Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling
Abstract
:1. Introduction
2. Materials and Methods
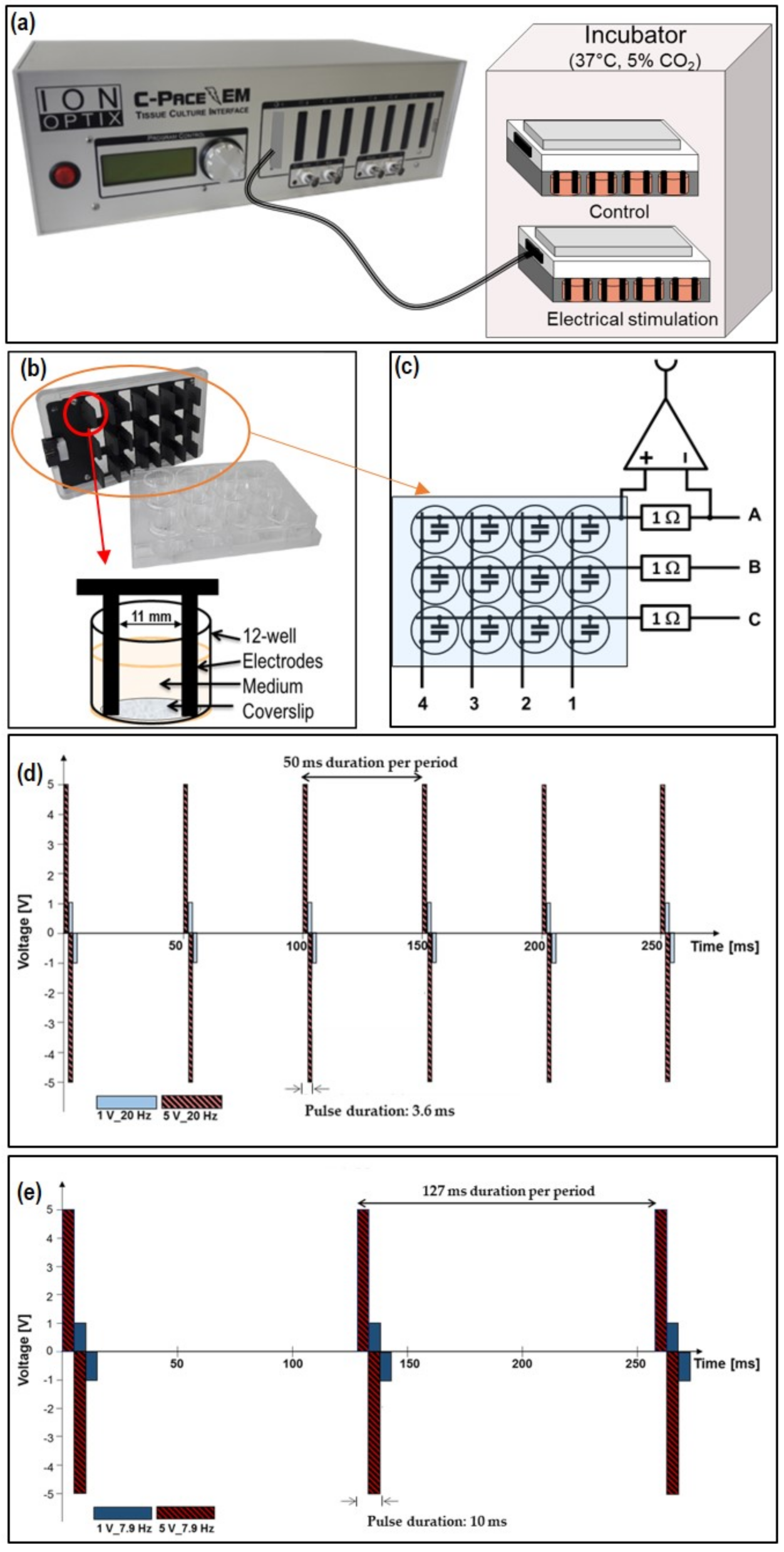
2.1. Electrical Stimulation (ES) Chamber C-Pace EM 100
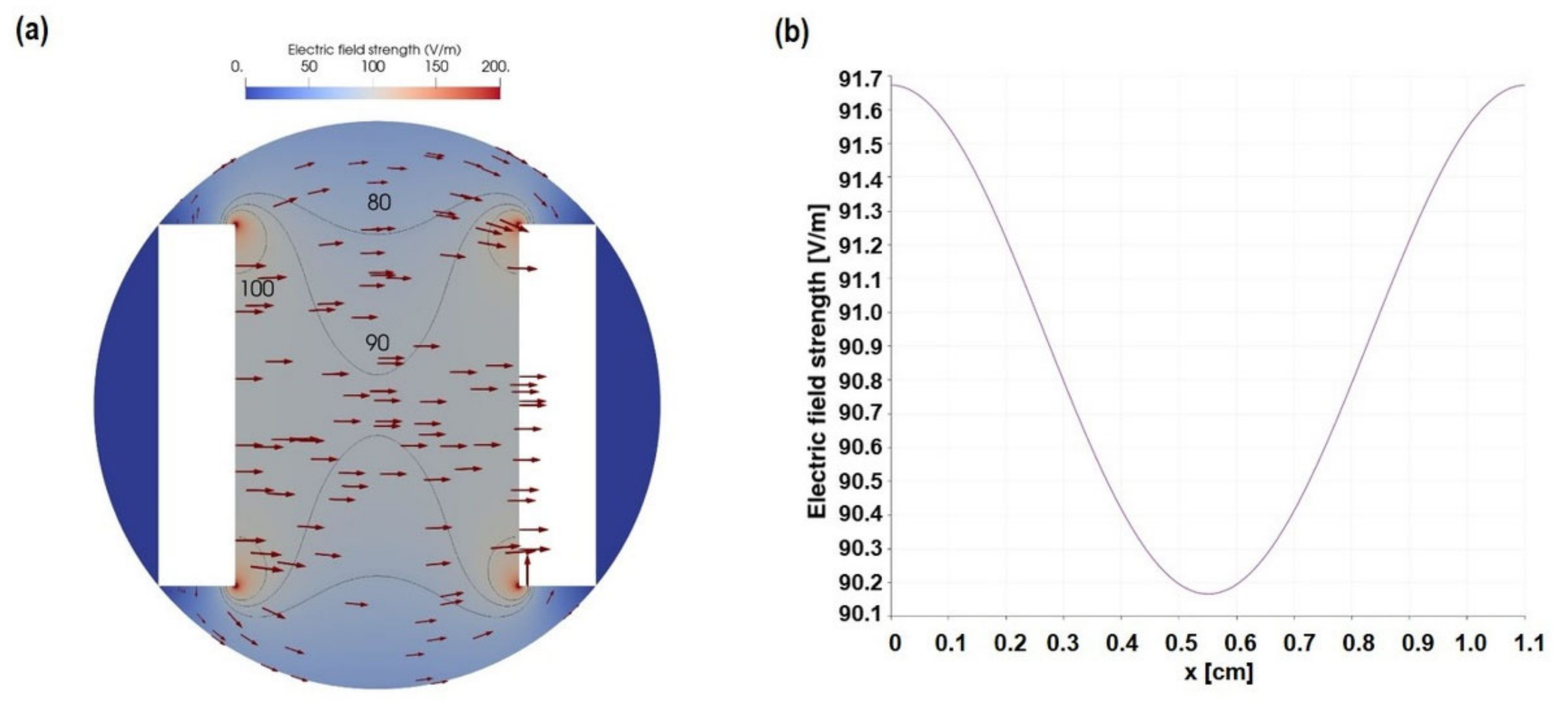
2.2. Calculation and Simulation of ES
2.3. Current Measurement
2.4. Characterization of AC-Stimulated Liquid
2.5. Cell Culture
2.6. AC-Activated Medium and Online Monitoring of Cell Adhesion
2.7. AC stimulation of Cells and Initial Adhesion
2.8. Cell Morphology and Spreading
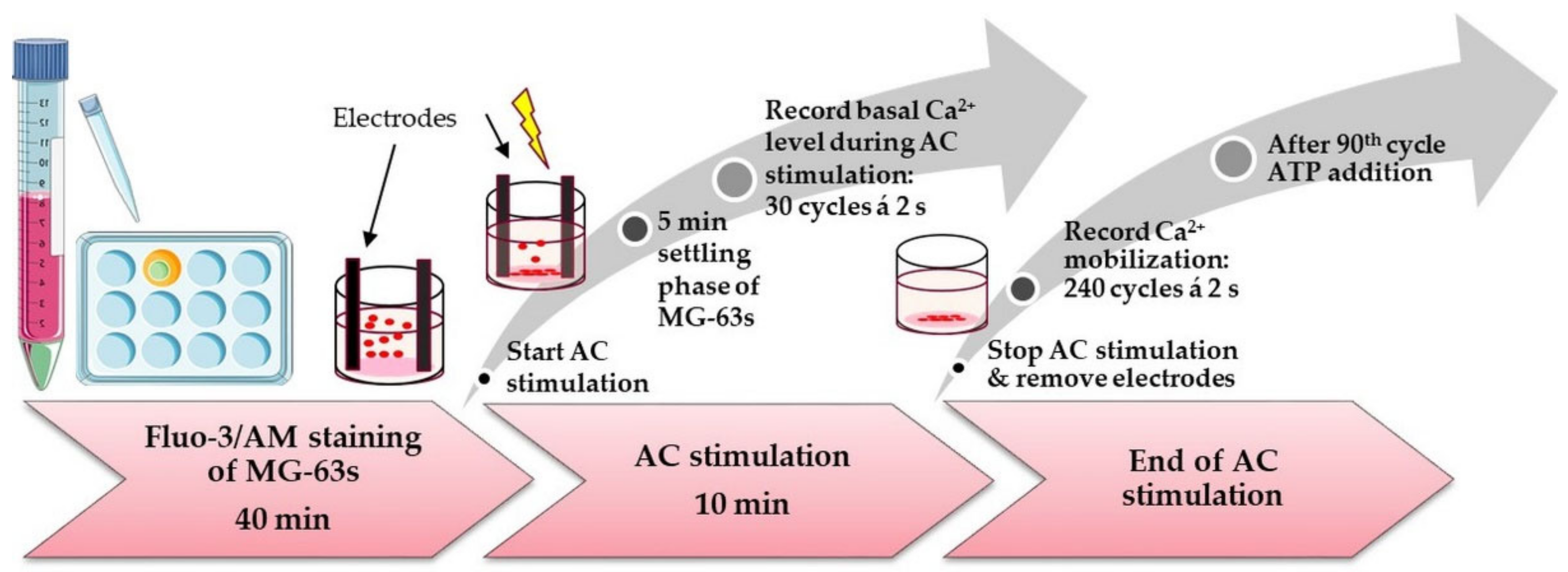
2.9. Calcium Mobilization and AC stimulation
2.10. Cellular Reactive Oxygen Species (ROS)
2.11. Statistics
3. Results
3.1. Characterization of AC Electrical Stimulation (ES) Parameter
3.2. Acellular Medium Characteristics—AC-Stimulated Liquid
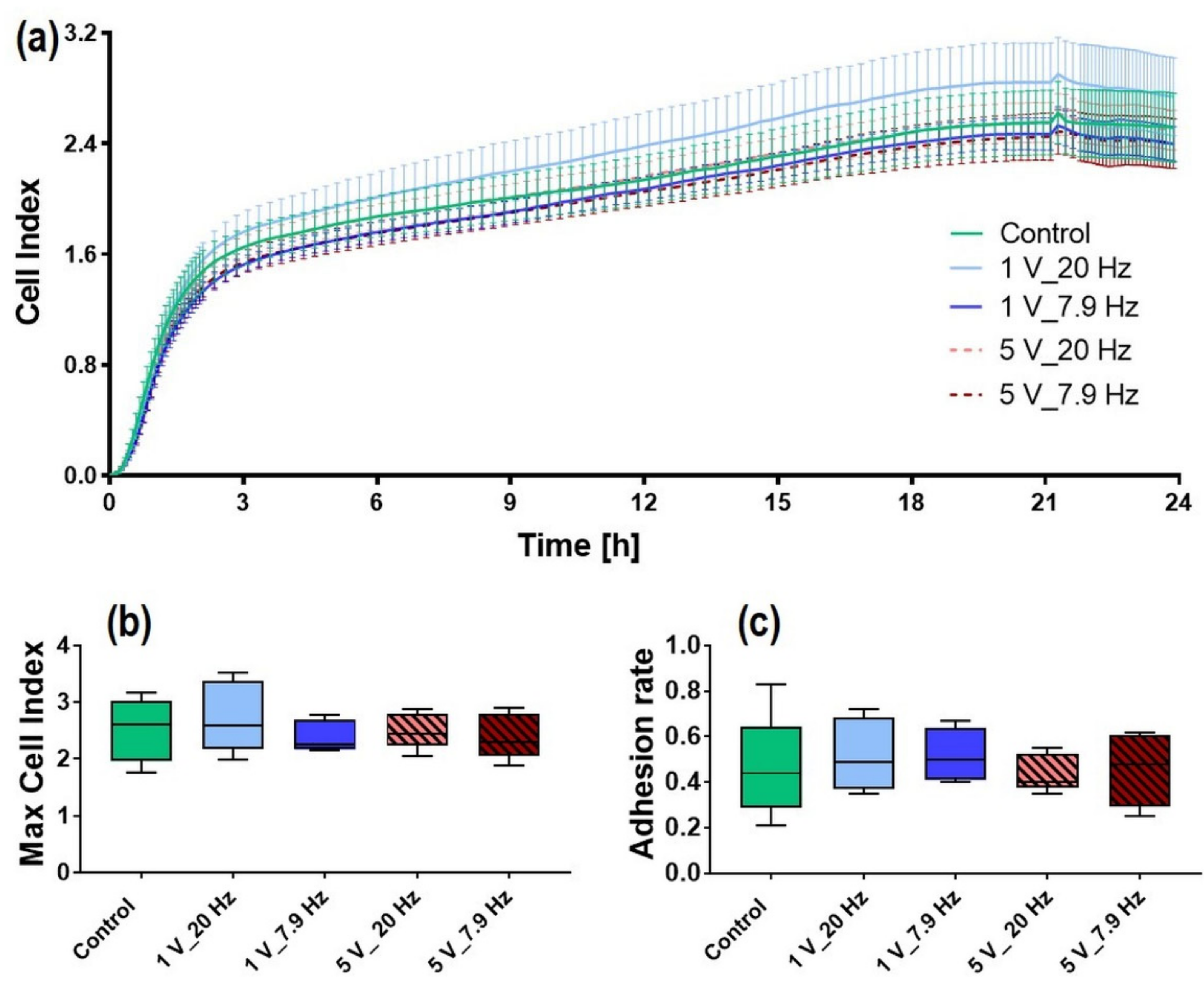
3.3. AC-Stimulated Liquids and Long-Time Cell Adhesion
3.4. AC-Stimulated Cells
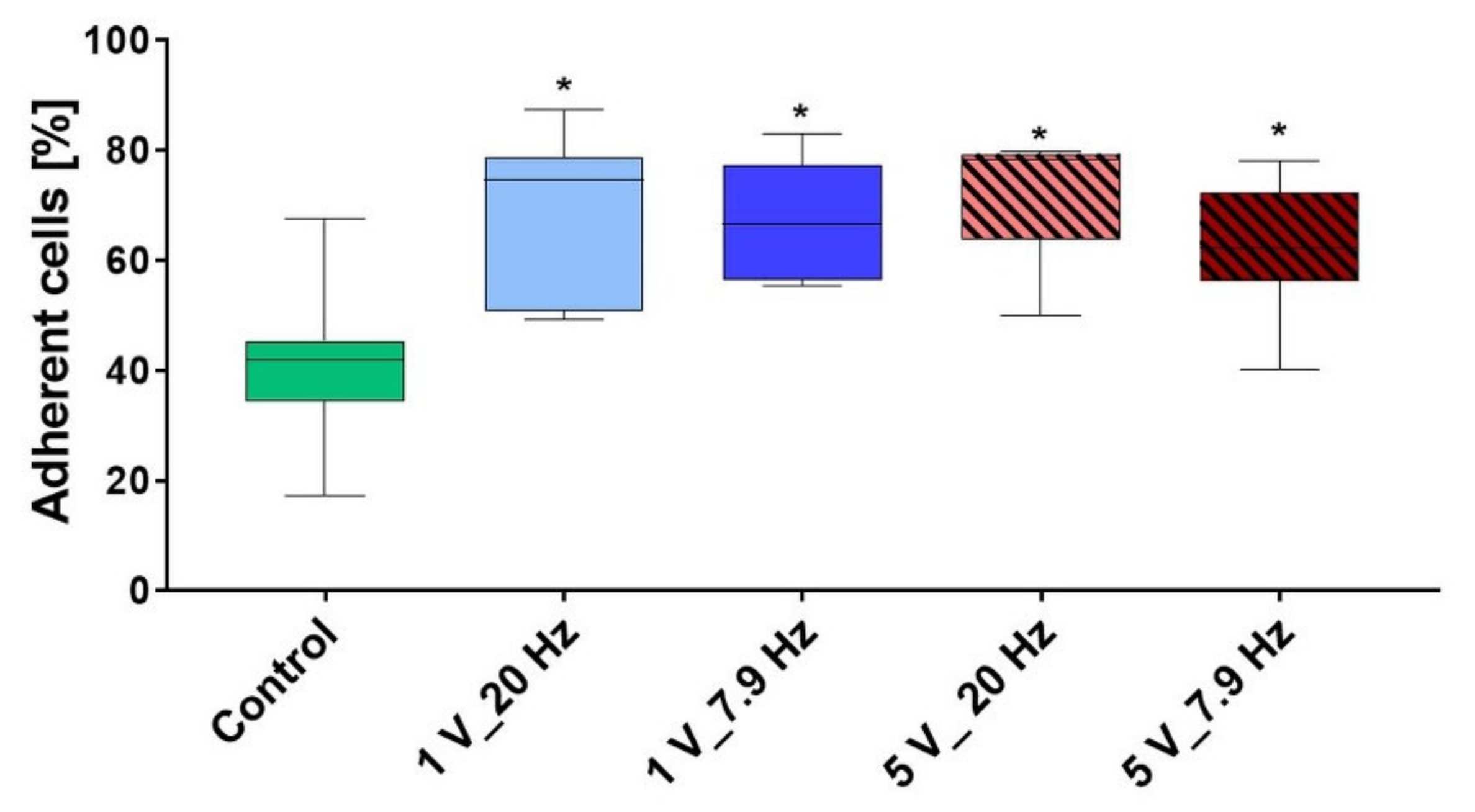
3.4.1. Initial Cell Adhesion
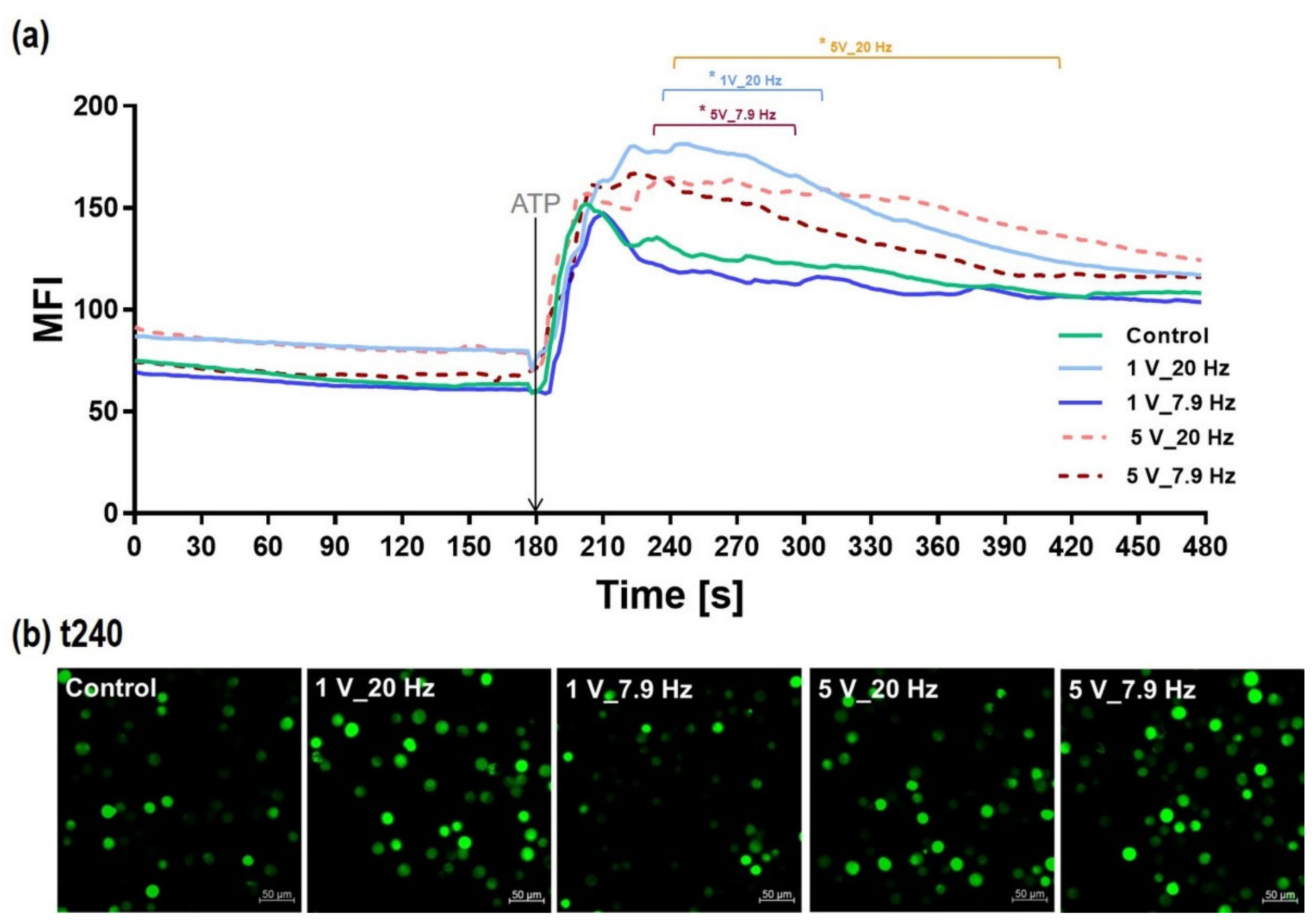
3.4.2. Intracellular Calcium Ion (Ca2+) Level during AC stimulation
3.4.3. Calcium Ion Mobilization in AC-Pre-Stimulated Cells
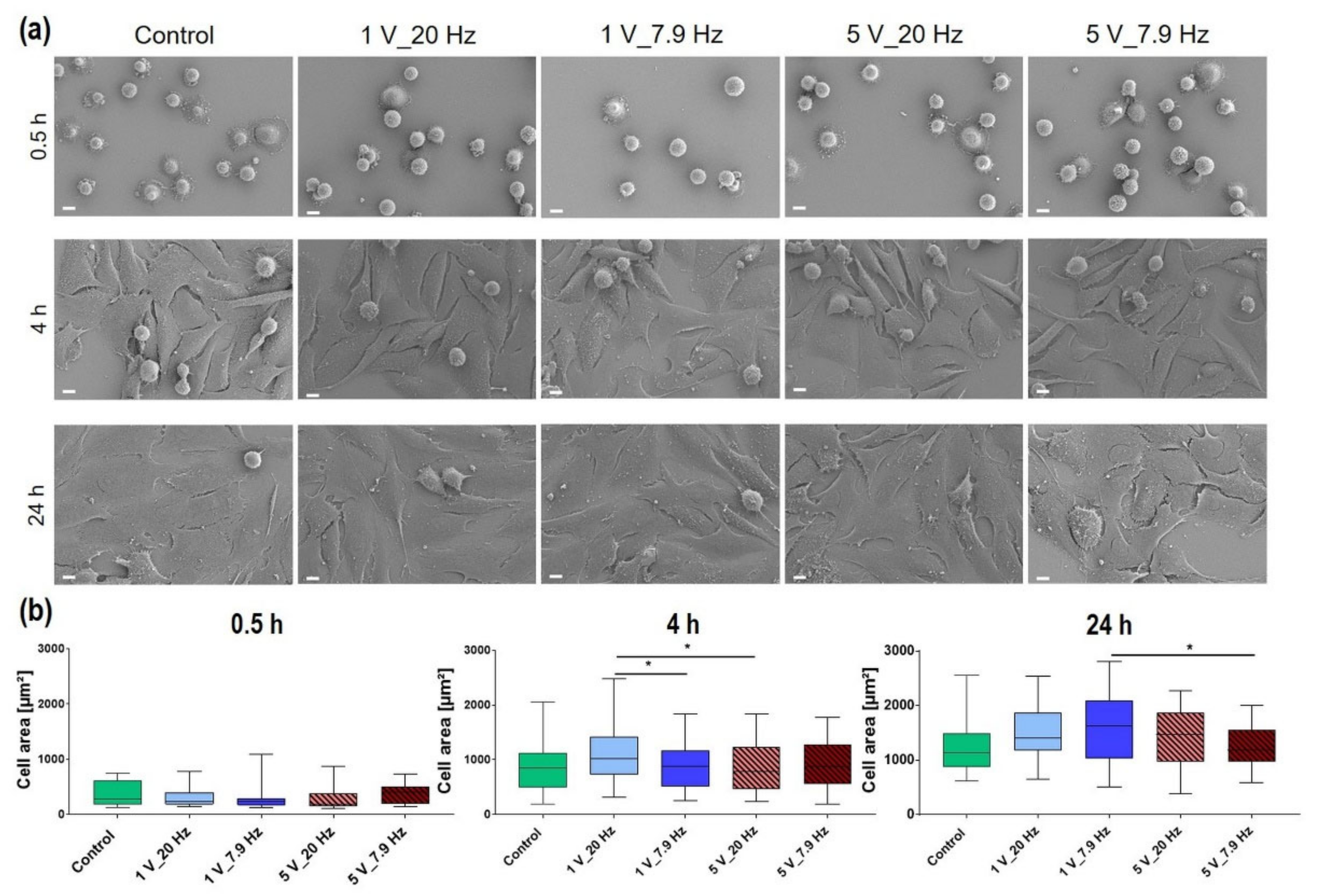
3.4.4. Cell Morphology
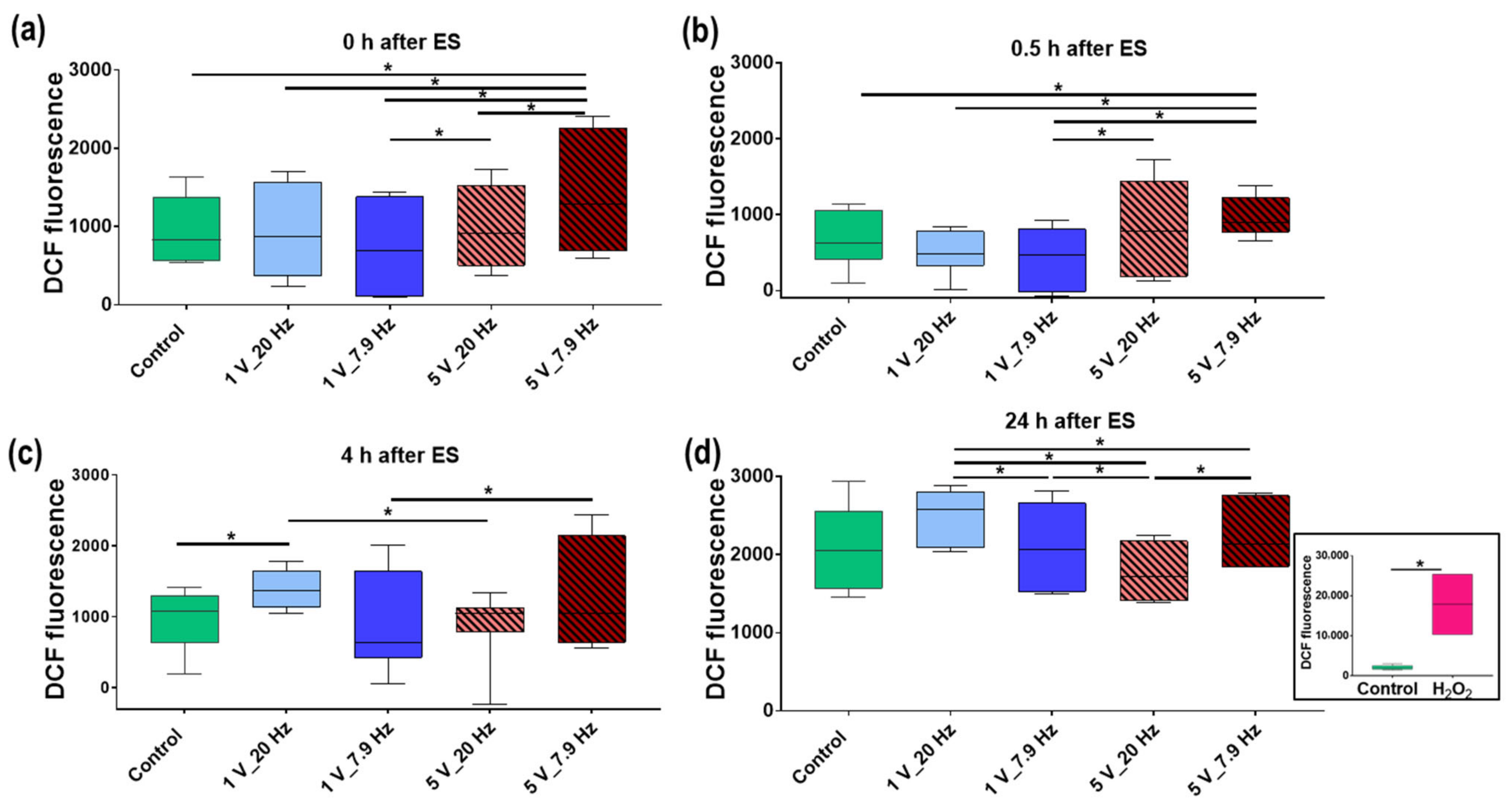
3.4.5. Reactive Oxygen Species (ROS) Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffin, M.; Bayat, A. Electrical Stimulation in Bone Healing: Critical Analysis by Evaluating Levels of Evidence. Eplasty 2011, 11, e34. [Google Scholar]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Bassett, C.A.; Becker, R.O. Generation of Electric Potentials by Bone in Response to Mechanical Stress. Science 1962, 137, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.; Anderson, J.; Ortiz-Catalan, M. Electrical stimulation to promote osseointegration of bone anchoring implants: A topical review. J. Neuro Eng. Rehabil. 2022, 19, 31. [Google Scholar] [CrossRef]
- O’Hearn, S.F.; Ackerman, B.J.; Mower, M.M. Paced monophasic and biphasic waveforms alter transmembrane potentials and metabolism of human fibroblasts. Biochem. Biophys. Rep. 2016, 8, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Khitrin, A.J.; Khitrin, K.A.; Model, M.A. A model for membrane potential and intracellular ion distribution. Chem. Phys. Lipids 2014, 184, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Love, M.R.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of electrical stimulation on cell proliferation and apoptosis. J. Cell. Physiol. 2018, 233, 1860–1876. [Google Scholar] [CrossRef] [PubMed]
- Pangalos, M.; Bintig, W.; Schlingmann, B.; Feyerabend, F.; Witte, F.; Begandt, D.; Heisterkamp, A.; Ngezahayo, A. Action potentials in primary osteoblasts and in the MG-63 osteoblast-like cell line. J. Bioenerg. Biomembr. 2011, 43, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Rebl, H.; Finke, B.; Schmidt, J.; Mohamad, H.S.; Ihrke, R.; Helm, C.A.; Nebe, J.B. Accelerated cell-surface interlocking on plasma polymer-modified porous ceramics. Mater. Sci. Eng. C Mater Biol. Appl. 2016, 69, 1116–1124. [Google Scholar] [CrossRef]
- Kirson, E.D.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef] [Green Version]
- Ercan, B.; Webster, T.J. Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int. J. Nanomed. 2008, 3, 477–485. [Google Scholar] [CrossRef]
- Bhavsar, M.B.; Cato, G.; Hauschild, A.; Leppik, L.; Oliveira, K.M.; Eischen-Loges, M.J.; Barker, J.H. Membrane potential (Vmem) measurements during mesenchymal stem cell (MSC) proliferation and osteogenic differentiation. PeerJ 2019, 7, e6341. [Google Scholar] [CrossRef]
- Ercan, B.; Webster, T.J. The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces. Biomaterials 2010, 31, 3684–3693. [Google Scholar] [CrossRef]
- Kloth, L.C. Electrical Stimulation Technologies for Wound Healing. Adv. Wound Care 2014, 3, 81–90. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Almeida, M. Gone with the Wnts: β-Catenin, T-Cell Factor, Forkhead Box O, and Oxidative Stress in Age-Dependent Diseases of Bone, Lipid, and Glucose Metabolism. Mol. Endocrinol. 2007, 21, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Pink, M.; Schmitz-Spanke, S. A new perspective on calmodulin-regulated calcium and ROS homeostasis upon carbon black nanoparticle exposure. Arch. Toxicol. 2021, 95, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Tandon, N.; Cannizzaro, C.; Figallo, E.; Voldman, J.; Vunjak-Novakovic, G. Characterization of Electrical Stimulation Electrodes for Cardiac Tissue Engineering. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 845–848. [Google Scholar] [CrossRef]
- Zimmermann, J.; Budde, K.; Arbeiter, N.; Molina, F.; Storch, A.; Uhrmacher, A.M.; van Rienen, U. Using a Digital Twin of an Electrical Stimulation Device to Monitor and Control the Electrical Stimulation of Cells in vitro. Front. Bioeng. Biotechnol. 2021, 9, 765516. [Google Scholar] [CrossRef]
- Budde, K.; Zimmermann, J.; Neuhaus, E.; Schroder, M.; Uhrmacher, A.M.; van Rienen, U. Requirements for Documenting Electrical Cell Stimulation Experiments for Replicability and Numerical Modeling. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 41, 1082–1088. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. Part A 2014, 102, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Artun, F.T.; Karagöz, A. Antiproliferative and apoptosis inducing effects of the methanolic extract of Centaurea hermannii in human cervical cancer cell line. Biotech. Histochem. 2021, 96, 1–10. [Google Scholar] [CrossRef]
- Bergemann, C.; Waldner, A.-C.; Emmert, S.; Nebe, J.B. The Hyaluronan Pericellular Coat and Cold Atmospheric Plasma Treatment of Cells. Appl. Sci. 2020, 10, 5024. [Google Scholar] [CrossRef]
- Staehlke, S.; Oster, P.; Seemann, S.; Kruse, F.; Brief, J.; Nebe, B. Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation. Materials 2022, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Koertge, A.; Nebe, B. Intracellular calcium dynamics dependent on defined microtopographical features of titanium. Biomaterials 2015, 46, 48–57. [Google Scholar] [CrossRef]
- Schröder, M.; Staehlke, S.; Groth, P.; Nebe, J.B.; Spors, S.; Krüger, F. Structure-based knowledge acquisition from electronic lab notebooks for research data provenance documentation. J. Biomed. Semant. 2022, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Nebe, J.B. Research data of Calcium Imaging after electrical stimulation [Data set]. Zenodo 2021, 1, 1. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Gruening, M.; Neuber, S.; Nestler, P.; Lehnfeld, J.; Dubs, M.; Fricke, K.; Schnabelrauch, M.; Helm, C.A.; Müller, R.; Staehlke, S.; et al. Enhancement of Intracellular Calcium Ion Mobilization by Moderately but Not Highly Positive Material Surface Charges. Front. Bioeng. Biotechnol. 2020, 8, 1016. [Google Scholar] [CrossRef] [PubMed]
- Hoentsch, M.; Bussiahn, R.; Rebl, H.; Bergemann, C.; Eggert, M.; Frank, M.; von Woedtke, T.; Nebe, B. Persistent Effectivity of Gas Plasma-Treated, Long Time-Stored Liquid on Epithelial Cell Adhesion Capacity and Membrane Morphology. PLoS ONE 2014, 9, e104559. [Google Scholar] [CrossRef] [PubMed]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1995, 15, 585–591. [Google Scholar] [CrossRef]
- Jin, G.; Yang, G.-H.; Kim, G. Tissue engineering bioreactor systems for applying physical and electrical stimulations to cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Nicksic, P.J.; Donnelly, D.T.; Hesse, M.; Bedi, S.; Verma, N.; Seitz, A.J.; Shoffstall, A.J.; Ludwig, K.A.; Dingle, A.M.; Poore, S.O. Electronic Bone Growth Stimulators for Augmentation of Osteogenesis in In Vitro and In Vivo Models: A Narrative Review of Electrical Stimulation Mechanisms and Device Specifications. Front. Bioeng. Biotechnol. 2022, 10, 793945. [Google Scholar] [CrossRef]
- Aleem, I.S.; Aleem, I.; Evaniew, N.; Busse, J.W.; Yaszemski, M.; Agarwal, A.; Einhorn, T.; Bhandari, M. Efficacy of Electrical Stimulators for Bone Healing: A Meta-Analysis of Randomized Sham-Controlled Trials. Sci. Rep. 2016, 6, 31724. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef]
- Haglin, J.M.; Jain, S.; Eltorai, A.E.; Daniels, A.H. Bone Growth Stimulation: A critical analysis review. JBJS Rev. 2017, 5, e8. [Google Scholar] [CrossRef] [PubMed]
- Brighton, C.T.; Wang, W.; Seldes, R.; Zhang, G.H.; Pollack, S.R. Signal Transduction in Electrically Stimulated Bone Cells. J. Bone Jt. Surg. 2001, 83, 1514–1523. [Google Scholar] [CrossRef]
- Bydak, B.; Pierdoná, T.M.; Seif, S.; Sidhom, K.; Obi, P.O.; Labouta, H.I.; Gordon, J.W.; Saleem, A. Characterizing Extracellular Vesicles and Particles Derived from Skeletal Muscle Myoblasts and Myotubes and the Effect of Acute Contractile Activity. Membranes 2022, 12, 464. [Google Scholar] [CrossRef]
- Taghian, T.; Narmoneva, D.A.; Kogan, A.B. Modulation of cell function by electric field: A high-resolution analysis. J. R. Soc. Interface 2015, 12, 20150153. [Google Scholar] [CrossRef]
- Asadi, M.R.; Torkaman, G. Bacterial Inhibition by Electrical Stimulation. Adv. Wound Care 2014, 3, 91–97. [Google Scholar] [CrossRef]
- Contu, F.; Elsener, B.; Böhni, H. Characterization of implant materials in fetal bovine serum and sodium sulfate by electrochemical impedance spectroscopy. I. Mechanically polished samples. J. Biomed. Mater. Res. 2002, 62, 412–421. [Google Scholar] [CrossRef]
- Aaron, R.K.; Ciombor, D.M.; Wang, S.; Simon, B. Clinical Biophysics: The Promotion of Skeletal Repair by Physical Forces. Ann. N. Y. Acad. Sci. 2006, 1068, 513–531. [Google Scholar] [CrossRef]
- Dauben, T.J.; Ziebart, J.; Bender, T.; Zaatreh, S.; Kreikemeyer, B.; Bader, R. A Novel In Vitro System for Comparative Analyses of Bone Cells and Bacteria under Electrical Stimulation. BioMed Res. Int. 2016, 2016, 5178640. [Google Scholar] [CrossRef]
- Reissis, Y.; García-Gareta, E.; Korda, M.; Blunn, G.W.; Hua, J. The effect of temperature on the viability of human mesenchymal stem cells. Stem Cell Res. Ther. 2013, 4, 139. [Google Scholar] [CrossRef] [Green Version]
- Srirussamee, K.; Mobini, S.; Cassidy, N.J.; Cartmell, S.H. Direct electrical stimulation enhances osteogenesis by inducing Bmp2 and Spp1 expressions from macrophages and preosteoblasts. Biotechnol. Bioeng. 2019, 116, 3421–3432. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical Stimulation in Tissue Regeneration. In Applied Biomedical Engineering; Gargiulo, G.D., McEwan, A., Eds.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Stephan, M.; Zimmermann, J.; Klinder, A.; Sahm, F.; Van Rienen, U.; Kämmerer, P.W.; Bader, R.; Jonitz-Heincke, A. Establishment and Evaluation of an In Vitro System for Biophysical Stimulation of Human Osteoblasts. Cells 2020, 9, 1995. [Google Scholar] [CrossRef]
- Leppik, L.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Bodhak, S.; Bose, S.; Kinsel, W.C.; Bandyopadhyay, A. Investigation of in vitro bone cell adhesion and proliferation on Ti using direct current stimulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 2163–2168. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhao, R.; Wang, C.; Qiu, F.; Sun, B.; Ji, H.; Qiu, J.; Wang, C. Enhanced adhesion and proliferation of human umbilical vein endothelial cells on conductive PANI-PCL fiber scaffold by electrical stimulation. Mater. Sci. Eng. C 2017, 72, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Gupta, S.D.; Basu, B. Optimization of electrical stimulation parameters for enhanced cell proliferation on biomaterial surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Sjaastad, M.D.; Nelson, W.J. Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. BioEssays 1997, 19, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Titushkin, I.; Cho, M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry 2006, 69, 133–141. [Google Scholar] [CrossRef]
- Katoh, K. Effects of Electrical Stimulation on the Signal Transduction-Related Proteins, c-Src and Focal Adhesion Kinase, in Fibroblasts. Life 2022, 12, 531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nune, K.C.; Misra, R. Understanding the response of pulsed electric field on osteoblast functions in three-dimensional mesh structures. J. Biomater. Appl. 2016, 31, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.D.; Daniels, R.L. Live-cell calcium imaging of adherent and non-adherent GL261 cells reveals phenotype-dependent differences in drug responses. BMC Cancer 2017, 17, 516. [Google Scholar] [CrossRef] [PubMed]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Y.; Lipsky, S.; Cho, M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007, 21, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Khatib, L.; Golan, D.E.; Cho, M. Physiologic electrical stimulation provokes intracellular calcium increase mediated by phospholipase C activation in human osteoblasts. FASEB J. 2004, 18, 1903–1905. [Google Scholar] [CrossRef]
- Zhou, P.; He, F.; Han, Y.; Liu, B.; Wei, S. Nanosecond pulsed electric field induces calcium mobilization in osteoblasts. Bioelectrochemistry 2018, 124, 7–12. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Hou, H.-S.; Sun, Y.-S.; Cheng, J.-Y.; Lo, K.-Y. Correlation between cell migration and reactive oxygen species under electric field stimulation. Biomicrofluidics 2015, 9, 054120. [Google Scholar] [CrossRef]
- Díaz-Vegas, A.; Campos, C.A.; Contreras-Ferrat, A.; Casas, M.; Buvinic, S.; Jaimovich, E.; Espinosa, A. ROS Production via P2Y1-PKC-NOX2 Is Triggered by Extracellular ATP after Electrical Stimulation of Skeletal Muscle Cells. PLoS ONE 2015, 10, e0129882. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahm, F.; Ziebart, J.; Jonitz-Heincke, A.; Hansmann, D.; Dauben, T.; Bader, R. Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes. Int. J. Mol. Sci. 2020, 21, 6944. [Google Scholar] [CrossRef] [PubMed]
- Bagne, L.; Oliveira, M.A.; Pereira, A.T.; Caetano, G.F.; Oliveira, C.A.; Aro, A.A.; Chiarotto, G.B.; Santos, G.M.T.; Mendonça, F.A.S.; Santamaria-Jr, M. Electrical therapies act on the Ca2+/CaM signaling pathway to enhance bone regeneration with bioactive glass [S53P4] and allogeneic grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2104–2116. [Google Scholar] [CrossRef] [PubMed]



| Voltage [V] | Frequency [Hz] | Pulse Duration [ms] | Electric Field [V/m] * | Theoretical Electric Current [A] * | Measured Electric Current [A] ** | Code | |
|---|---|---|---|---|---|---|---|
| Control | - | - | - | - | - | - | |
| 1 V_20 Hz | 1 V | 20 | 3.6 | 90 | 0.0077 | 0.012 | |
| 1 V_7.9 Hz | 1 V | 7.9 | 10 | 90 | 0.0077 | 0.012 | |
| 5 V_20 Hz | 5 V | 20 | 3.6 | 450 | 0.0385 | 0.067 | |
| 5 V_7.9 Hz | 5 V | 7.9 | 10 | 450 | 0.0385 | 0.069 |
| Time after AC Stimulation | Control (in µm2) | 1 V_20 Hz (in µm2) | 1 V_7.9 Hz (in µm2) | 5 V_20 Hz (in µm2) | 5 V_7.9 Hz (in µm2) |
|---|---|---|---|---|---|
| 0.5 h | 319 ± 5.1 | 295 ± 4.9 | 311.3 ± 10.0 | 322.6 ± 7.1 | 375.4 ± 5.7 |
| 4 h | 903 ± 16.4 | 1075.9 ± 16.3 | 947.7 ± 10.9 | 890.6 ± 12.2 | 955.7 ± 13.1 |
| 24 h | 1422.7 ± 14.2 | 1450.5 ± 17.4 | 1517.3 ± 23.2 | 1422.6 ± 23.7 | 1315.3 ± 11.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staehlke, S.; Bielfeldt, M.; Zimmermann, J.; Gruening, M.; Barke, I.; Freitag, T.; Speller, S.; Van Rienen, U.; Nebe, B. Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling. Cells 2022, 11, 2650. https://doi.org/10.3390/cells11172650
Staehlke S, Bielfeldt M, Zimmermann J, Gruening M, Barke I, Freitag T, Speller S, Van Rienen U, Nebe B. Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling. Cells. 2022; 11(17):2650. https://doi.org/10.3390/cells11172650
Chicago/Turabian StyleStaehlke, Susanne, Meike Bielfeldt, Julius Zimmermann, Martina Gruening, Ingo Barke, Thomas Freitag, Sylvia Speller, Ursula Van Rienen, and Barbara Nebe. 2022. "Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling" Cells 11, no. 17: 2650. https://doi.org/10.3390/cells11172650
APA StyleStaehlke, S., Bielfeldt, M., Zimmermann, J., Gruening, M., Barke, I., Freitag, T., Speller, S., Van Rienen, U., & Nebe, B. (2022). Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling. Cells, 11(17), 2650. https://doi.org/10.3390/cells11172650









