Enhancement of Tendon Repair Using Tendon-Derived Stem Cells in Small Intestinal Submucosa via M2 Macrophage Polarization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Material Preparation
2.2.1. SIS Scaffold
2.2.2. SIS Hydrogel Coating
2.3. Assessments of Biocompatibility
2.3.1. Isolation and Culture of TDSCs
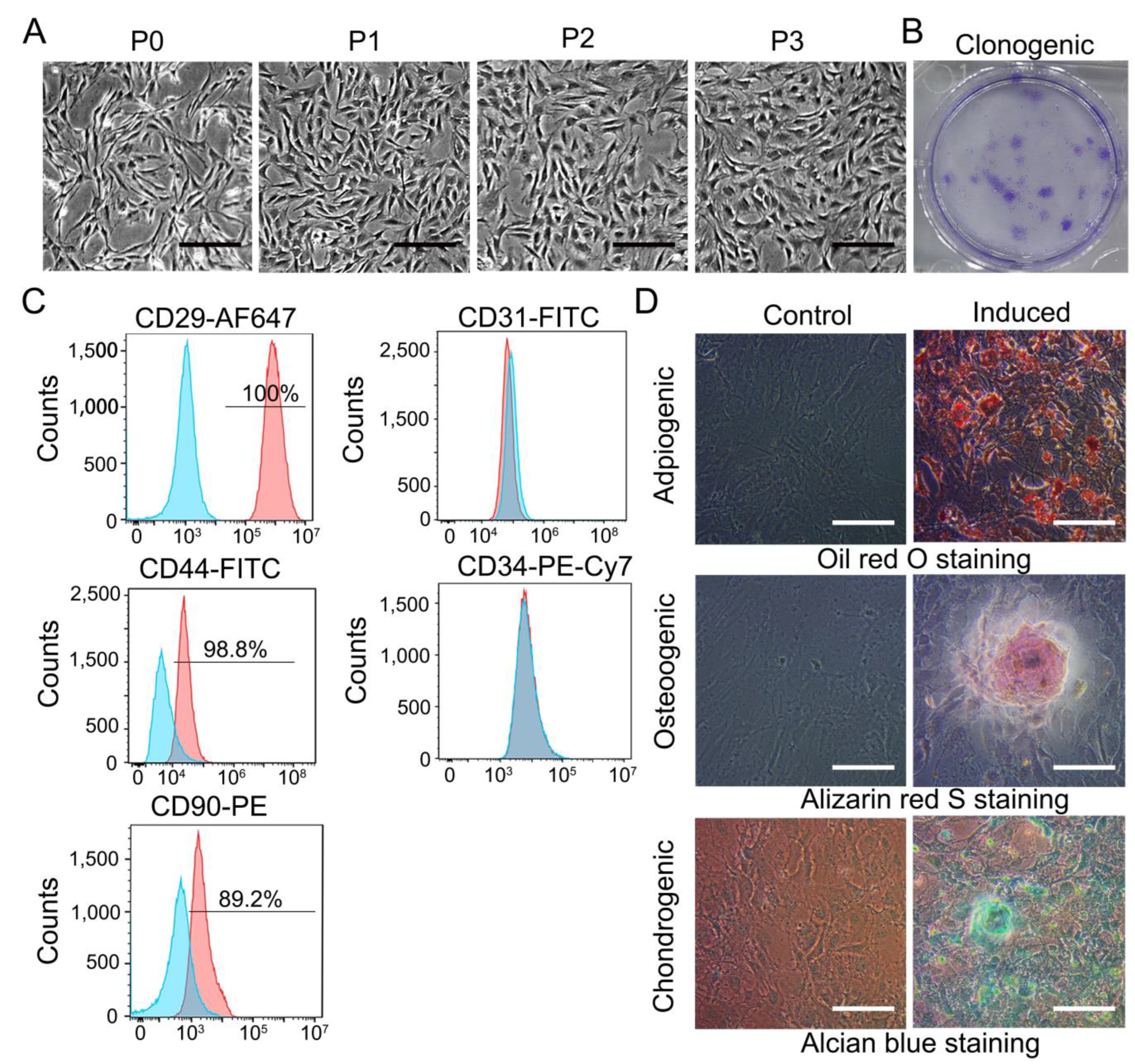
2.3.2. Flow Cytometry Assay of TDSCs
2.3.3. Trilineage Differentiation of TDSCs
2.3.4. Colony Formation Assay
2.3.5. Proliferation, Adhesion, and Differentiation of Tendon Stem Cells on SIS
2.4. M1 and M2 Polarization Model
2.5. Induction of RAW264.7 Cells by Culture Media
2.6. qRT-PCR
2.7. Immunofluorescence
2.8. Assessments of In Vivo Repair
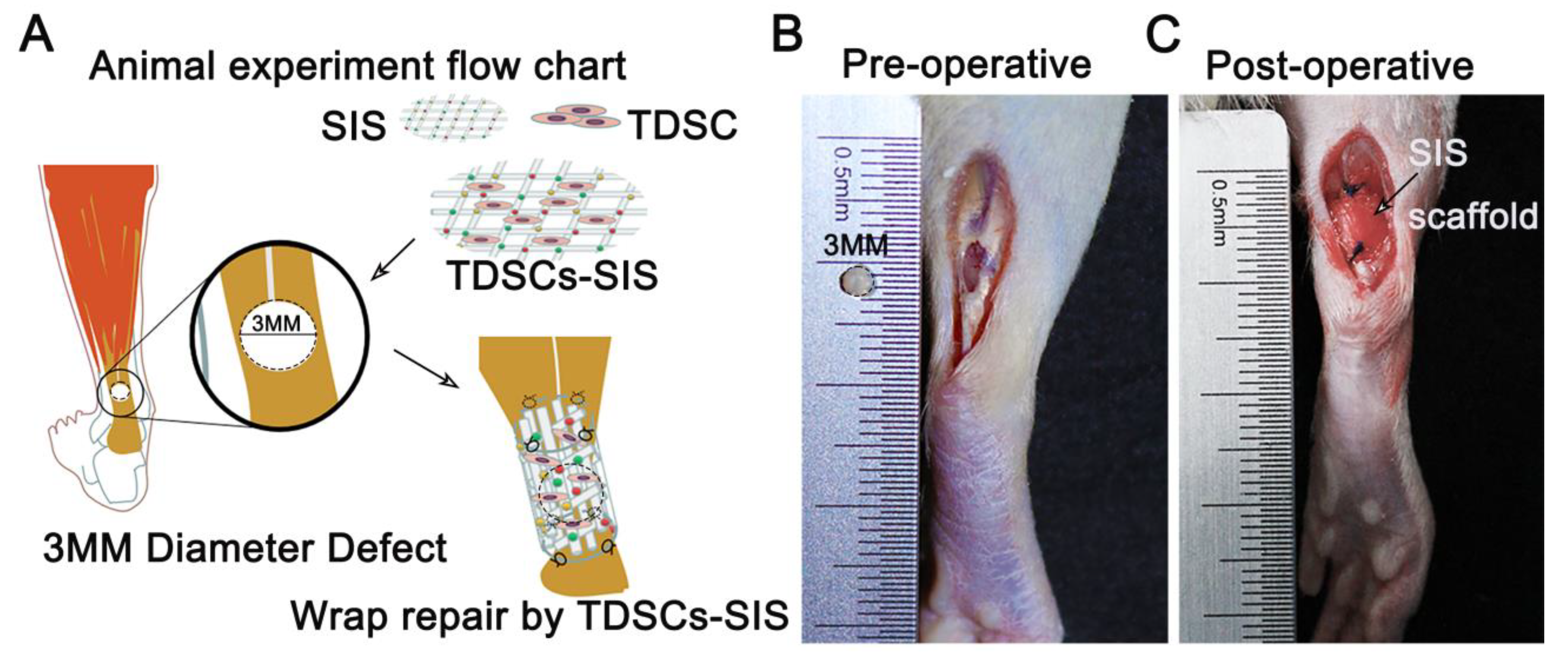
2.8.1. Rats and Experimental Design
2.8.2. Surgical Protocol
2.8.3. Macrographic Examination
2.8.4. Tissue Sample Preparation
2.8.5. Histopathological Analysis
2.8.6. Biomechanical Testing
2.9. Statistical Analysis
3. Results
3.1. Characterization of TDSCs
3.2. SIS Scaffold Facilitated Cell Adhesion and Tenogenic Differentiation of TDSCs, While SIS Hydrogel Coating Promoted Proliferation of TDSCs
3.3. Transplantation of TDSCs–SIS Scaffold to Repair Achilles Tendon Defect in Rats
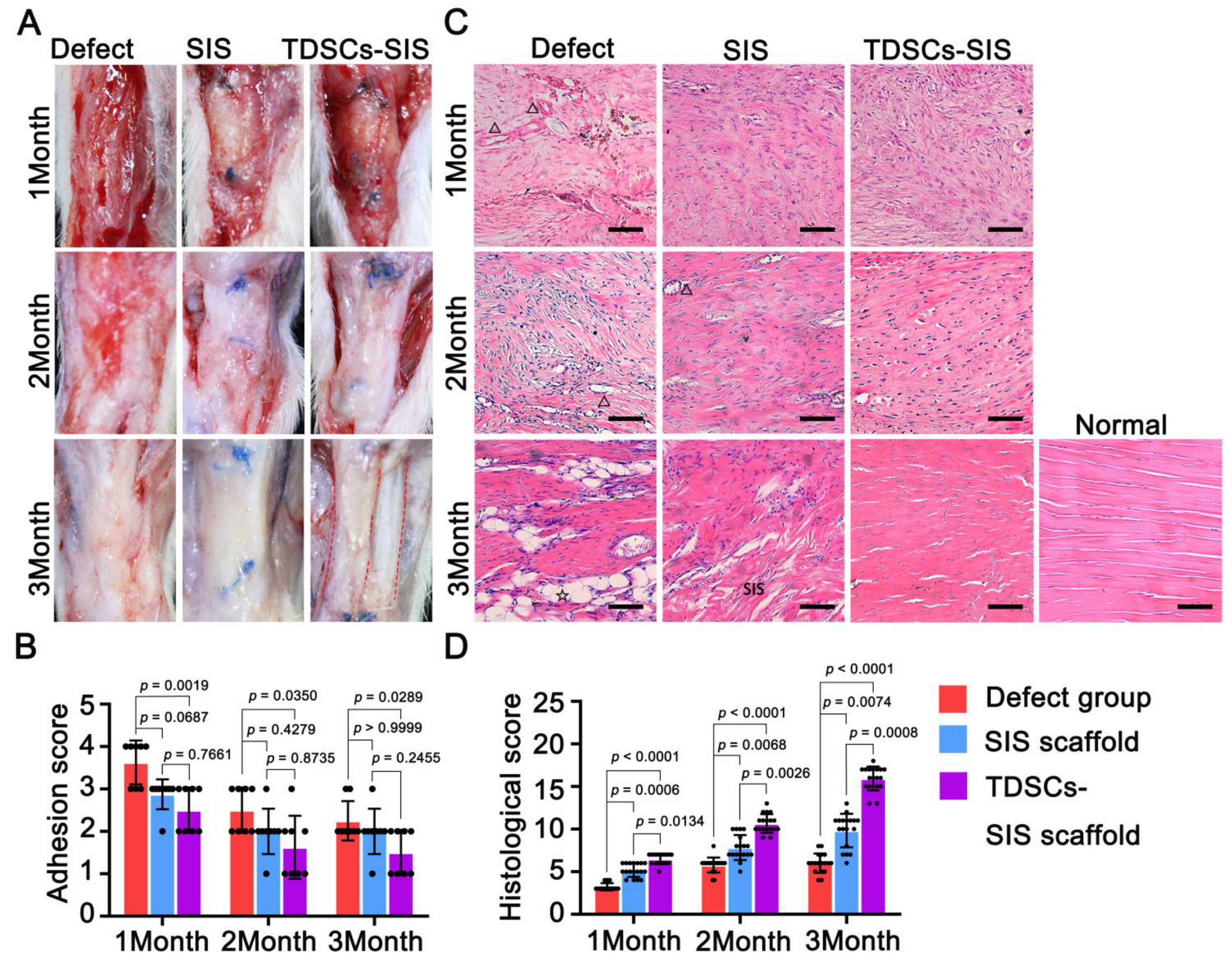
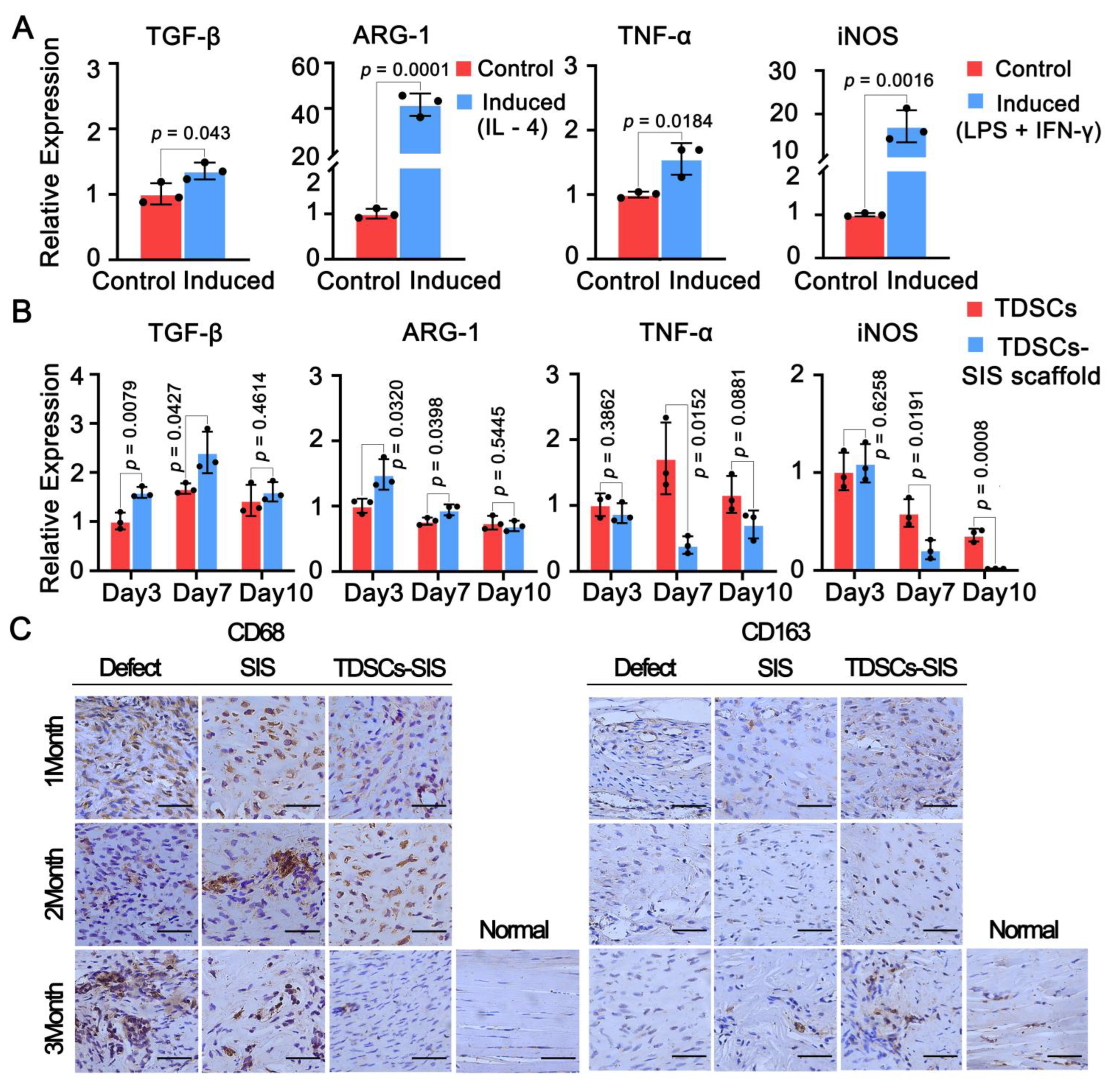
3.4. TDSCs–SIS Scaffold Improved Tendon Healing through Gross and Histological Evaluation
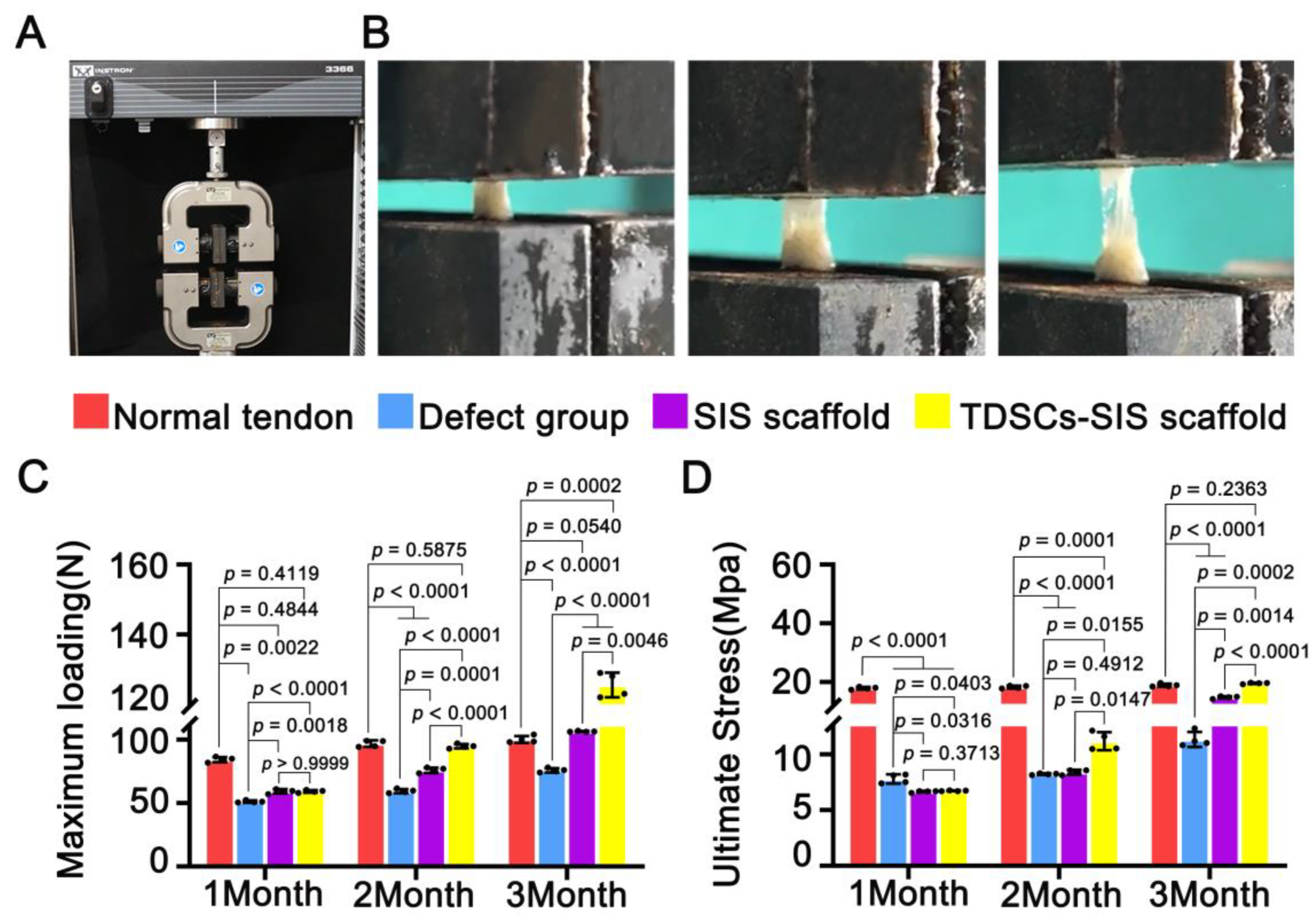
3.5. TDSCs–SIS Scaffold Contributes to Biomechanical Property Recovery
3.6. TDSCs–SIS Scaffold Promoted Regenerated Achilles Tendon and Regulated Extracellular Matrix Formation
3.7. Effect of TDSCs and SIS Scaffold on Macrophage Polarization toward the M2 Phenotype
4. Discussion
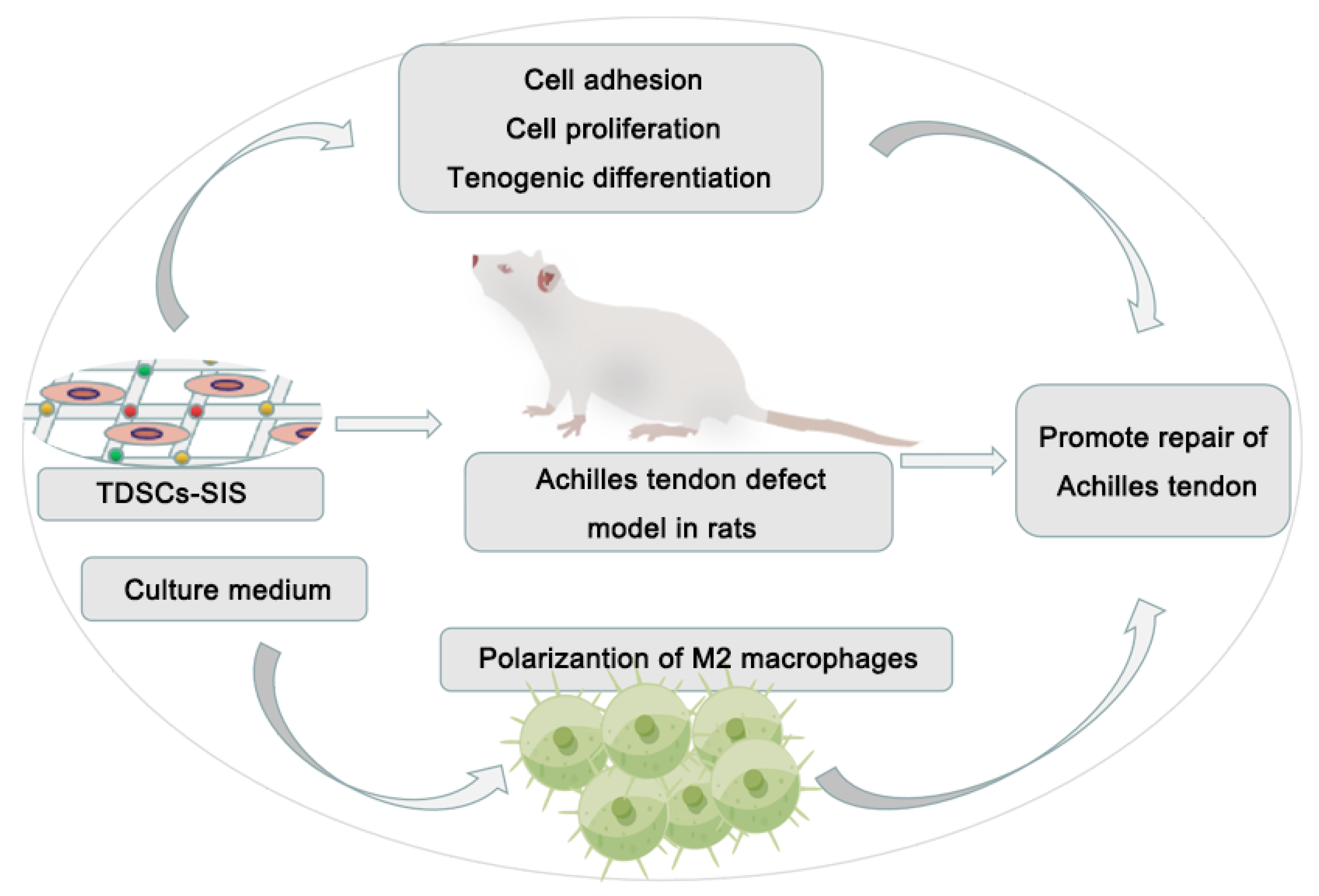
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maffulli, N.; Via, A.G.; Oliva, F. Chronic Achilles Tendon Rupture. Open Orthop. J. 2017, 11, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.E.; Nunley, J.A. Treatment of Neglected Achilles Tendon Ruptures with Interpositional Allograft. Foot Ankle Clin. 2017, 22, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, J.; Yu, K.; Liu, G.; Tian, S.; Tian, D. Biological Amnion Prevents Flexor Tendon Adhesion in Zone II: A Controlled, Multicentre Clinical Trial. BioMed. Res. Int. 2019, 2019, 2354325. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.N.; Havlioglu, N.; Knutsen ESakiyama-Elbert, S.E.; Silva, M.J.; Thomopoulos, S.; Gelberman, R.H. The early inflammatory response after flexor tendon healing: A gene expression and histological analysis. J. Orthop. Res. 2014, 32, 645–652. [Google Scholar] [CrossRef]
- Vieira, C.P.; Guerra Fda, R.; de Oliveira, L.P.; de Almeida Mdos, S.; Pimentel, E.R. Alterations in the Achilles tendon after inflammation in surrounding tissue. Acta Ortop. Bras. 2012, 20, 266–269. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, R.; das Neves, J.; Tang, J.; Xiao, J.; Ni, Q.; Liu, X.; Pan, G.; Li, D.; Cui, W.; et al. Advances in biomaterials for preventing tissue adhesion. J. Control. Release 2017, 261, 318–336. [Google Scholar] [CrossRef]
- Hong, G.S.; Schwandt, T.; Stein, K.; Schneiker, B.; Kummer, M.P.; Heneka, M.T.; Kitamura, K.; Kalff, J.C.; Wehner, S. Effects of macrophage-dependent peroxisome proliferator-activated receptor γ signalling on adhesion formation after abdominal surgery in an experimental model. Br. J. Surg. 2015, 102, 1506–1516. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, Y.; Tang, Y.; Chen, C.; Li, S.; Zhao, C.; Hu, J.; Lu, H. Book-shaped decellularized tendon matrix scaffold combined with bone marrow mesenchymal stem cells-sheets for repair of achilles tendon defect in rabbit. J. Orthop. Res. 2019, 37, 887–897. [Google Scholar] [CrossRef]
- Deng, D.; Wang, W.; Wang, B.; Zhang, P.; Zhou, G.; Zhang, W.J.; Cao, Y.; Liu, W. Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials 2014, 35, 8801–8809. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, Z.; Wang, C.; Li, M.; Zhang, H.; Qu, J. Electrohydrodynamic 3D Printing Scaffolds for Repair of Achilles Tendon Defect in Rats. Tissue Eng. Part A 2021, 27, 1343–1354. [Google Scholar] [CrossRef]
- Zhang, C.H.; Jiang, Y.L.; Ning, L.J.; Li, Q.; Fu, W.L.; Zhang, Y.J.; Zhang, Y.J.; Xia, C.C.; Li, J.; Qin, T.W. Evaluation of Decellularized Bovine Tendon Sheets for Achilles Tendon Defect Reconstruction in a Rabbit Model. Am. J. Sports Med. 2018, 46, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, X.; Liu, B.; Yu, Y.; Sun, L.; Liu, T.; Wang, Y.; Ding, J.; Chen, X. Polymer materials for prevention of postoperative adhesion. Acta Biomater. 2017, 61, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Wang, S.; Hu, D.; Fu, W.; Wu, J.; Hong, H.; Domian, I.J.; Li, F.; Liu, J. Bioresorbable electrospun gelatin/polycaprolactone nanofibrous membrane as a barrier to prevent cardiac postoperative adhesion. Acta Biomater. 2019, 83, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, S.; Bai, J.; Yu, K.; Liu, L.; Liu, G.; Dong, R.; Tian, D. Regulation of ERK1/2 and SMAD2/3 Pathways by Using Multi-Layered Electrospun PCL-Amnion Nanofibrous Membranes for the Prevention of Post-Surgical Tendon Adhesion. Int. J. Nanomed. 2020, 15, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Sheu, C.; Chen, C.H.; Chen, S.H.; Jose, G.; Kuo, C.Y.; Chen, J.P. Multi-functional electrospun antibacterial core-shell nanofibrous membranes for prolonged prevention of post-surgical tendon adhesion and inflammation. Acta Biomater. 2018, 72, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Li, L.; Song, Y.; Dong, L.; Chen, P.; Li, X.; Cai, K. Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials 2018, 164, 22–37. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Yin, Y.; Hao, H.; Cheng, Y.; Gao, J.; Liu, J.; Xie, Z.; Zhang, Q.; Zang, L.; Han, W.; Mu, Y. The homing of human umbilical cord-derived mesenchymal stem cells and the subsequent modulation of macrophage polarization in type 2 diabetic mice. Int. Immunopharmacol. 2018, 60, 235–245. [Google Scholar] [CrossRef]
- Yin, Y.; Hao, H.; Cheng, Y.; Zang, L.; Liu, J.; Gao, J.; Xue, J.; Xie, Z.; Zhang, Q.; Han, W.; et al. Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell Death Dis. 2018, 9, 760. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Chen, B.; Zhao, J.; Peng, Z.; Xu, W.; Yu, G. Effect of intravenous transplantation of hUCB-MSCs on M1/M2 subtype conversion in monocyte/macrophages of AMI mice. Biomed. Pharmacother. 2019, 111, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, L.; Song, Y.; Fang, Y.; Liu, J.; Chen, P.; Wang, S.; Wang, C.; Xia, T.; Liu, W.; et al. MSC-derived immunomodulatory extracellular matrix functionalized electrospun fibers for mitigating foreign-body reaction and tendon adhesion. Acta Biomater. 2021, 133, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, Z.; Cho, E.; Huang, K.; Zhao, J.; Jiang, J.; Huangfu, X. Use of a Novel, Reinforced, Low Immunogenic, Porcine Small Intestine Submucosa Patch to Repair a Supraspinatus Tendon Defect in a Rabbit Model. BioMed. Res. Int. 2019, 2019, 9346567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, Y.; Fang, Y.; Yao, M.; Redmond, R.W.; Ni, T. No midterm advantages in the middle term using small intestinal submucosa and human amniotic membrane in Achilles tendon transverse tenotomy. J. Orthop. Surg. Res. 2016, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Stewart-Akers, A.M.; Simmons-Byrd, A.; Badylak, S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J. Bone Jt. Surg. Am. 2007, 89, 621–630. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Tang, H.; Shi, Y.; Zhu, M.; Kang, X.; Bian, X.; Lyu, J.; Zhou, M.; Yang, M.; et al. Aspirin promotes tenogenic differentiation of tendon stem cells and facilitates tendinopathy healing through regulating the GDF7/Smad1/5 signaling pathway. J. Cell. Physiol. 2020, 235, 4778–4789. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.; Flamenco, S.; Fan, C.M. A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 2019, 21, 1490–1503. [Google Scholar] [CrossRef]
- Guo, X.; Lv, H.; Fan, Z.; Duan, K.; Liang, J.; Zou, L.; Xue, H.; Huang, D.; Wang, Y.; Tan, M. Effects of hypoxia on Achilles tendon repair using adipose tissue-derived mesenchymal stem cells seeded small intestinal submucosa. J. Orthop. Surg. Res. 2021, 16, 570. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Viganò, M.; Perucca Orfei, C.; Colombini, A.; Stanco, D.; Randelli, P.; Sansone, V.; de Girolamo, L. Different culture conditions affect the growth of human tendon stem/progenitor cells (TSPCs) within a mixed tendon cells (TCs) population. J. Exp. Orthop. 2017, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Lui, P.P. Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ-update and future development. Stem Cell Res. Ther. 2015, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.X.; Mao, Y.; Cao, Q.; Chen, Y.; Zhou, L.B.; Li, S.; Chen, H.; Chen, J.H.; Zhou, G.P.; Jin, R. Exosome-mediated pyroptosis of miR-93-TXNIP-NLRP3 leads to functional difference between M1 and M2 macrophages in sepsis-induced acute kidney injury. J. Cell. Mol. Med. 2021, 25, 4786–4799. [Google Scholar] [CrossRef] [PubMed]
- Périz, M.; Pérez-Cano, F.J.; Cambras, T.; Franch, À.; Best, I.; Pastor-Soplin, S.; Castell, M.; Massot-Cladera, M. Attenuating Effect of Peruvian Cocoa Populations on the Acute Asthmatic Response in Brown Norway Rats. Nutrients 2020, 12, 2301. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; John, T.; Conrad, C.; Lohan, A.; Hondke, S.; Ertel, W.; Kaps, C.; Endres, M.; Sittinger, M.; Ringe, J.; et al. Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials 2011, 32, 4806–4815. [Google Scholar] [CrossRef]
- Lui, P.P.; Kong, S.K.; Lau, P.M.; Wong, Y.M.; Lee, Y.W.; Tan, C.; Wong, O.T. Allogeneic tendon-derived stem cells promote tendon healing and suppress immunoreactions in hosts: In vivo model. Tissue Eng. Part A 2014, 20, 2998–3009. [Google Scholar] [CrossRef]
- Han, P.; Cui, Q.; Lu, W.; Yang, S.; Shi, M.; Li, Z.; Gao, P.; Xu, B.; Li, Z. Hepatocyte growth factor plays a dual role in tendon-derived stem cell proliferation, migration, and differentiation. J. Cell. Physiol. 2019, 234, 17382–17391. [Google Scholar] [CrossRef]
- Lui, P.P.; Wong, O.T.; Lee, Y.W. Transplantation of tendon-derived stem cells pre-treated with connective tissue growth factor and ascorbic acid in vitro promoted better tendon repair in a patellar tendon window injury rat model. Cytotherapy 2016, 18, 99–112. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Hou, J.; Yang, R.; Vuong, I.; Li, F.; Kong, J.; Mao, H.Q. Biomaterials strategies to balance inflammation and tenogenesis for tendon repair. Acta Biomater. 2021, 130, 1–16. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021, 6, 2491–2510. [Google Scholar] [CrossRef] [PubMed]
- Andrée, B.; Bär, A.; Haverich, A.; Hilfiker, A. Small intestinal submucosa segments as matrix for tissue engineering: Review. Tissue Eng. Part B Rev. 2013, 19, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Zantop, T.; Gilbert, T.W.; Yoder, M.C.; Badylak, S.F. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J. Orthop. Res. 2006, 24, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Peng, Z.; Liu, Z.; Yang, J.; Tang, R.; Gu, Y. Reconstruction of abdominal wall musculofascial defects with small intestinal submucosa scaffolds seeded with tenocytes in rats. Tissue Eng. Part A 2013, 19, 1543–1553. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Bao, L.; Liu, J.; Zhu, X.; Mo, X.; Tang, R. The evaluation of functional small intestinal submucosa for abdominal wall defect repair in a rat model: Potent effect of sequential release of VEGF and TGF-β1 on host integration. Biomaterials 2021, 276, 120999. [Google Scholar] [CrossRef]
- Spang, C.; Chen, J.; Backman, L.J. The tenocyte phenotype of human primary tendon cells in vitro is reduced by glucocorticoids. BMC Musculoskelet. Disord. 2016, 17, 467. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Novak, M.L.; Koh, T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 2013, 183, 1352–1363. [Google Scholar] [CrossRef]
- Lu, J.; Chamberlain, C.S.; Ji, M.L.; Saether, E.E.; Leiferman, E.M.; Li, W.J.; Vanderby, R. Tendon-to-Bone Healing in a Rat Extra-articular Bone Tunnel Model: A Comparison of Fresh Autologous Bone Marrow and Bone Marrow-Derived Mesenchymal Stem Cells. Am. J. Sports Med. 2019, 47, 2729–2736. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhang, L.; Zhou, Y.L.; Tang, J.B. Morphological changes of macrophages and their potential contribution to tendon healing. Colloids Surf. B Biointerfaces 2022, 209, 112145. [Google Scholar] [CrossRef]
- Wang, H.; Morales, R.T.; Cui, X.; Huang, J.; Qian, W.; Tong, J.; Chen, W. A Photoresponsive Hyaluronan Hydrogel Nanocomposite for Dynamic Macrophage Immunomodulation. Adv. Healthc. Mater. 2019, 8, e1801234. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Morales, R.T.; Qian, W.; Wang, H.; Gagner, J.P.; Dolgalev, I.; Placantonakis, D.; Zagzag, D.; Cimmino, L.; Snuderl, M.; et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 2018, 161, 164–178. [Google Scholar] [CrossRef] [PubMed]


| Genes | Forward Primer Sequences | Reverse Primer Sequences |
|---|---|---|
| β-Actin | AGATGTGGATCAGCAAGCAG | GCGCAAGTTAGGTTTTGTCA |
| SCX | GACCCGCTTTCTTCCACAGC | GTCACGGTCTTTGCTCAACTTT |
| TNMD | GTGATTTGGGTTCCCGCAGAA | GTGGGATTGATCCAGTACATGG |
| COL1A1 | ACGTCCTGGTGAAGTTGGTC | CAGGGAAGCCTCTTTCTCCT |
| COL3A1 | CTGTAACATGGAAACTGGGGAAA | CCATAGCTGAACTGAAAACCACC |
| ARG-1 | GGCTTGCTTCGGAACTCAAC | CATGTGGCGCATTCACAGTC |
| TGF-β | CCACCTGCAAGACCATCGAC | CTGGCGAGCCTTAGTTTGGAC |
| TNF-α | AGGCACTCCCCCAAAAGATG | TTGCTACGACGTGGGCTAC |
| iNOS | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Yao, L.; Li, M.; Zhang, X.; Weng, B.; Zhu, W.; Ni, R.; Chen, K.; Yi, L.; Zhao, J.; et al. Enhancement of Tendon Repair Using Tendon-Derived Stem Cells in Small Intestinal Submucosa via M2 Macrophage Polarization. Cells 2022, 11, 2770. https://doi.org/10.3390/cells11172770
Mao X, Yao L, Li M, Zhang X, Weng B, Zhu W, Ni R, Chen K, Yi L, Zhao J, et al. Enhancement of Tendon Repair Using Tendon-Derived Stem Cells in Small Intestinal Submucosa via M2 Macrophage Polarization. Cells. 2022; 11(17):2770. https://doi.org/10.3390/cells11172770
Chicago/Turabian StyleMao, Xufeng, Liwei Yao, Mei Li, Xiqian Zhang, Bowen Weng, Weilai Zhu, Renhao Ni, Kanan Chen, Linhua Yi, Jiyuan Zhao, and et al. 2022. "Enhancement of Tendon Repair Using Tendon-Derived Stem Cells in Small Intestinal Submucosa via M2 Macrophage Polarization" Cells 11, no. 17: 2770. https://doi.org/10.3390/cells11172770
APA StyleMao, X., Yao, L., Li, M., Zhang, X., Weng, B., Zhu, W., Ni, R., Chen, K., Yi, L., Zhao, J., & Mao, H. (2022). Enhancement of Tendon Repair Using Tendon-Derived Stem Cells in Small Intestinal Submucosa via M2 Macrophage Polarization. Cells, 11(17), 2770. https://doi.org/10.3390/cells11172770






