; Prinz, I.; Lindenstrøm, T.; Hölscher, C. Interleukin-23 instructs protective multifunctional CD4 T cell responses after immunization with the Mycobacterium tuberculosis subunit vaccine H1 DDA/TDB independently of interleukin-17A. J. Mol. Med. 2021, 99, 1585–1602. [Google Scholar] [CrossRef]
- Ritter, K.; Rousseau, J.; Hölscher, C. Interleukin-27 in Tuberculosis: A Sheep in Wolf’s Clothing? Front. Immunol. 2021, 12, 810602. [Google Scholar] [CrossRef]
- Nakae, S.; Komiyama, Y.; Nambu, A.; Sudo, K.; Iwase, M.; Homma, I.; Sekikawa, K.; Asano, M.; Iwakura, Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002, 17, 375–387. [Google Scholar] [CrossRef]
- Haas, J.D.; Ravens, S.; Düber, S.; Sandrock, I.; Oberdörfer, L.; Kashani, E.; Chennupati, V.; Föhse, L.; Naumann, R.; Weiss, S.; et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity 2012, 37, 48–59. [Google Scholar] [CrossRef]
- Keller, C.; Hoffmann, R.; Lang, R.; Brandau, S.; Hermann, C.; Ehlers, S. Genetically determined susceptibility to tuberculosis in mice causally involves accelerated and enhanced recruitment of granulocytes. Infect. Immun. 2006, 74, 4295–4309. [Google Scholar] [CrossRef]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef]
- D’Souza, C.D.; Cooper, A.M.; Frank, A.A.; Ehlers, S.; Turner, J.; Bendelac, A.; Orme, I.M. A novel nonclassic beta2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am. J. Respir. Cell Mol. Biol. 2000, 23, 188–193. [Google Scholar] [CrossRef]
- Behrends, J.; Renauld, J.-C.; Ehlers, S.; Hölscher, C. IL-22 Is Mainly Produced by IFNγ-Secreting Cells but Is Dispensable for Host Protection against Mycobacterium tuberculosis Infection. PLoS ONE 2013, 8, e57379. [Google Scholar] [CrossRef]
- Dubin, P.J.; Kolls, J.K. Interleukin-17A and interleukin-17F: A tale of two cytokines. Immunity 2009, 30, 9–11. [Google Scholar] [CrossRef]
- Cho, J.S.; Pietras, E.M.; Garcia, N.C.; Ramos, R.I.; Farzam, D.M.; Monroe, H.R.; Magorien, J.E.; Blauvelt, A.; Kolls, J.K.; Cheung, A.L.; et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Investig. 2010, 120, 1762–1773. [Google Scholar] [CrossRef]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001, 194, 519–527. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Wang, H.; Chen, J.; Zheng, M.J.; Chen, X.G.; Zhang, L.; Liang, C.Z.; Zhan, C.S. IL-17 exacerbates experimental autoimmune prostatitis via CXCL1/CXCL2-mediated neutrophil infiltration. Andrologia 2022. [Google Scholar] [CrossRef]
- Laan, M.; Cui, Z.H.; Hoshino, H.; Lötvall, J.; Sjöstrand, M.; Gruenert, D.C.; Skoogh, B.E.; Lindén, A. Neutrophil recruitment by human IL-17 via C-X-C ch.h.hemokine release in the airways. J. Immunol. 1999, 162, 2347–2352. [Google Scholar]
- Brown, A.E.; Holzer, T.J.; Andersen, B.R. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J. Infect. Dis. 1987, 156, 985–989. [Google Scholar] [CrossRef]
- Jones, G.S.; Amirault, H.J.; Andersen, B.R. Killing of Mycobacterium tuberculosis by neutrophils: A nonoxidative process. J. Infect. Dis. 1990, 162, 700–704. [Google Scholar] [CrossRef]
- Lyadova, I.V. Neutrophils in Tuberculosis: Heterogeneity Shapes the Way? Mediat. Inflamm. 2017, 2017, 8619307. [Google Scholar] [CrossRef]
- Corleis, B.; Korbel, D.; Wilson, R.; Bylund, J.; Chee, R.; Schaible, U.E. Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell. Microbiol. 2012, 14, 1109–1121. [Google Scholar] [CrossRef]
- Sutherland, J.S.; Jeffries, D.J.; Donkor, S.; Walther, B.; Hill, P.C.; Adetifa, I.M.; Adegbola, R.A.; Ota, M.O. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis 2009, 89, 398–404. [Google Scholar] [CrossRef]
- Seiler, P.; Aichele, P.; Bandermann, S.; Hauser, A.E.; Lu, B.; Gerard, N.P.; Gerard, C.; Ehlers, S.; Mollenkopf, H.J.; Kaufmann, S.H. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur. J. Immunol. 2003, 33, 2676–2686. [Google Scholar] [CrossRef]
- Ishigame, H.; Kakuta, S.; Nagai, T.; Kadoki, M.; Nambu, A.; Komiyama, Y.; Fujikado, N.; Tanahashi, Y.; Akitsu, A.; Kotaki, H.; et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009, 30, 108–119. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, K.; Behrends, J.; Rückerl, D.; Hölscher, A.; Volz, J.; Prinz, I.; Hölscher, C. High-Dose Mycobacterium tuberculosis H37rv Infection in IL-17A- and IL-17A/F-Deficient Mice. Cells 2022, 11, 2875. https://doi.org/10.3390/cells11182875
Ritter K, Behrends J, Rückerl D, Hölscher A, Volz J, Prinz I, Hölscher C. High-Dose Mycobacterium tuberculosis H37rv Infection in IL-17A- and IL-17A/F-Deficient Mice. Cells. 2022; 11(18):2875. https://doi.org/10.3390/cells11182875
Chicago/Turabian StyleRitter, Kristina, Jochen Behrends, Dominik Rückerl, Alexandra Hölscher, Johanna Volz, Immo Prinz, and Christoph Hölscher. 2022. "High-Dose Mycobacterium tuberculosis H37rv Infection in IL-17A- and IL-17A/F-Deficient Mice" Cells 11, no. 18: 2875. https://doi.org/10.3390/cells11182875
APA StyleRitter, K., Behrends, J., Rückerl, D., Hölscher, A., Volz, J., Prinz, I., & Hölscher, C. (2022). High-Dose Mycobacterium tuberculosis H37rv Infection in IL-17A- and IL-17A/F-Deficient Mice. Cells, 11(18), 2875. https://doi.org/10.3390/cells11182875






