Golgi Dysfunctions in Ciliopathies
Abstract
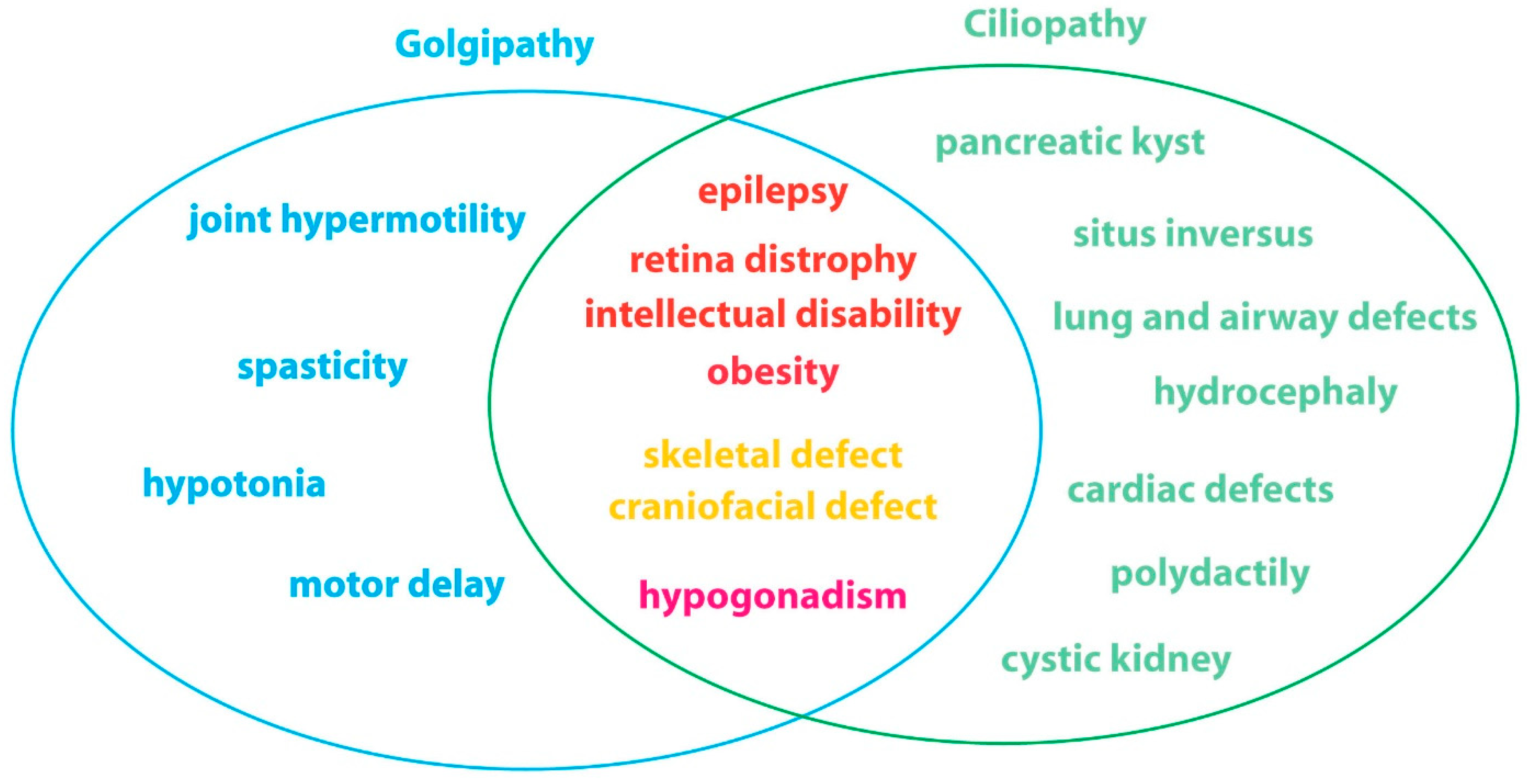
1. Introduction
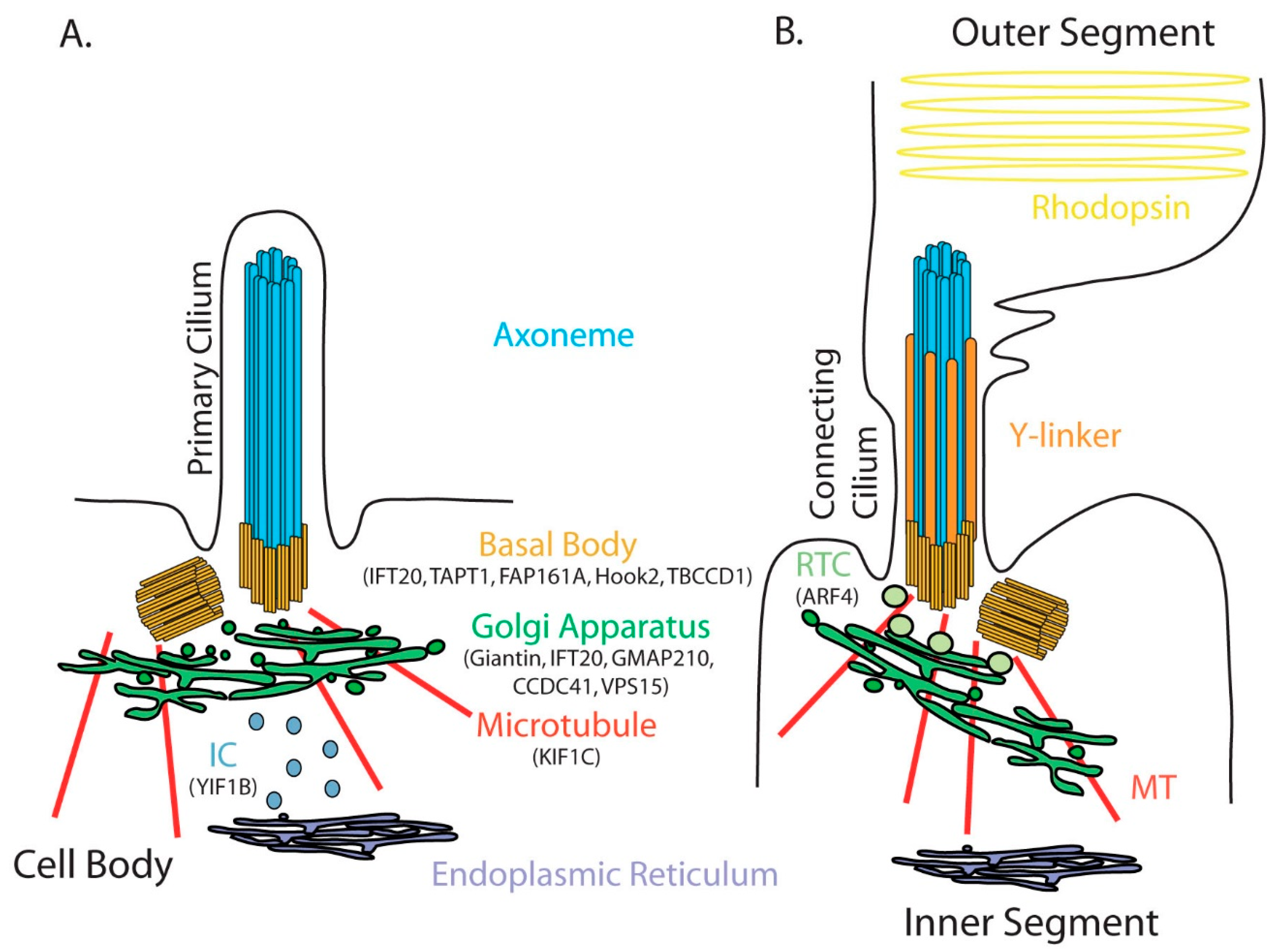
2. Golgi Apparatus and Primary Cilium Are Closely Linked
3. Targeting Proteins to the Cilium
4. GA Related Proteins Necessary for Normal Ciliogenesis/Ciliary Function
4.1. Resident GA Proteins
4.1.1. GIANTIN
4.1.2. GALNT11
4.1.3. IFT20 and Its Associated Complex
4.1.4. VPS15/PIK3R4
4.1.5. KIF1C
4.2. Golgi Proteins Involved in Post-Golgi Transport
4.3. Proteins Required for Golgi Integrity but Not Directly Located at the GA
4.3.1. YIF1B
4.3.2. TAPT1
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Primers 2020, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, A.; Witkos, T.M.; Unger, S.; Schneider, J.; Follit, J.A.; Hermann, J.; Welting, T.; Fano, V.; Hietala, M.; Vatanavicharn, N.; et al. Hypomorphic mutations of TRIP11 cause odontochondrodysplasia. JCI Insight 2019, 4, e124701. [Google Scholar] [CrossRef] [PubMed]
- Upadhyai, P.; Radhakrishnan, P.; Guleria, V.S.; Kausthubham, N.; Nayak, S.S.; Superti-Furga, A.; Girisha, K.M. Biallelic deep intronic variant c.5457+81T>A in TRIP11 causes loss of function and results in achondrogenesis 1A. Hum. Mutat. 2021, 42, 1005–1014. [Google Scholar] [CrossRef]
- Stoetzel, C.; Bar, S.; De Craene, J.O.; Scheidecker, S.; Etard, C.; Chicher, J.; Reck, J.R.; Perrault, I.; Geoffroy, V.; Chennen, K.; et al. A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi. Nat. Commun. 2016, 7, 13586. [Google Scholar] [CrossRef] [PubMed]
- Dor, T.; Cinnamon, Y.; Raymond, L.; Shaag, A.; Bouslam, N.; Bouhouche, A.; Gaussen, M.; Meyer, V.; Durr, A.; Brice, A.; et al. KIF1C mutations in two families with hereditary spastic paraparesis and cerebellar dysfunction. J. Med. Genet. 2014, 51, 137–142. [Google Scholar] [CrossRef]
- Marchionni, E.; Meneret, A.; Keren, B.; Melki, J.; Denier, C.; Durr, A.; Apartis, E.; Boespflug-Tanguy, O.; Mochel, F. KIF1C Variants Are Associated with Hypomyelination, Ataxia, Tremor, and Dystonia in Fraternal Twins. Tremor Other Hyperkinetic Mov. 2019, 9. [Google Scholar] [CrossRef]
- Santos, M.; Damasio, J.; Carmona, S.; Neto, J.L.; Dehghani, N.; Guedes, L.C.; Barbot, C.; Barros, J.; Bras, J.; Sequeiros, J.; et al. Molecular Characterization of Portuguese Patients with Hereditary Cerebellar Ataxia. Cells 2022, 11, 981. [Google Scholar] [CrossRef]
- Diaz, J.; Gerard, X.; Emerit, M.B.; Areias, J.; Geny, D.; Degardin, J.; Simonutti, M.; Guerquin, M.J.; Collin, T.; Viollet, C.; et al. YIF1B mutations cause a post-Natal neurodevelopmental syndrome associated with Golgi and primary cilium alterations. Brain 2020, 143, 2911–2928. [Google Scholar] [CrossRef]
- Symoens, S.; Barnes, A.M.; Gistelinck, C.; Malfait, F.; Guillemyn, B.; Steyaert, W.; Syx, D.; D’Hondt, S.; Biervliet, M.; De Backer, J.; et al. Genetic Defects in TAPT1 Disrupt Ciliogenesis and Cause a Complex Lethal Osteochondrodysplasia. Am. J. Hum. Genet. 2015, 97, 521–534. [Google Scholar] [CrossRef]
- Katayama, K.; Sasaki, T.; Goto, S.; Ogasawara, K.; Maru, H.; Suzuki, K.; Suzuki, H. Insertional mutation in the Golgb1 gene is associated with osteochondrodysplasia and systemic edema in the OCD rat. Bone 2011, 49, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zhang, N.; Liu, H.; Xu, J.; Jiang, R. Golgb1 regulates protein glycosylation and is crucial for mammalian palate development. Development 2016, 143, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, J.A.; San Agustin, J.; Follit, J.A.; Pazour, G.J. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J. Cell Biol. 2008, 183, 377–384. [Google Scholar] [CrossRef]
- Le, Y.Z.; Ash, J.D.; Al-Ubaidi, M.R.; Chen, Y.; Ma, J.X.; Anderson, R.E. Targeted expression of Cre recombinase to cone photoreceptors in transgenic mice. Mol. Vis. 2004, 10, 1011–1018. [Google Scholar]
- Keady, B.T.; Le, Y.Z.; Pazour, G.J. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol. Biol. Cell 2011, 22, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, W.; Zhang, Y.; Zhang, L.; Teves, M.E.; Liu, H.; Strauss, J.F., 3rd; Pazour, G.J.; Foster, J.A.; Hess, R.A. Intraflagellar transport protein IFT20 is essential for male fertility and spermiogenesis in mice. Mol. Biol. Cell 2016, 27, 3705–3716. [Google Scholar] [CrossRef] [PubMed]
- Follit, J.A.; San Agustin, J.T.; Xu, F.; Jonassen, J.A.; Samtani, R.; Lo, C.W.; Pazour, G.J. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 2008, 4, e1000315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, Y.; Ma, S.; Huang, Q.; Yap, Y.T.; Shi, L.; Zhang, S.; Zhou, T.; Li, W.; Hu, B.; et al. Abnormal fertility, acrosome formation, IFT20 expression and localization in conditional Gmap210 knockout mice. Am. J. Physiol. Cell Physiol. 2020, 318, C174–C190. [Google Scholar] [CrossRef]
- Alterio, J.; Masson, J.; Diaz, J.; Chachlaki, K.; Salman, H.; Areias, J.; Al Awabdh, S.; Emerit, M.B.; Darmon, M. Yif1B Is Involved in the Anterograde Traffic Pathway and the Golgi Architecture. Traffic 2015, 16, 978–993. [Google Scholar] [CrossRef]
- Howell, G.R.; Shindo, M.; Murray, S.; Gridley, T.; Wilson, L.A.; Schimenti, J.C. Mutation of a ubiquitously expressed mouse transmembrane protein (Tapt1) causes specific skeletal homeotic transformations. Genetics 2007, 175, 699–707. [Google Scholar] [CrossRef]
- Wachten, D.; Mick, D.U. Signal transduction in primary cilia—Analyzing and manipulating GPCR and second messenger signaling. Pharmacol. Ther. 2021, 224, 107836. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.A.; Flint, M.H.; Beaumont, B.W. Analysis of the morphology and function of primary cilia in connective tissues: A cellular cybernetic probe? Cell Motil. 1985, 5, 175–193. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kataoka, K. Electron microscopic observation of the primary cilium in the pancreatic islets. Arch. Histol. Jpn. Nihon Soshikigaku Kiroku 1986, 49, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Tenkova, T.; Chaldakov, G.N. Golgi-Cilium complex in rabbit ciliary process cells. Cell Struct. Funct. 1988, 13, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Silhavy, J.L.; Lee, J.E.; Al-Gazali, L.; Thomas, S.; Davis, E.E.; Bielas, S.L.; Hill, K.J.; Iannicelli, M.; Brancati, F.; et al. Evolutionarily assembled cis-Regulatory module at a human ciliopathy locus. Science 2012, 335, 966–969. [Google Scholar] [CrossRef]
- Ghossoub, R.; Molla-Herman, A.; Bastin, P.; Benmerah, A. The ciliary pocket: A once-Forgotten membrane domain at the base of cilia. Biol. Cell 2011, 103, 131–144. [Google Scholar] [CrossRef]
- Focsa, I.O.; Budisteanu, M.; Balgradean, M. Clinical and genetic heterogeneity of primary ciliopathies (Review). Int. J. Mol. Med. 2021, 48, 1–15. [Google Scholar] [CrossRef]
- Rasika, S.; Passemard, S.; Verloes, A.; Gressens, P.; El Ghouzzi, V. Golgipathies in Neurodevelopment: A New View of Old Defects. Dev. Neurosci. 2018, 40, 396–416. [Google Scholar] [CrossRef]
- Deretic, D.; Schmerl, S.; Hargrave, P.A.; Arendt, A.; McDowell, J.H. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-Terminal sequence QVS(A)PA. Proc. Natl. Acad. Sci. USA 1998, 95, 10620–10625. [Google Scholar] [CrossRef]
- Berbari, N.F.; Johnson, A.D.; Lewis, J.S.; Askwith, C.C.; Mykytyn, K. Identification of ciliary localization sequences within the third intracellular loop of G protein-Coupled receptors. Mol. Biol. Cell 2008, 19, 1540–1547. [Google Scholar] [CrossRef]
- Deretic, D.; Williams, A.H.; Ransom, N.; Morel, V.; Hargrave, P.A.; Arendt, A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-Ribosylation factor 4 (ARF4). Proc. Natl. Acad. Sci. USA 2005, 102, 3301–3306. [Google Scholar] [CrossRef] [PubMed]
- Domire, J.S.; Green, J.A.; Lee, K.G.; Johnson, A.D.; Askwith, C.C.; Mykytyn, K. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell. Mol. Life Sci. 2011, 68, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Okuhara, D.; Yu, Z.; Tian, X.; Cai, Y.; Shibazaki, S.; Somlo, S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-Terminal RVxP motif. J. Cell Sci. 2006, 119, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Soetedjo, L.; Glover, D.A.; Jin, H. Targeting of vasoactive intestinal peptide receptor 2, VPAC2, a secretin family G-Protein coupled receptor, to primary cilia. Biol. Open 2013, 2, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Linstedt, A.D.; Hauri, H.P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell 1993, 4, 679–693. [Google Scholar] [CrossRef]
- Puthenveedu, M.A.; Linstedt, A.D. Evidence that Golgi structure depends on a p115 activity that is independent of the vesicle tether components giantin and GM130. J. Cell Biol. 2001, 155, 227–238. [Google Scholar] [CrossRef]
- Stevenson, N.L.; Bergen, D.J.M.; Skinner, R.E.H.; Kague, E.; Martin-Silverstone, E.; Robson Brown, K.A.; Hammond, C.L.; Stephens, D.J. Giantin-Knockout models reveal a feedback loop between Golgi function and glycosyltransferase expression. J. Cell Sci. 2017, 130, 4132–4143. [Google Scholar] [CrossRef]
- Koreishi, M.; Gniadek, T.J.; Yu, S.; Masuda, J.; Honjo, Y.; Satoh, A. The golgin tether giantin regulates the secretory pathway by controlling stack organization within Golgi apparatus. PLoS ONE 2013, 8, e59821. [Google Scholar] [CrossRef]
- Bergen, D.J.M.; Stevenson, N.L.; Skinner, R.E.H.; Stephens, D.J.; Hammond, C.L. The Golgi matrix protein giantin is required for normal cilia function in zebrafish. Biol. Open 2017, 6, 1180–1189. [Google Scholar] [CrossRef]
- Rottger, S.; White, J.; Wandall, H.H.; Olivo, J.C.; Stark, A.; Bennett, E.P.; Whitehouse, C.; Berger, E.G.; Clausen, H.; Nilsson, T. Localization of three human polypeptide GalNAc-Transferases in HeLa cells suggests initiation of O-Linked glycosylation throughout the Golgi apparatus. J. Cell Sci. 1998, 111, 45–60. [Google Scholar] [CrossRef]
- Fakhro, K.A.; Choi, M.; Ware, S.M.; Belmont, J.W.; Towbin, J.A.; Lifton, R.P.; Khokha, M.K.; Brueckner, M. Rare copy number variations in congenital heart disease patients identify unique genes in left-Right patterning. Proc. Natl. Acad. Sci. USA 2011, 108, 2915–2920. [Google Scholar] [CrossRef] [PubMed]
- Boskovski, M.T.; Yuan, S.; Pedersen, N.B.; Goth, C.K.; Makova, S.; Clausen, H.; Brueckner, M.; Khokha, M.K. The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality. Nature 2013, 504, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Follit, J.A.; Tuft, R.A.; Fogarty, K.E.; Pazour, G.J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 2006, 17, 3781–3792. [Google Scholar] [CrossRef] [PubMed]
- Infante, C.; Ramos-Morales, F.; Fedriani, C.; Bornens, M.; Rios, R.M. GMAP-210, A cis-Golgi network-Associated protein, is a minus end microtubule-Binding protein. J. Cell Biol. 1999, 145, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Broekhuis, J.R.; Rademakers, S.; Burghoorn, J.; Jansen, G. SQL-1, homologue of the Golgi protein GMAP210, modulates intraflagellar transport in C. elegans. J. Cell Sci. 2013, 126, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.; Bolton, A.D.; Funari, V.; Hong, M.; Boyden, E.D.; Lu, L.; Manning, D.K.; Dwyer, N.D.; Moran, J.L.; Prysak, M.; et al. Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N. Engl. J. Med. 2010, 362, 206–216. [Google Scholar] [CrossRef]
- Joo, K.; Kim, C.G.; Lee, M.S.; Moon, H.Y.; Lee, S.H.; Kim, M.J.; Kweon, H.S.; Park, W.Y.; Kim, C.H.; Gleeson, J.G.; et al. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc. Natl. Acad. Sci. USA 2013, 110, 5987–5992. [Google Scholar] [CrossRef]
- Tanos, B.E.; Yang, H.J.; Soni, R.; Wang, W.J.; Macaluso, F.P.; Asara, J.M.; Tsou, M.F. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013, 27, 163–168. [Google Scholar] [CrossRef]
- Herman, P.K.; Stack, J.H.; DeModena, J.A.; Emr, S.D. A novel protein kinase homolog essential for protein sorting to the yeast lysosome-Like vacuole. Cell 1991, 64, 425–437. [Google Scholar] [CrossRef]
- Nemazanyy, I.; Blaauw, B.; Paolini, C.; Caillaud, C.; Protasi, F.; Mueller, A.; Proikas-Cezanne, T.; Russell, R.C.; Guan, K.L.; Nishino, I.; et al. Defects of Vps15 in skeletal muscles lead to autophagic vacuolar myopathy and lysosomal disease. EMBO Mol. Med. 2013, 5, 870–890. [Google Scholar] [CrossRef]
- Senatore, E.; Iannucci, R.; Chiuso, F.; Delle Donne, R.; Rinaldi, L.; Feliciello, A. Pathophysiology of Primary Cilia: Signaling and Proteostasis Regulation. Front. Cell Dev. Biol. 2022, 10, 833086. [Google Scholar] [CrossRef] [PubMed]
- She, Z.Y.; Pan, M.Y.; Tan, F.Q.; Yang, W.X. Minus end-Directed kinesin-14 KIFC1 regulates the positioning and architecture of the Golgi apparatus. Oncotarget 2017, 8, 36469–36483. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Joo, K.; Jung, E.J.; Hong, H.; Seo, J.; Kim, J. Export of membrane proteins from the Golgi complex to the primary cilium requires the kinesin motor, KIFC1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Morita, Y.; Mazelova, J.; Deretic, D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-Mediated ciliary receptor targeting. EMBO J. 2012, 31, 4057–4071. [Google Scholar] [CrossRef] [PubMed]
- Yucel-Yilmaz, D.; Yucesan, E.; Yalnizoglu, D.; Oguz, K.K.; Sagiroglu, M.S.; Ozbek, U.; Serdaroglu, E.; Bilgic, B.; Erdem, S.; Iseri, S.A.U.; et al. Clinical phenotype of hereditary spastic paraplegia due to KIF1C gene mutations across life span. Brain Dev. 2018, 40, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Mazelova, J.; Astuto-Gribble, L.; Inoue, H.; Tam, B.M.; Schonteich, E.; Prekeris, R.; Moritz, O.L.; Randazzo, P.A.; Deretic, D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009, 28, 183–192. [Google Scholar] [CrossRef]
- Lorente-Rodriguez, A.; Barlowe, C. Entry and exit mechanisms at the cis-Face of the Golgi complex. Cold Spring Harb. Perspect. Biol. 2011, 3, a005207. [Google Scholar] [CrossRef][Green Version]
- Tormanen, K.; Ton, C.; Waring, B.M.; Wang, K.; Sutterlin, C. Function of Golgi-Centrosome proximity in RPE-1 cells. PLoS ONE 2019, 14, e0215215. [Google Scholar] [CrossRef]
- Carrel, D.; Masson, J.; Al Awabdh, S.; Capra, C.B.; Lenkei, Z.; Hamon, M.; Emerit, M.B.; Darmon, M. Targeting of the 5-HT1A serotonin receptor to neuronal dendrites is mediated by Yif1B. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 8063–8073. [Google Scholar] [CrossRef]
- Goncalves, J.; Nolasco, S.; Nascimento, R.; Lopez Fanarraga, M.; Zabala, J.C.; Soares, H. TBCCD1, a new centrosomal protein, is required for centrosome and Golgi apparatus positioning. EMBO Rep. 2010, 11, 194–200. [Google Scholar] [CrossRef]
| Gene | Nucleotide Change | Predicted Amino Acid Change | Protein Level | Reference |
|---|---|---|---|---|
| GMAP210/Trip11 | c.[1314+5G>A]; [chr14:g. (?_ 92.474.069)_ (92.597.431_?)del | p.[(Glu439Valfs*20)]; [(?)]A | N.D | [3] |
| c.[1228G>T]; [4815_4818delAGAG] | p.[Asp410_ Lys438del]; [Glu1606Leufs*3] | Strongly reduced (reduction of approximately 90%) | ||
| c.[586C>T];[4534C>T] | p.[Gln196*]; (Gln1512*] | N.D | ||
| c.[1228G>T]; [2128_2129delAT] | p.[Asp410Tyr]; [Ile710Cysfs*19] | Complete absence | ||
| c.[1622delA]; [5416A>G] | p.[Lys541Argfs*17]; [Met1806Val] | Complete absence | ||
| c.[586C>T]; [2993_2994delAA] | p.[Gln196*]; [(ys998Serfs*5] | N.D | ||
| c.[586C>T]; [790C>T] | p.[Gln196*]; [Arg264*] | Reduced (30% of control) | ||
| c.[5457+81T>A] | N.D | Complete absence | [4] | |
| VPS15 | c.[2993G>A] | p.[Arg998Gl] | N.D | [5] |
| KIF1C | c.[4925567C>T] | N.D | Complete absence | [6] |
| c.[1019+1dup] | N.D | N.D | [7] | |
| c.[1166-2A>G] | N.D | N.D | [8] | |
| YIF1B | c.[598G>T] | p.[Glu200*] | N.D | [9] |
| c.[539+1G>A] | p.[Ala161Glyfs*18] | Complete absence | ||
| c.[367A>C] | p.[Lys123Gln] | Strongly reduced | ||
| c.[177dupT] | p.[Ala63Cysfs*13] | N.D | ||
| c.[539+1G>A]; [696-2A>C] | p.[Ala161Glyfs*18]; [Met233Phefs*40] | Complete absence | ||
| c.[577_592del); [501C>A] | p.[Ala193Profs*36]; [Tyr167*] | N.D | ||
| TAPT1 | c.[1108-1G>C] | p.[Val370_Asn389del] | N.D | [10] |
| Gene | Model | Silencing Strategy | Protein Level | Reference |
|---|---|---|---|---|
| GIANTIN | OCD rat (mutant inbred strain) | 10-bp (CTGCAGGCAG) duplication in the 13th exon resulting in a frame shift mutation and introducing a premature termination codon at codon 1082 | Absent | [11] |
| Golgb1 KO mice (CRISPR/Cas9-mediated genome editing) | Extension of exon 9 introducing a premature termination codon | Absent | [12] | |
| IFT20 | Ift20 flox/flox [13]/HRGP-Cre mice [14] | Homologous recombination with a disrupting cassette | Absent in cone photoreceptor | [15] |
| Ift20 flox/flox [13]/Stra8-iCre (Jackson Laboratory) mice | Homologous recombination with a disrupting cassette | Absent in male germ cell | [16] | |
| GMAP210/Trip11 | Gmap210 KO mice | Insertion of a splice acceptor site and a βgalactosidase-neomycin resistance gene fusion into intron 4 and duplication of Chr16 | Absent | [17] |
| Gmap210flox/flox [17]/Stra8-iCre (Jackson Laboratory) mice | Homologous recombination with a disrupting cassette | Absent in male germ cell | [18] | |
| YIF1B | Yif1B KO mice [19] | Homologous recombination with a disrupting cassette | Absent | [9] |
| TAPT1 | mice carrying L5Jcs1 mutation | T>A in the 5′ splice site of intron 7 resulting in the introduction of 12 aa after N279 and a premature termination codon | N.D | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masson, J.; El Ghouzzi, V. Golgi Dysfunctions in Ciliopathies. Cells 2022, 11, 2773. https://doi.org/10.3390/cells11182773
Masson J, El Ghouzzi V. Golgi Dysfunctions in Ciliopathies. Cells. 2022; 11(18):2773. https://doi.org/10.3390/cells11182773
Chicago/Turabian StyleMasson, Justine, and Vincent El Ghouzzi. 2022. "Golgi Dysfunctions in Ciliopathies" Cells 11, no. 18: 2773. https://doi.org/10.3390/cells11182773
APA StyleMasson, J., & El Ghouzzi, V. (2022). Golgi Dysfunctions in Ciliopathies. Cells, 11(18), 2773. https://doi.org/10.3390/cells11182773







