LL-37, a Multi-Faceted Amphipathic Peptide Involved in NETosis
Abstract
:1. Introduction
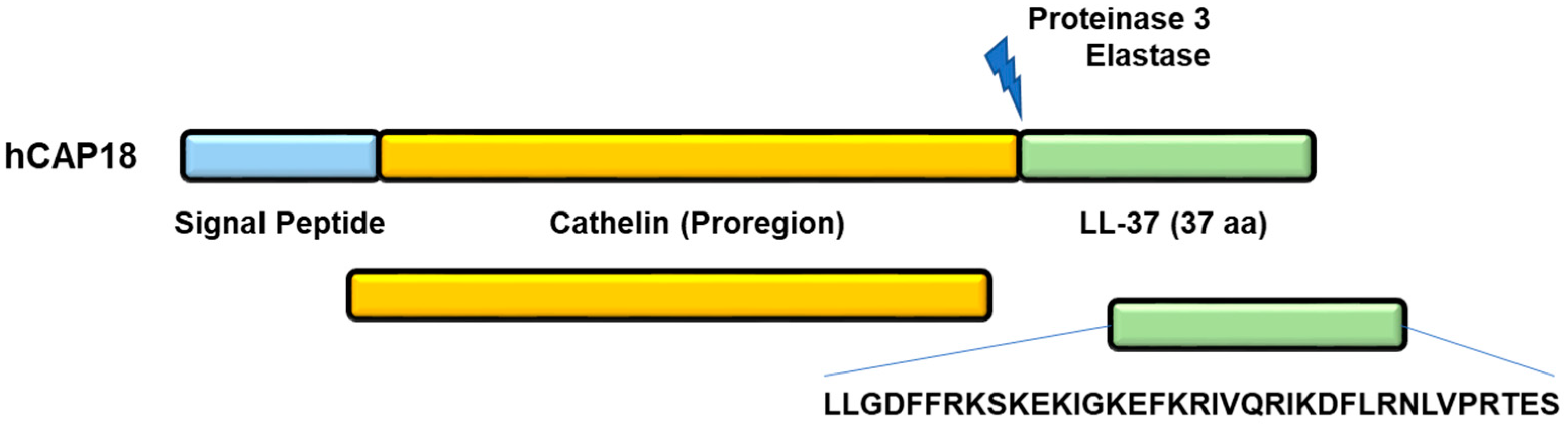
2. Generation of LL-37 Peptide
3. Structure of Peptide LL-37
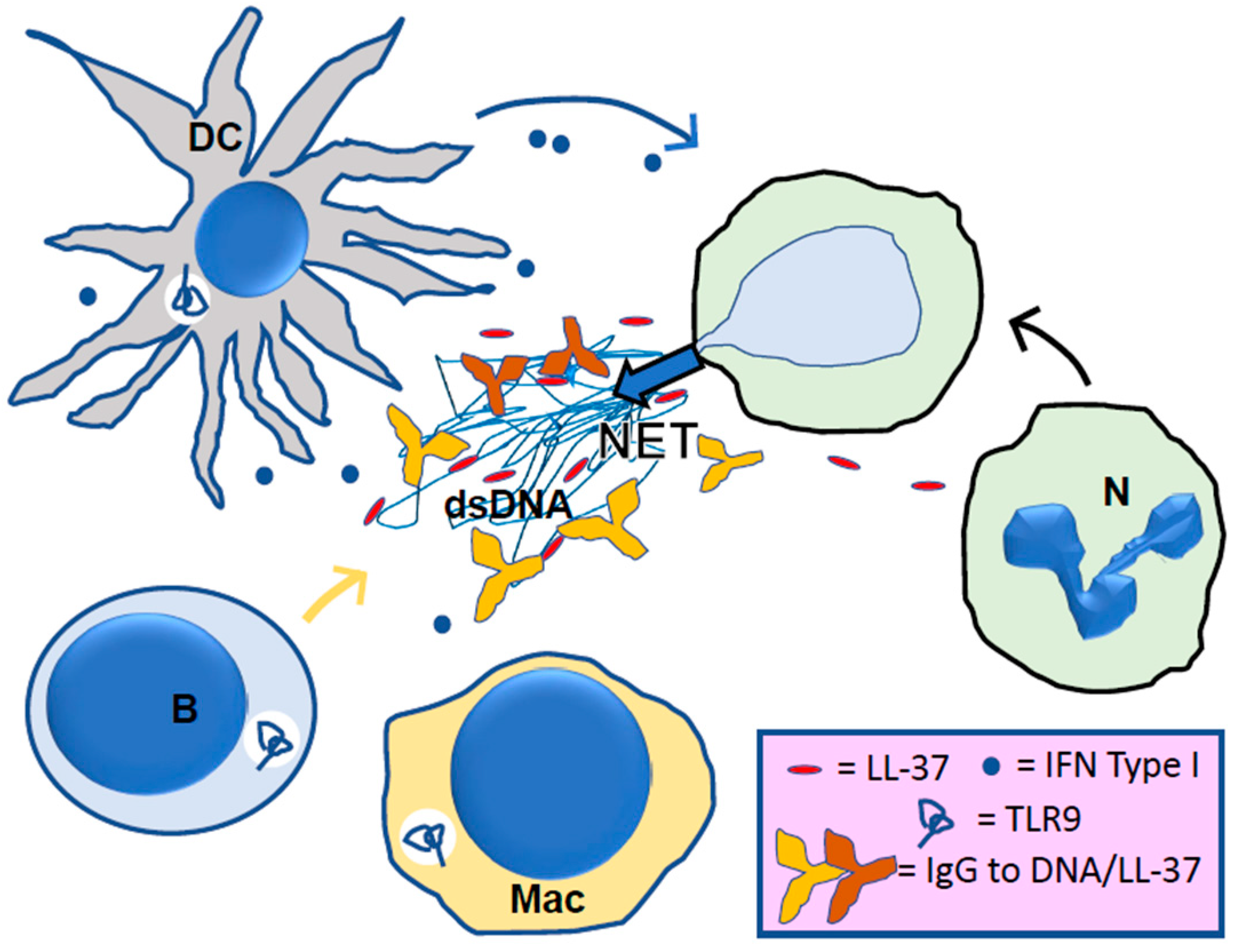
4. LL-37, Its Central Role in NETosis
5. LL-37 in Lupus
6. LL-37 in other Autoimmune and Inflammatory Settings
7. Final Comments and Driving Forces
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kahlenberg, J.M.; Kaplan, M.J. Little peptide, big effects: The role of LL-37 in inflammation and autoimmune disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Lakshmaiah, J.; Biswajit, N.; Yingxia, Z.; Fangyu, W.; Chunfeng, W.; Zarena, D.; Lushnikova, T.; Xiuqing, W. Design of antimicrobial peptides: Progress made with human cathelicidin LL-37. Adv. Exp. Med. Biol. 2019, 1117, 215–240. [Google Scholar] [PubMed]
- Biswas, D.; Ambalavanan, P.; Ravins, M.; Anand, A.; Sharma, A.; Lim, K.; Tan, R.; Lim, H.Y.; Sol, A.; Bachrach, G.; et al. LL-37-mediated activation of host receptors is critical for defense against group A streptococcal infection. Cell Rep. 2021, 34, 108766. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Zhang, X.; Li, M.; Zhang, Q.; Wang, Y. Antibacterial and anti-inflammatory properties of a novel antimicrobial peptide derived from LL-37. Antibiotics 2022, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef]
- Porcelli, F.; Verardi, R.; Shi, L.; Henzler-Wildman, K.A.; Ramamoorthy, A.; Veglia, G. NMR structure of the cathelicidin-derived human antimicrobial peptide LL-37 in dodecylphosphocholine micelles. Biochemistry 2008, 47, 5565–5572. [Google Scholar] [CrossRef] [Green Version]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [Green Version]
- Sancho-Vaello, E.; Gil-Carton, D.; François, P.; Bonetti, E.J.; Kreir, M.; Pothula, K.R.; Kleinekathöfer, U.; Zeth, K. The structure of the antimicrobial human cathelicidin LL-37 shows oligomerization and channel formation in the presence of membrane mimics. Sci. Rep. 2020, 10, 17356. [Google Scholar] [CrossRef]
- Engelberg, Y.; Landau, M. The Human LL-37(17-29) antimicrobial peptide reveals a functional supramolecular structure. Nat. Commun. 2020, 11, 3894. [Google Scholar] [CrossRef]
- Zeth, K.; Sancho-Vaello, E. Structural Plasticity of LL-37 indicates elaborate functional adaptation mechanisms to bacterial target structures. Int. J. Mol. Sci. 2021, 22, 5200. [Google Scholar] [CrossRef]
- Johansson, J.; Gudmundsson, G.H.; Rottenberg, M.E.; Berndt, K.D.; Agerberth, B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 1998, 273, 3718–3724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, S.; Li, D.; Chen, P.; Han, S.; Zhao, G.; Chen, Y.; Zhao, J.; Xiong, J.; Qiu, J.; et al. Human Cathelicidin Inhibits SARS-CoV-2 Infection: Killing Two Birds with One Stone. ACS Infect. Dis. 2021, 7, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Gracia, P.; Navarro, S.; Peña-Díaz, S.; Pujols, J.; Cremades, N.; Pallarès, I.; Ventura, S. α-Helical peptidic scaffolds to target α-synuclein toxic species with nanomolar affinity. Nat. Commun. 2021, 12, 3752. [Google Scholar] [CrossRef]
- Neumann, A.; Berends, E.T.; Nerlich, A.; Molhoek, E.M.; Gallo, R.L.; Meerloo, T.; Nizet, V.; Naim, H.Y.; von Köckritz-Blickwede, M. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem. J. 2014, 464, 3–11. [Google Scholar] [CrossRef]
- Neumann, A.; Völlger, L.; Berends, E.T.; Molhoek, E.M.; Stapels, D.A.; Midon, M.; Friães, A.; Pingoud, A.; Rooijakkers, S.H.; Gallo, R.L.; et al. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J. Innate Immun. 2014, 6, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef]
- Herster, F.; Bittner, Z.; Archer, N.K.; Dickhöfer, S.; Eisel, D.; Eigenbrod, T.; Knorpp, T.; Schneiderhan-Marra, N.; Löffler, M.W.; Kalbacher, H.; et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat. Commun. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lande, R.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; Bassett, R. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Angarita, A.; Aragón, C.C.; Tobón, G.J. Cathelicidin LL-37: A new important molecule in the pathophysiology of systemic lupus erythematosus. J. Transl. Autoimmun. 2019, 3, 100029. [Google Scholar] [CrossRef]
- Gestermann, N.; Di Domizio, J.; Lande, R.; Demaria, O.; Frasca, L.; Feldmeyer, L.; Di Lucca, J.; Gilliet, M. Netting neutrophils activate autoreactive B cells in lupus. J. Immunol. 2018, 200, 3364–3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory role of the antimicrobial ll-37 peptide in autoimmune diseases and viral infections. Vaccines 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, J.; Absalón-Aguilar, A.; Nuñez-Aguirre, M.; Pérez-Fragoso, A.; Carrillo-Vázquez, D.A.; Maravillas-Montero, J.L.; Mejía-Domínguez, N.R.; Llorente, L.; Alcalá-Carmona, B.; Lira-Luna, J.; et al. Neutrophil Extracellular Traps Contribute to COVID-19 Hyperinflammation and Humoral Autoimmunity. Cells 2021, 10, 2545. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Adhikarakunnathu, S.; Bhardwaj, K.; Ranjith-Kumar, C.T.; Wen, Y.; Jordan, J.L.; Wu, L.H.; Dragnea, B.; Mateo, L.S.; Kao, C.C. LL37 and cationic peptides enhance TLR3 signaling by viral double-stranded RNAs. PLoS ONE 2011, 6, e26632. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Horton, C.G.; Farris, A.D. Toll-like receptors in systemic lupus erythematosus: Potential targets for therapeutic intervention. Curr. Allergy Asthma Rep. 2012, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kienhöfer, D.; Hahn, J.; Stoof, J.; Csepregi, J.Z.; Reinwald, C.; Urbonaviciute, V.; Johnsson, C.; Maueröder, C.; Podolska, M.J.; Biermann, M.H.; et al. Experimental lupus is aggravated in mouse strains with impaired induction of neutrophil extracellular traps. JCI Insight 2017, 2, e92920. [Google Scholar] [CrossRef] [Green Version]
- Fillatreau, S.; Manfroi, B.; Dörner, T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Jaouhar, M.; Skrygan, M.; Tigges, C.; Stücker, M.; Altmeyer, P.; Gläser, R.; Gambichler, T. Expression of antimicrobial peptides in different subtypes of cutaneous lupus erythematosus. J. Am. Acad. Dermatol. 2011, 65, 125–133. [Google Scholar] [CrossRef]
- Kienhöfer, D.; Hahn, J.; Schubert, I.; Reinwald, C.; Ipseiz, N.; Lang, S.C.; Borràs, B.; Amann, K.; Sjöwall, C.; Barron, A.E.; et al. No evidence of pathogenic involvement of cathelicidins in patient cohorts and mouse models of lupus and arthritis. PLoS ONE 2014, 9, e115474. [Google Scholar] [CrossRef] [PubMed]
- Sahebari, M.; Roshandel, G.; Saadati, N.; Saghafi, M.; Abdolahi, N.; Rezaieyazdi, Z. Cathelicidin (LL-37) and its correlation with pro-oxidant, antioxidant balance and disease activity in systemic lupus erythematosus: A cross-sectional human study. Lupus 2017, 26, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Koro, C.; Hellvard, A.; Delaleu, N.; Binder, V.; Scavenius, C.; Bergum, B.; Główczyk, I.; Roberts, H.M.; Chapple, I.L.; Grant, M.M.; et al. Carbamylated LL-37 as a modulator of the immune response. Innate Immun. 2016, 22, 218–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lande, R.; Palazzo, R.; Hammel, P.; Pietraforte, I.; Surbeck, I.; Gilliet, M.; Chizzolini, C.; Frasca, L. Generation of monoclonal antibodies specific for native LL37 and citrullinated LL37 that discriminate the two LL37 forms in the skin and circulation of cutaneous/systemic lupus erythematosus and rheumatoid arthritis patients. Antibodies 2020, 9, 14. [Google Scholar] [CrossRef]
- Lu, X.; Tang, Q.; Lindh, M.; Dastmalchi, M.; Alexanderson, H.; Silwerfeldt, K.P.; Agerberth, B.; Lundberg, I.E.; Wick, C. The host defense peptide LL-37 a possible inducer of the type I interferon system in patients with polymyositis and dermatomyositis. J. Autoimmun. 2017, 78, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Negreros, M.; Flores-Suárez, L.F. A proposed role of neutrophil extracellular traps and their interplay with fibroblasts in ANCA-associated vasculitis lung fibrosis. Autoimmun. Rev. 2021, 20, 102781. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Alessandrini, V.; Hardisty, G.; Melrose, L.; Jackson-Jones, L.; MacDonald, A.S.; Davidson, D.J.; Gwyer Findlay, E. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat. Commun. 2021, 12, 1285. [Google Scholar] [CrossRef]
- Säll, J.; Carlsson, M.; Gidlöf, O.; Holm, A.; Humlén, J.; Öhman, J.; Svensson, D.; Nilsson, B.-O.; Jönsson, D. The antimicrobial peptide LL-37 alters human osteoblast Ca2+ handling and induces Ca2+-independent apoptosis. J. Innate Immun. 2013, 5, 290–300. [Google Scholar] [CrossRef]
- Parackova, Z.; Zentsova, I.; Vrabcova, P.; Klocperk, A.; Sumnik, Z.; Pruhova, S.; Petruzelkova, L.; Hasler, R.; Sediva, A. Neutrophil Extracellular Trap Induced Dendritic Cell Activation Leads to Th1 Polarization in Type 1 Diabetes. Front. Immunol. 2020, 11, 661. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, M.; Yang, Z.; Pan, X.; Zhang, Y.; Wang, Q.; Xiao, W. LL-37 secreted by epithelium promotes fibroblast collagen production: A potential mechanism of small airway remodeling in chronic obstructive pulmonary disease. Lab. Investig. 2014, 94, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Zhang, Y.; Zhu, Y.; Li, C.; Zhou, L.; Li, X.; Zhang, F.; Qiu, X.; Qu, Y. Cathelicidin induces epithelial-mesenchymal transition to promote airway remodeling in smoking-related chronic obstructive pulmonary disease. Ann. Transl. Med. 2021, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Qi, R.; Jordan, J.L.; San Mateo, L.; Kao, C.C. The human antimicrobial peptide LL-37, but not the mouse ortholog, mCRAMP, can stimulate signaling by poly(I:C) through a FPRL1-dependent pathway. J. Biol. Chem. 2013, 288, 8258–8268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, O.E.; Clemmensen, S.N.; Dahl, S.L.; Dahl, S.L.; Østergaard, O.; Heegaard, N.H.; Glenthøj, A.; Nielsen, F.C.; Borregaard, N. Papillon-Lefèvre syndrome patient reveals species-dependent requirements for neutrophil defenses. J. Clin. Investig. 2014, 124, 4539–4548. [Google Scholar] [CrossRef] [Green Version]
- Puklo, M.; Guentsch, A.; Hiemstra, P.S.; Eick, S.; Potempa, J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol. Immunol. 2008, 23, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhou, Y.; Ren, B.; Zou, L.; He, B.; Li, M. The Role of Neutrophil Extracellular Traps in Periodontitis. Front. Cell Infect. Microbiol. 2021, 11, 639144. [Google Scholar] [CrossRef]
- Stricher, F.; Macri, C.; Ruff, M.; Muller, S. HSPA8/HSC70 chaperone protein: Structure, function, and chemical targeting. Autophagy 2013, 9, 1937–1954. [Google Scholar] [CrossRef] [Green Version]
- Toto, A.; Malagrinò, F.; Visconti, L.; Troilo, F.; Pagano, L.; Brunori, M.; Jemth, P.; Gianni, S. Templated folding of intrinsically disordered proteins. J. Biol. Chem. 2020, 295, 6586–6593. [Google Scholar] [CrossRef] [Green Version]
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef] [Green Version]
- Aloul, K.M.; Nielsen, J.E.; Defensor, E.B.; Lin, J.S.; Fortkort, J.A.; Shamloo, M.; Cirillo, J.D.; Gombart, A.F.; Barron, A.E. Upregulating Human Cathelicidin Antimicrobial Peptide LL-37 Expression May Prevent Severe COVID-19 Inflammatory Responses and Reduce Microthrombosis. Front. Immunol. 2022, 13, 880961. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radic, M.; Muller, S. LL-37, a Multi-Faceted Amphipathic Peptide Involved in NETosis. Cells 2022, 11, 2463. https://doi.org/10.3390/cells11152463
Radic M, Muller S. LL-37, a Multi-Faceted Amphipathic Peptide Involved in NETosis. Cells. 2022; 11(15):2463. https://doi.org/10.3390/cells11152463
Chicago/Turabian StyleRadic, Marko, and Sylviane Muller. 2022. "LL-37, a Multi-Faceted Amphipathic Peptide Involved in NETosis" Cells 11, no. 15: 2463. https://doi.org/10.3390/cells11152463
APA StyleRadic, M., & Muller, S. (2022). LL-37, a Multi-Faceted Amphipathic Peptide Involved in NETosis. Cells, 11(15), 2463. https://doi.org/10.3390/cells11152463





