Faster Serotonin Clearance in CA3 Region of Hippocampus and Antidepressant-like Effect of Decynium-22 in Juvenile Mice Are Putatively Linked to Increased Plasma Membrane Monoamine Transporter Function: Implications for Efficacy of Antidepressants in Juveniles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tail Suspension Test
2.3. High-Speed Chronoamperometry
2.4. Cell Density
2.5. Western Blot
2.6. Immunohistochemistry
2.7. Drugs
2.8. Data Analysis
3. Results
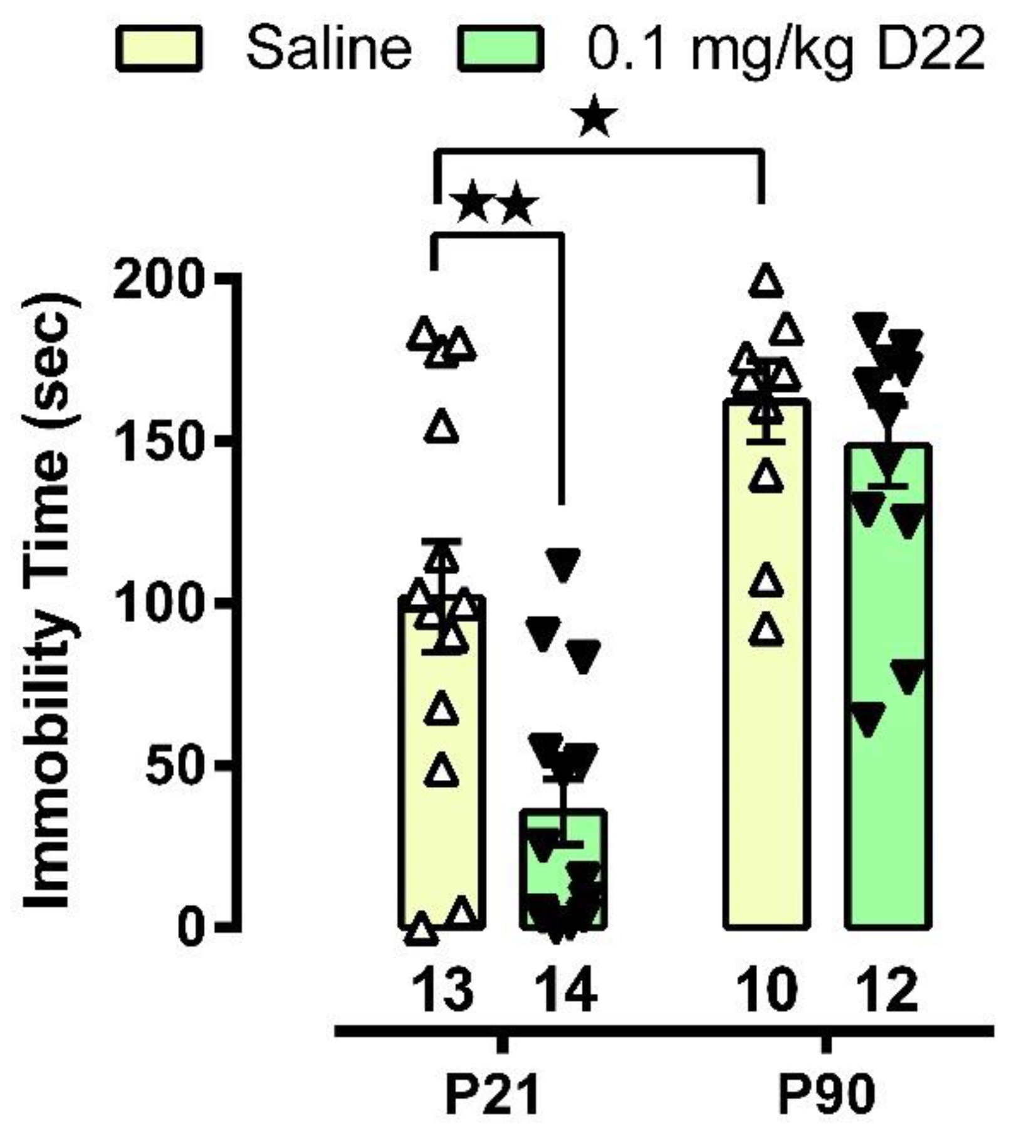
3.1. D22 Produces an Antidepressant-like Effect in Juvenile, but Not Adult, Mice
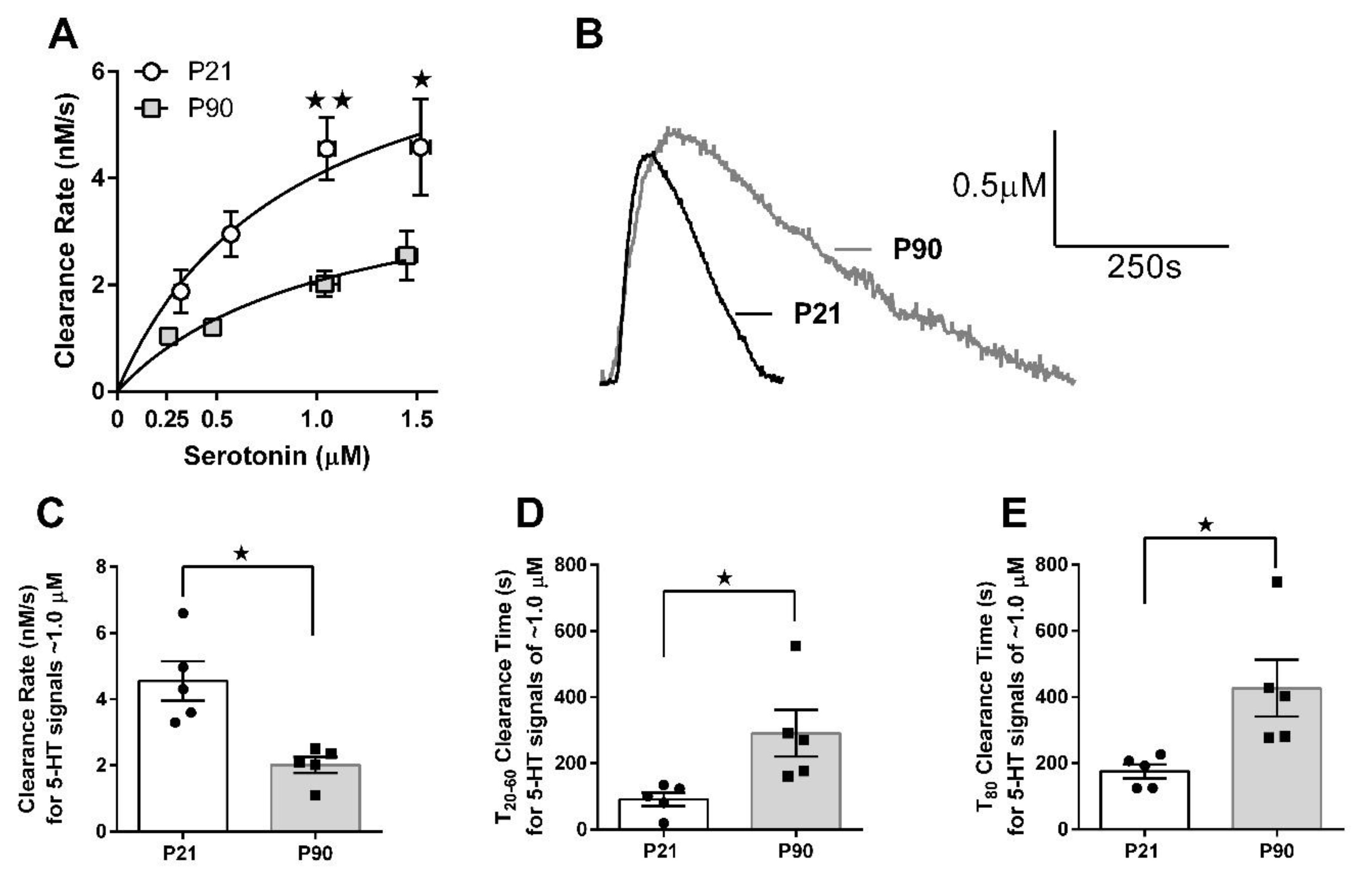
3.2. Juvenile Mice Clear Serotonin from Extracellular Fluid Faster than Adult Mice
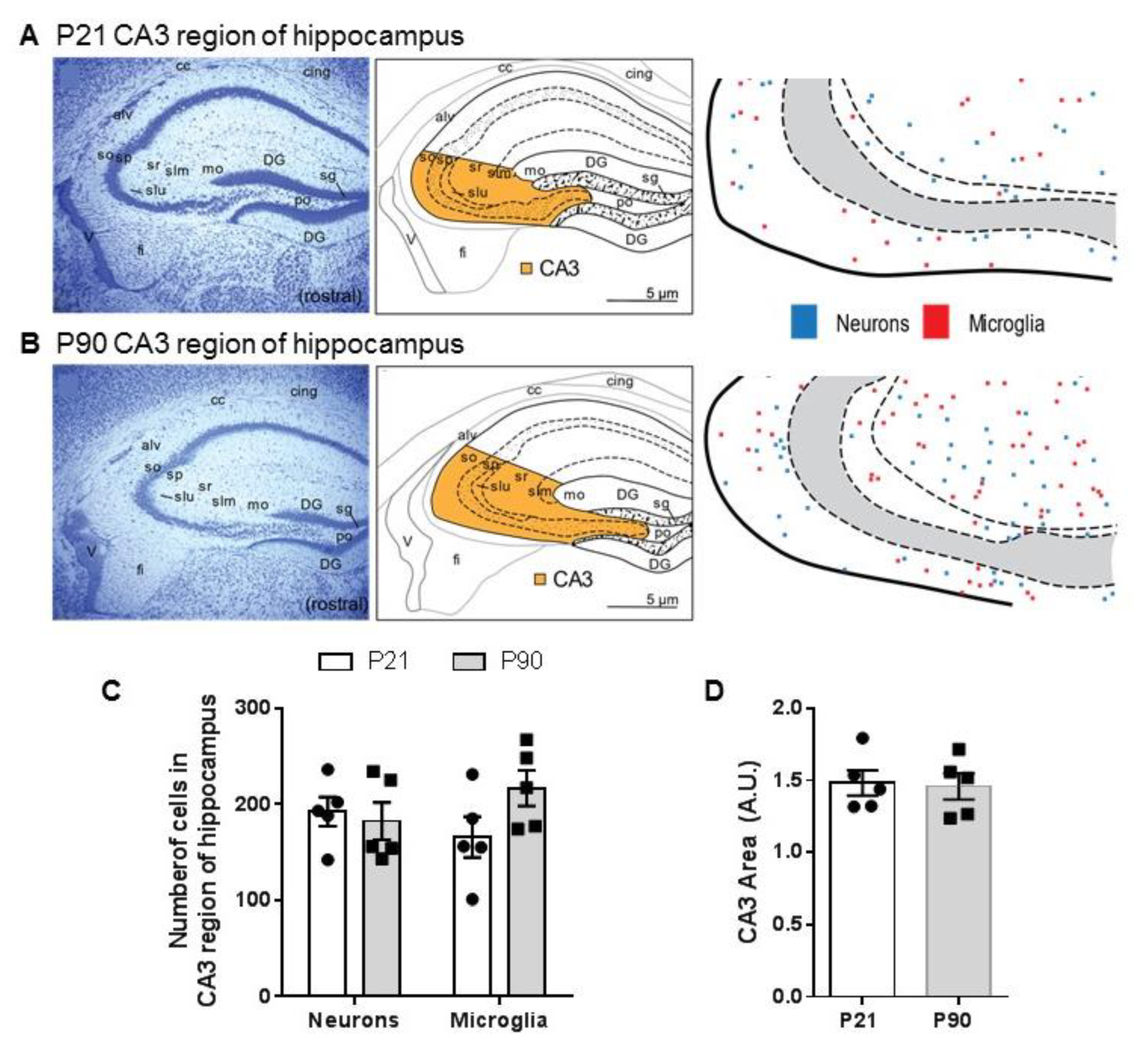
3.3. Density of Neurons and Microglia in CA3 Region of Hippocampus Does Not Differ between Juvenile and Adult Mice
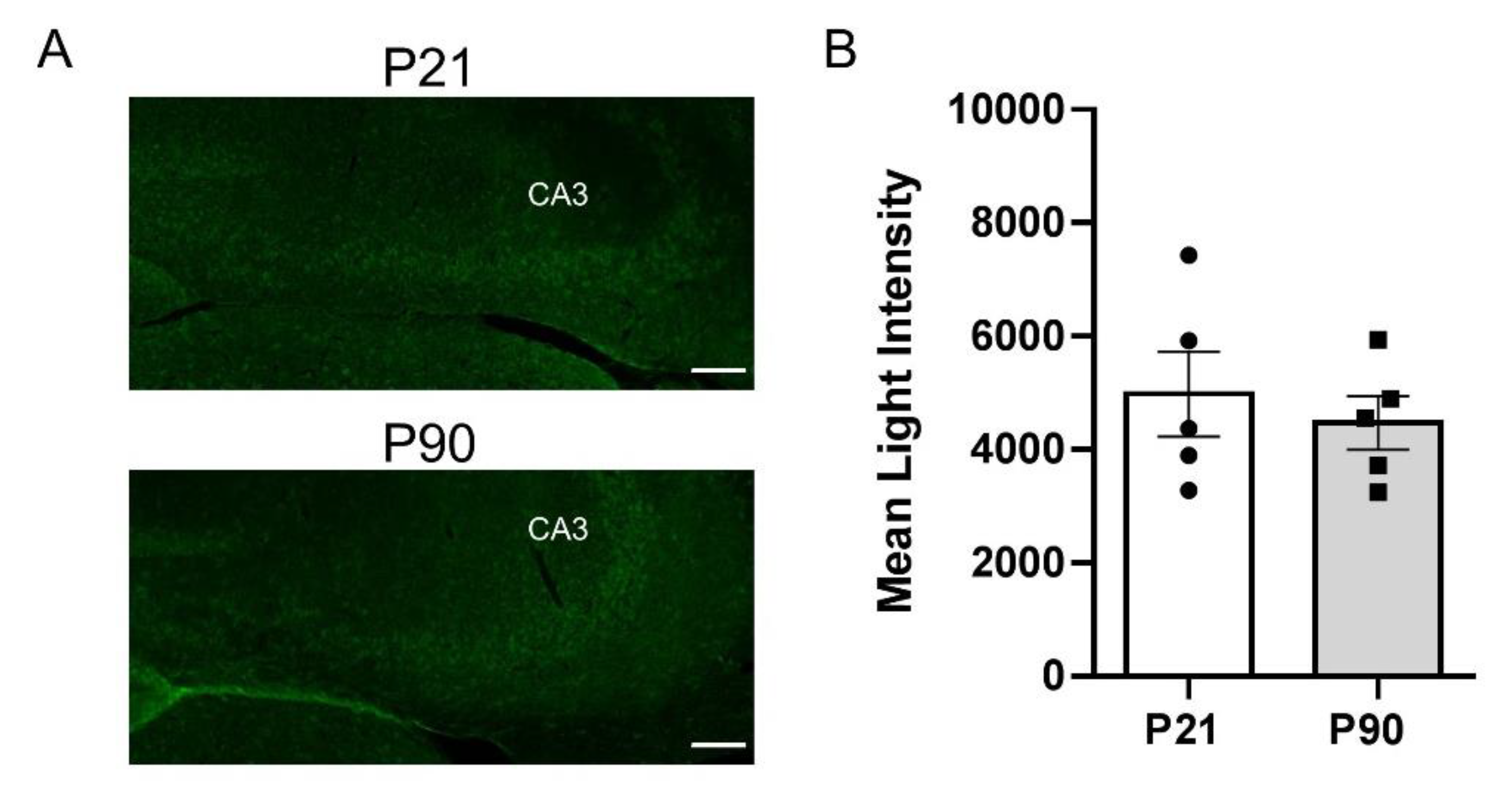
3.4. Greater PMAT, but Not OCT3, Expression in Juvenile Hippocampus than in Adult
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bylund, D.B.; Reed, A.L. Childhood and adolescent depression: Why do children and adults respond differently to antidepressant drugs? Neurochem. Int. 2007, 51, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Amphoux, A.; Vialou, V.; Drescher, E.; Brüss, M.; La Cour, C.M.; Rochat, C.; Millan, M.J.; Giros, B.; Bönisch, H.; Gautron, S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology 2006, 50, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.E.; Karbach, U.; Miska, D.; Gorboulev, V.; Akhoundova, A.; Volk, C.; Arndt, P.; Ulzheimer, J.C.; Sonders, M.S.; Baumann, C.; et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol. Pharmacol. 1998, 54, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, A.E.; Quester, S.; Ulzheimer, J.C.; Gorboulev, V.; Akhoundova, A.; Waldegger, S.; Lang, F.; Koepsell, H. Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS Lett. 1996, 395, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Wang, J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J. Pharmacol. Exp. Ther. 2010, 335, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Engel, K.; Zhou, M.; Wang, J. Identification and characterization of a novel monoamine transporter in the human brain. J. Biol. Chem. 2004, 279, 50042–50049. [Google Scholar] [CrossRef] [Green Version]
- Gasser, P.J.; Lowry, C.A.; Orchinik, M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: Potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J. Neurosci. 2006, 26, 8758–8766. [Google Scholar] [CrossRef]
- Koepsell, H.; Endou, H. The SLC22 drug transporter family. Eur. J. Physiol. 2004, 447, 666–676. [Google Scholar] [CrossRef]
- Koepsell, H.; Schmitt, B.M.; Gorboulev, V. Organic cation transporters. Rev. Physiol. Biochem. Pharmacol. 2003, 150, 36–90. [Google Scholar] [CrossRef]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef]
- Koepsell, H. Organic cation transporters in health and disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef]
- Koepsell, H. General overview of organic cation transporters in brain. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2021; Volume 266, pp. 1–39. [Google Scholar] [CrossRef]
- Vieira, L.S.; Wang, J. Brain plasma membrane monoamine transporter in health and disease. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2021; Volume 266, pp. 253–280. [Google Scholar] [CrossRef]
- Wu, X.; Kekuda, R.; Huang, W.; Fei, Y.-J.; Leibach, F.H.; Chen, J.; Conway, S.J.; Ganapathy, V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J. Biol. Chem. 1998, 273, 32776–32786. [Google Scholar] [CrossRef] [Green Version]
- Horton, R.E.; Apple, D.M.; Owens, W.A.; Baganz, N.L.; Cano, S.; Mitchell, N.C.; Vitela, M.; Gould, G.G.; Koek, W.; Daws, L.C. Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: Uncovering novel targets to treat depression. J. Neurosci. 2013, 33, 10534–10543. [Google Scholar] [CrossRef]
- Baganz, N.L.; Horton, R.E.; Calderon, A.S.; Owens, W.A.; Munn, J.L.; Watts, L.T.; Koldzic-Zivanovic, N.; Jeske, N.A.; Koek, W.; Toney, G.M.; et al. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc. Natl. Acad. Sci. USA 2008, 105, 18976–18981. [Google Scholar] [CrossRef] [Green Version]
- Alnouti, Y.; Petrick, J.S.; Klaassen, C.D. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab. Dispos. 2006, 34, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Bönisch, H. Substrates and inhibitors of organic cation transporters (OCTs) and plasma membrane monoamine transporter (PMAT) and therapeutic implications. Handb. Exp. Pharmacol. 2021, 266, 119–167. [Google Scholar] [CrossRef]
- Fraser-Spears, R.; Krause-Heuer, A.M.; Basiouny, M.; Mayer, F.P.; Manishimwe, R.; Wyatt, N.A.; Dobrowolski, J.C.; Roberts, M.P.; Greguric, I.; Kumar, N.; et al. Comparative analysis of novel decynium-22 analogs to inhibit transport by the low-affinity, high-capacity monoamine transporters, organic cation transporters 2 and 3, and plasma membrane monoamine transporter. Eur. J. Pharmacol. 2019, 842, 351–364. [Google Scholar] [CrossRef]
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Behav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Castagné, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent models of depression: Forced swim and tail suspension behavioral despair test in rats and mice. Curr. Protoc. Neurosci. 2010, 49, 5–8. [Google Scholar] [CrossRef]
- Bowman, M.A.; Daws, L.C. Targeting serotonin transporters in the treatment of juvenile and adolescent depression. Front. Neurosci. 2019, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Daws, L.C.; Toney, G.M. High-speed chronoamperometry to study kinetics and mechanisms for serotonin clearance in vivo. In Electrochemical Methods for Neuroscience; Michael, A.C., Borland, L.M., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Daws, L.C.; Owens, W.A.; Toney, G.M. High-speed chronoamperometry to measure biogenic amine release and uptake in vivo. In Neurotransmitter Transporters—Investigate Methods; Sitte, H., Bonisch, H., Eds.; Springer Science & Business Media: New York, NY, USA, 2016; pp. 53–81. [Google Scholar]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- García-Cabezas, M.Á.; John, Y.J.; Barbas, H.; Zikopoulos, B. Distinction of neurons, glia and endothelial cells in the cerebral cortex: An algorithm based on cytological features. Front. Neuroanat. 2016, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attili, S.M.; Silva, M.F.M.; Nguyen, T.-V.; Ascoli, G.A. Cell numbers, distribution, shape, and regional variation throughout the murine hippocampal formation from the adult brain Allen Reference Atlas. Brain Struct. Funct. 2019, 224, 2883–2897. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abdi, H. The Bonferroni and Sidak corrections for multiple comparisons. In Encyclopedia of Measurement and Statistics; Salkind, N.J., Ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2007; pp. 103–107. [Google Scholar]
- Mitchell, N.C.; Gould, G.G.; Smolik, C.M.; Koek, W.; Daws, L.C. Antidepressant-like drug effects in juvenile and adolescent mice in the tail suspension test: Relationship with hippocampal serotonin and norepinephrine transporter expression and function. Front. Pharmacol. 2013, 4, 131. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.C. Multiple Comparisons. Theory and Methods; Chapman & Hall/CRC: Boca Raton, FL, USA, 1996. [Google Scholar]
- Maxwell, S.E.; Delaney, H.D.; Kelley, K. Designing Experiments and Analyzing Data. A Model Comparison Perspective, 3rd ed.; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Mitchell, N.C.; Gould, G.G.; Koek, W.; Daws, L.C. Ontogeny of SERT expression and antidepressant-like response to escitalopram in wild-type and SERT mutant mice. J. Pharmacol. Exp. Ther. 2016, 358, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Sweet, D.H. Organic cation transporter expression and function in the CNS. Handb. Exp. Pharmacol. 2021, 266, 41–80. [Google Scholar] [CrossRef]
- Lehmenkühler, A.; Syková, E.; Svoboda, J.; Zilles, K.; Nicholson, C. Extracellular space parameters in the rat neocortex and subcortical white matter during postnatal development determined by diffusion analysis. Neuroscience 1993, 55, 339–351. [Google Scholar] [CrossRef]
- Nicholson, C.; Syková, E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998, 21, 207–215. [Google Scholar] [CrossRef]
- Syková, E.; Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, P.D.; Irvine, R.J.; Daws, L.C. Differences in the in vivo dynamics of neurotransmitter release and serotonin uptake after acute para-methoxyamphetamine and 3,4-methylenedioxymethamphetamine revealed by chronoamperometry. Neurochem. Int. 2005, 47, 350–361. [Google Scholar] [CrossRef]
- Daws, L.C.; Toney, G.M.; Gerhardt, G.A.; Frazer, A. In vivo chronoamperometric measures of extracellular serotonin clearance in rat dorsal hippocampus: Contribution of serotonin and norepinephrine transporters. J. Pharmacol. Exp. Ther. 1998, 286, 967–976. [Google Scholar]
- Daws, L.C.; Montañez, S.; Owens, W.A.; Gould, G.G.; Frazer, A.; Toney, G.M.; Gerhardt, G.A. Transport mechanisms governing serotonin clearance in vivo revealed by high-speed chronoamperomerty. J. Neurosci. Methods 2005, 143, 49–62. [Google Scholar] [CrossRef]
- Jackson, B.P.; Wightman, R.M. Dynamics of 5-hydroxytryptamine released from dopamine neurons in caudate putamen of the rat. Brain Res. 1995, 674, 163–166. [Google Scholar] [CrossRef]
- Pan, Y.; Gembom, E.; Peng, W.; Lesch, K.P.; Mossner, R.; Simantov, R. Plasticity in serotonin uptake in primary neuronal cultures of serotonin transporter knockout mice. Dev. Brain Res. 2001, 126, 125–129. [Google Scholar] [CrossRef]
- Stamford, J.A.; Kruk, Z.L.; Millar, J. Striatal dopamine terminals release serotonin after 5-HTP pretreatment: In vivo voltammetric data. Brain Res. 1990, 515, 173–180. [Google Scholar] [CrossRef]
- Zhou, F.C.; Lesch, K.-P.; Murphy, D.L. Serotonin uptake into dopamine neurons via dopamine transporters: A compensatory alternative. Brain Res. 2002, 942, 109–119. [Google Scholar] [CrossRef]
- Kwon, O.B.; Paredes, D.; Gonzalez, C.M.; Buonanno, A. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 15587–15592. [Google Scholar] [CrossRef] [Green Version]
- Mennicken, F.; Savasta, M.; Peretti-Renucci, R.; Feuerstein, C. Autoradiographic localization of dopamine uptake sites in the rat brain with 3H-GBR-12935. J. Neural Transm. 1992, 87, 1–14. [Google Scholar] [CrossRef]
- Mitchell, N.C.; Bowman, M.A.; Gould, G.G.; Koek, W.; Daws, L.C. Ontogeny of norepinephrine transporter expression and antidepressant-like response to desipramine in wild-type and serotonin transporter mutant mice. J. Pharmacol. Exp. Ther. 2017, 360, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.; Santra, S.; Dutta, A. Triple reuptake inhibitors as potential next-generation antidepressants: A new hope? Future Med. Chem. 2015, 7, 2385–2406. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowman, M.A.; Gomez, J.A.; Mitchell, N.C.; Wells, A.M.; Vitela, M.; Clarke, K.M.; Horton, R.E.; Koek, W.; Daws, L.C. Faster Serotonin Clearance in CA3 Region of Hippocampus and Antidepressant-like Effect of Decynium-22 in Juvenile Mice Are Putatively Linked to Increased Plasma Membrane Monoamine Transporter Function: Implications for Efficacy of Antidepressants in Juveniles. Cells 2022, 11, 2454. https://doi.org/10.3390/cells11152454
Bowman MA, Gomez JA, Mitchell NC, Wells AM, Vitela M, Clarke KM, Horton RE, Koek W, Daws LC. Faster Serotonin Clearance in CA3 Region of Hippocampus and Antidepressant-like Effect of Decynium-22 in Juvenile Mice Are Putatively Linked to Increased Plasma Membrane Monoamine Transporter Function: Implications for Efficacy of Antidepressants in Juveniles. Cells. 2022; 11(15):2454. https://doi.org/10.3390/cells11152454
Chicago/Turabian StyleBowman, Melodi A., Jorge A. Gomez, Nathan C. Mitchell, Anne M. Wells, Melissa Vitela, Kyra M. Clarke, Rebecca E. Horton, Wouter Koek, and Lynette C. Daws. 2022. "Faster Serotonin Clearance in CA3 Region of Hippocampus and Antidepressant-like Effect of Decynium-22 in Juvenile Mice Are Putatively Linked to Increased Plasma Membrane Monoamine Transporter Function: Implications for Efficacy of Antidepressants in Juveniles" Cells 11, no. 15: 2454. https://doi.org/10.3390/cells11152454
APA StyleBowman, M. A., Gomez, J. A., Mitchell, N. C., Wells, A. M., Vitela, M., Clarke, K. M., Horton, R. E., Koek, W., & Daws, L. C. (2022). Faster Serotonin Clearance in CA3 Region of Hippocampus and Antidepressant-like Effect of Decynium-22 in Juvenile Mice Are Putatively Linked to Increased Plasma Membrane Monoamine Transporter Function: Implications for Efficacy of Antidepressants in Juveniles. Cells, 11(15), 2454. https://doi.org/10.3390/cells11152454







