Functional ERAP1 Variants Distinctively Associate with Ankylosing Spondylitis Susceptibility under the Influence of HLA-B27 in Taiwanese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. HLA-B27 Determination
2.3. HLA-B Allele Determination
2.4. Genomic DNA Isolation
2.5. RNA Isolation and cDNA Synthesis
2.6. Deep DNA Sequencing Analyses of ERAP1 SNVs in Taiwanese
2.7. ERPA1 SNV Genotype Analyses
2.8. ERAP1 Expression Constructs
2.9. Peripheral Blood Mononuclear Cell (PBMC) Isolation and Culture
2.10. Cell Culture
2.11. Transfection of CHO and U937 Cells
2.12. Western Blot Analysis
2.13. ERAP1 Protein Immunoprecipitation
2.14. ERAP1 Enzyme Activity Determination
2.15. Cytokine Determination
2.16. Statistical Analysis
3. Results
3.1. Associations of ERAP1 SNVs with AS Susceptibility
3.2. Association of ERAP1 SNVs with HLA-B27 Positivity among AS Patients
3.3. Association of ERAP1 Allelic Variants (SNV Haplotypes) with AS Susceptibility
3.4. ERAP1 Allelic Variants Interact with HLA-B27 to Affect AS Susceptibility
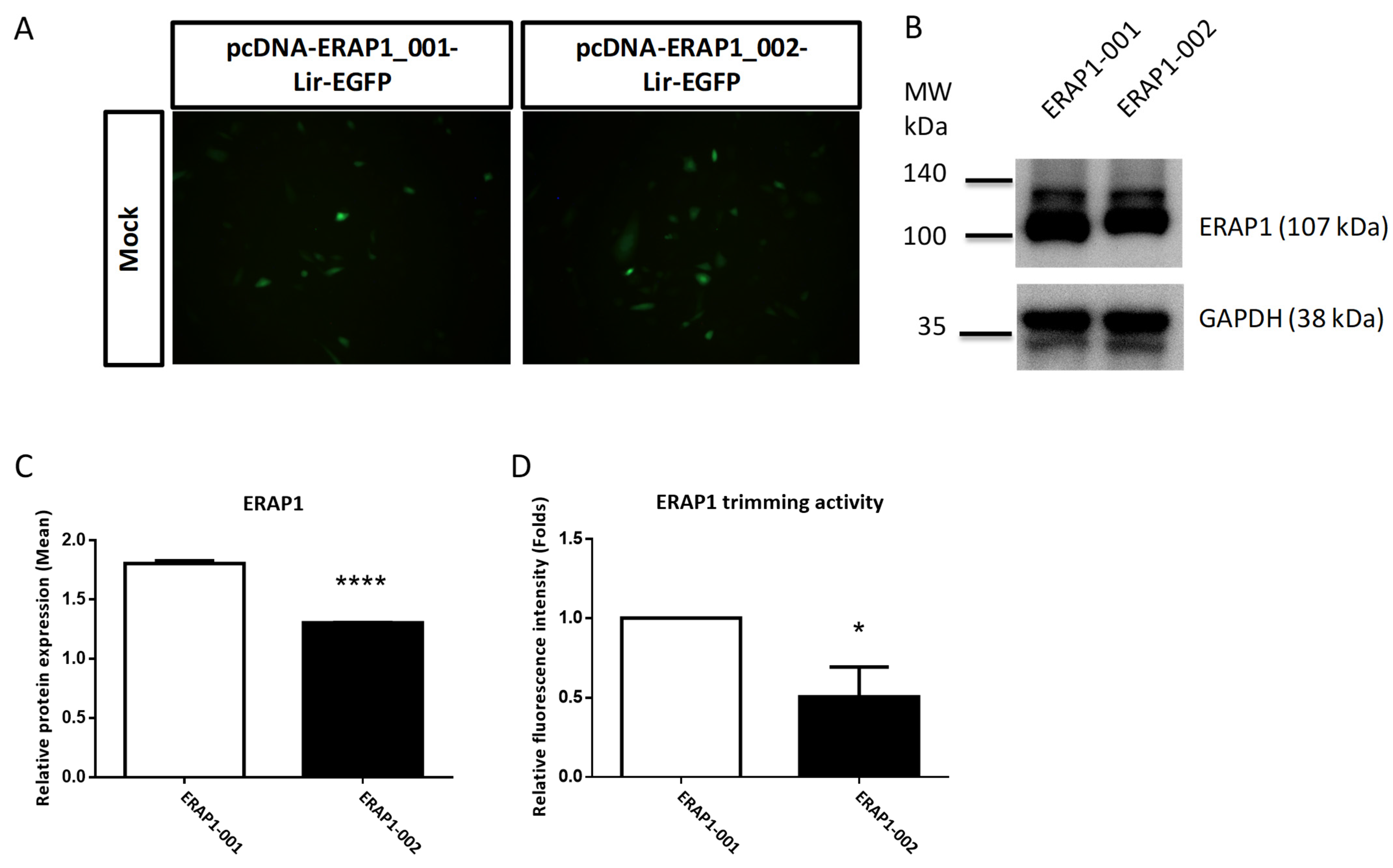
3.5. ERAP1 Variant Genotypes Affect Phenotypes
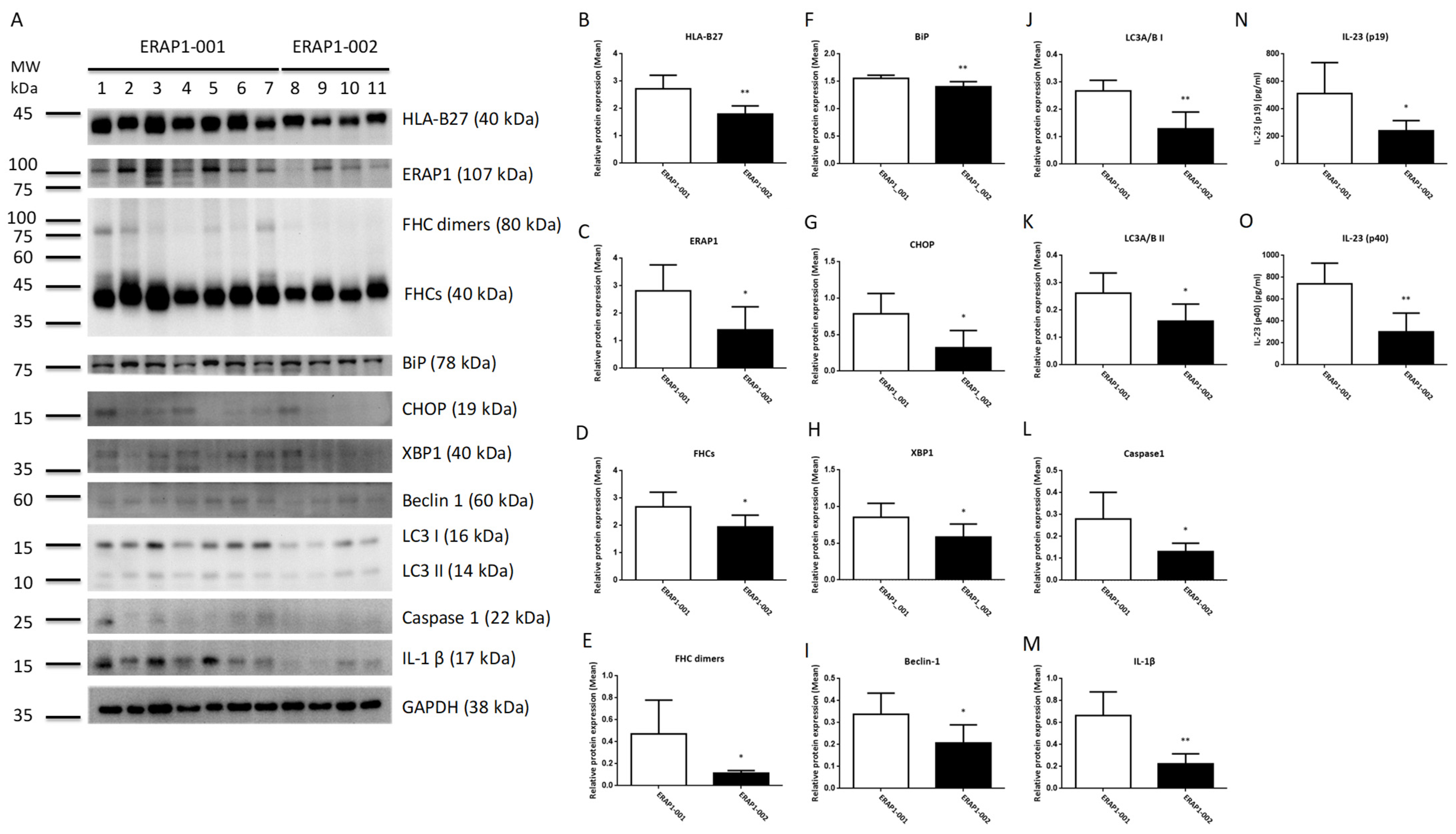
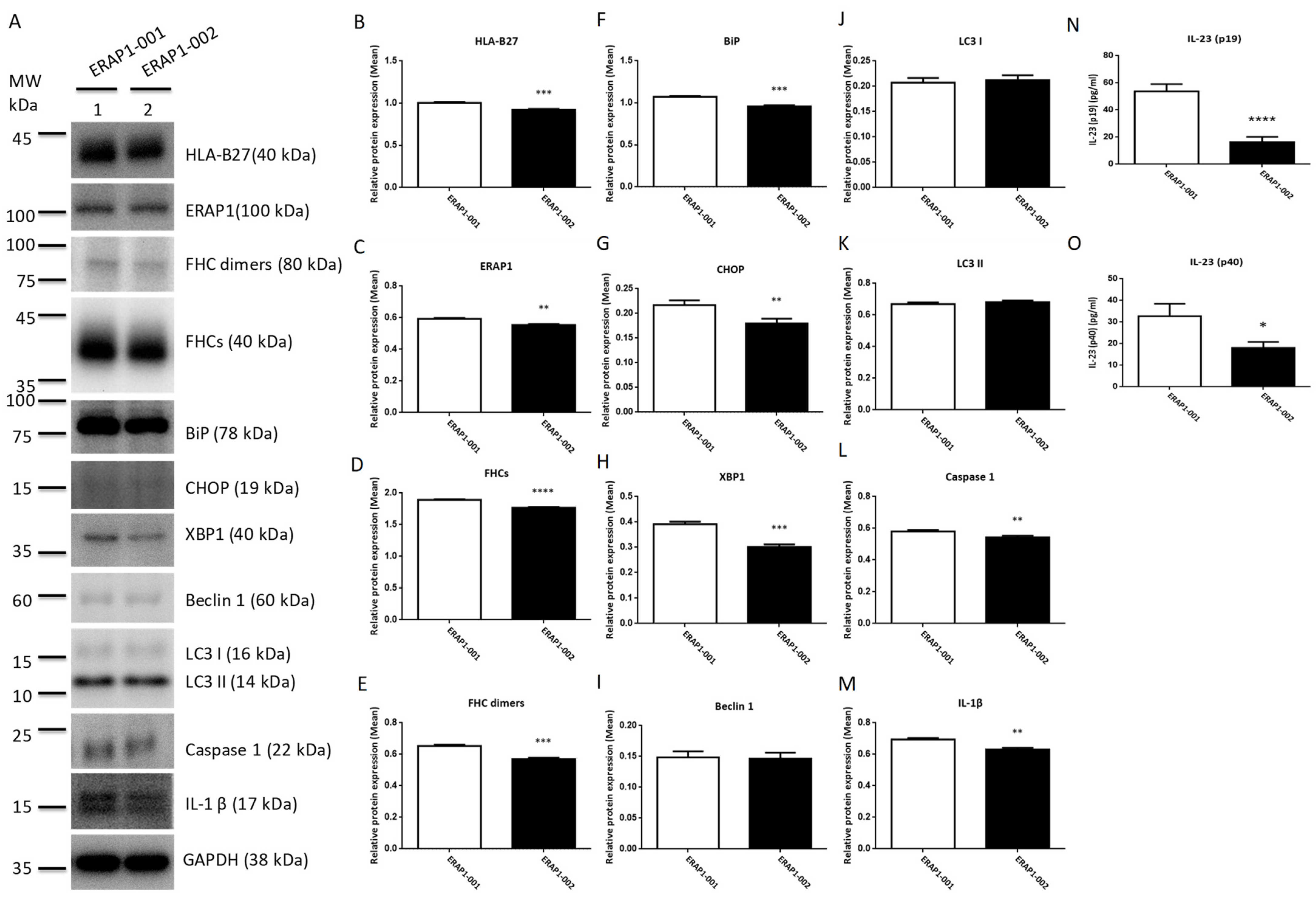
3.6. ERAP1 Allelic Variants Affect the Crosstalk of ER Stress (UPR), Autophagy, and Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tan, S.; Wang, R.; Ward, M.M. Syndesmophyte growth in ankylosing spondylitis. Curr. Opin. Rheumatol. 2015, 27, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, M.; Hellgren, K.; Frisell, T. Familial aggregation and heritability of ankylosing spondylitis—A Swedish nested case-control study. Rheumatology 2020, 59, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Costantino, F.; Breban, M.; Garchon, H.J. Genetics and Functional Genomics of Spondyloarthritis. Front. Immunol. 2018, 9, 2933. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.; Hadler, J.; Pointon, J.P.; Robinson, P.C.; Karaderi, T.; Leo, P.; Cremin, K.; Pryce, K.; Harris, J.; Lee, S.; et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 2013, 45, 730–738. [Google Scholar]
- Burton, P.R.; Clayton, D.G.; Cardon, L.R.; Craddock, N.; Deloukas, P.; Duncanson, A.; Kwiatkowski, D.P.; McCarthy, M.I.; Ouwehand, W.H.; Samani, N.J.; et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 2007, 39, 1329–1337. [Google Scholar]
- Cortes, A.; Pulit, S.L.; Leo, P.J.; Pointon, J.J.; Robinson, P.C.; Weisman, M.H.; Ward, M.; Gensler, L.S.; Zhou, X.; Garchon, H.J.; et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat. Commun. 2015, 6, 7146. [Google Scholar] [CrossRef] [Green Version]
- Reveille, J.D.; Sims, A.M.; Danoy, P.; Evans, D.M.; Leo, P.; Pointon, J.J.; Jin, R.; Zhou, X.; Bradbury, L.A.; Appleton, L.H.; et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat. Genet. 2010, 42, 123–127. [Google Scholar]
- Ellinghaus, D.; Jostins, L.; Spain, S.L.; Cortes, A.; Bethune, J.; Han, B.; Park, Y.R.; Raychaudhuri, S.; Pouget, J.G.; Hübenthal, M.; et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 2016, 48, 510–518. [Google Scholar] [CrossRef]
- Serwold, T.; Gonzalez, F.; Kim, J.; Jacob, R.; Shastri, N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature 2002, 419, 480–483. [Google Scholar] [CrossRef]
- Brouwenstijn, N.; Serwold, T.; Shastri, N. MHC class I molecules can direct proteolytic cleavage of antigenic precursors in the endoplasmic reticulum. Immunity 2001, 15, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Serwold, T.; Gaw, S.; Shastri, N. ER aminopeptidases generate a unique pool of peptides for MHC class I molecules. Nat. Immunol. 2001, 2, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Saric, T.; Chang, S.C.; Hattori, A.; York, I.A.; Markant, S.; Rock, K.L.; Tsujimoto, M.; Goldberg, A.L. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat. Immunol. 2002, 3, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Hammer, G.E.; Gonzalez, F.; Champsaur, M.; Cado, D.; Shastri, N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat. Immunol. 2006, 7, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.M.; Spencer, C.C.; Pointon, J.J.; Su, Z.; Harvey, D.; Kochan, G.; Oppermann, U.; Dilthey, A.; Pirinen, M.; Stone, M.A.; et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 2011, 43, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Reeves, E.; Elliott, T.; James, E.; Edwards, C.J. ERAP1 in the pathogenesis of ankylosing spondylitis. Immunol. Res. 2014, 60, 257–269. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, C.M.; Ma, C.C.; Luo, S.F.; Edberg, J.C.; Kimberly, R.P.; Wu, J. Association of a transmembrane polymorphism of Fcgamma receptor IIb (FCGR2B) with systemic lupus erythematosus in Taiwanese patients. Arthritis Rheum. 2006, 54, 3908–3917. [Google Scholar] [CrossRef]
- Evnouchidou, I.; Berardi, M.J.; Stratikos, E. A continuous fluorigenic assay for the measurement of the activity of endoplasmic reticulum aminopeptidase 1 competition kinetics as a tool for enzyme specificity investigation. Anal. Biochem. 2009, 395, 33–40. [Google Scholar] [CrossRef]
- Chen, R.; Yao, L.; Meng, T.; Xu, W. The association between seven ERAP1 polymorphisms and ankylosing spondylitis susceptibility. a meta-analysis involving 8,530 cases and 12,449 controls. Rheumatol. Int. 2012, 32, 909–914. [Google Scholar] [CrossRef]
- Wang, C.M.; Ho, H.H.; Chang, S.W.; Wu, Y.J.; Lin, J.C.; Chang, P.Y.; Wu, J.; Chen, J.Y. ERAP1 genetic variations associated with HLA-B27 interaction and disease severity of syndesmophytes formation in Taiwanese ankylosing spondylitis. Arthritis Res. Ther. 2012, 14, R125. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Batliwala, M.; Bouvier, M. ERAP1 enzyme-mediated trimming and structural analyses of MHC I--bound precursor peptides yield novel insights into antigen processing and presentation. J. Biol. Chem. 2019, 294, 18534–18544. [Google Scholar] [CrossRef] [Green Version]
- Mavridis, G.; Arya, R.; Domnick, A.; Zoidakis, J.; Makridakis, M.; Vlahou, A.; Mpakali, A.; Lelis, A.; Georgiadis, D.; Tampé, R.; et al. A systematic re-examination of processing of MHCI-bound antigenic peptide precursors by endoplasmic reticulum aminopeptidase 1. J. Biol. Chem. 2020, 295, 7193–7210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Medel, N.; Sanz-Bravo, A.; Van Nguyen, D.; Galocha, B.; Gomez-Molina, P.; Martin-Esteban, A.; Alvarez-Navarro, C.; de Castro, J.A. Functional interaction of the ankylosing spondylitis-associated endoplasmic reticulum aminopeptidase 1 polymorphism and HLA-B27 in vivo. Mol. Cell. Proteom. MCP 2012, 11, 1416–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Esteban, A.; Gomez-Molina, P.; Sanz-Bravo, A.; Lopez de Castro, J.A. Combined effects of ankylosing spondylitis-associated ERAP1 polymorphisms outside the catalytic and peptide-binding sites on the processing of natural HLA-B27 ligands. J. Biol. Chem. 2014, 289, 3978–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Navarro, C.; Lopez de Castro, J.A. ERAP1 in ankylosing spondylitis: Genetics, biology and pathogenetic role. Curr. Opin. Rheumatol. 2013, 25, 419–425. [Google Scholar] [CrossRef]
- Chen, L.; Fischer, R.; Peng, Y.; Reeves, E.; McHugh, K.; Ternette, N.; Hanke, T.; Dong, T.; Elliott, T.; Shastri, N.; et al. Critical role of endoplasmic reticulum aminopeptidase 1 in determining the length and sequence of peptides bound and presented by HLA-B27. Arthritis Rheumatol. 2014, 66, 284–294. [Google Scholar] [CrossRef]
- Seregin, S.S.; Rastall, D.P.; Evnouchidou, I.; Aylsworth, C.F.; Quiroga, D.; Kamal, R.P.; Godbehere-Roosa, S.; Blum, C.F.; York, I.A.; Stratikos, E.; et al. Endoplasmic reticulum aminopeptidase-1 alleles associated with increased risk of ankylosing spondylitis reduce HLA-B27 mediated presentation of multiple antigens. Autoimmunity 2013, 46, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Costantino, F.; Talpin, A.; Evnouchidou, I.; Kadi, A.; Leboime, A.; Said-Nahal, R.; Bonilla, N.; Letourneur, F.; Leturcq, T.; Ka, Z.; et al. ERAP1 Gene Expression Is Influenced by Nonsynonymous Polymorphisms Associated With Predisposition to Spondyloarthritis. Arthritis Rheumatol. 2015, 67, 1525–1534. [Google Scholar] [CrossRef]
- Sanz-Bravo, A.; Alvarez-Navarro, C.; Martín-Esteban, A.; Barnea, E.; Admon, A.; López de Castro, J.A. Ranking the Contribution of Ankylosing Spondylitis-associated Endoplasmic Reticulum Aminopeptidase 1 (ERAP1) Polymorphisms to Shaping the HLA-B*27 Peptidome. Mol. Cell. Proteom. MCP 2018, 17, 1308–1323. [Google Scholar] [CrossRef] [Green Version]
- Kochan, G.; Krojer, T.; Harvey, D.; Fischer, R.; Chen, L.; Vollmar, M.; von Delft, F.; Kavanagh, K.L.; Brown, M.A.; Bowness, P.; et al. Crystal structures of the endoplasmic reticulum aminopeptidase-1 (ERAP1) reveal the molecular basis for N-terminal peptide trimming. Proc. Natl. Acad. Sci. USA 2011, 108, 7745–7750. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Navarro, C.; Lopez de Castro, J.A. ERAP1 structure, function and pathogenetic role in ankylosing spondylitis and other MHC-associated diseases. Mol. Immunol. 2014, 57, 12–21. [Google Scholar] [CrossRef]
- Sanz-Bravo, A.; Campos, J.; Mazariegos, M.S.; Lopez de Castro, J.A. Dominant role of the ERAP1 polymorphism R528K in shaping the HLA-B27 Peptidome through differential processing determined by multiple peptide residues. Arthritis Rheumatol. 2015, 67, 692–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamogiannos, A.; Koumantou, D.; Papakyriakou, A.; Stratikos, E. Effects of polymorphic variation on the mechanism of Endoplasmic Reticulum Aminopeptidase 1. Mol. Immunol. 2015, 67, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Giastas, P.; Mpakali, A.; Papakyriakou, A.; Lelis, A.; Kokkala, P.; Neu, M.; Rowland, P.; Liddle, J.; Georgiadis, D.; Stratikos, E. Mechanism for antigenic peptide selection by endoplasmic reticulum aminopeptidase 1. Proc. Natl. Acad. Sci. USA 2019, 116, 26709–26716. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ridley, A.; Hammitzsch, A.; Al-Mossawi, M.H.; Bunting, H.; Georgiadis, D.; Chan, A.; Kollnberger, S.; Bowness, P. Silencing or inhibition of endoplasmic reticulum aminopeptidase 1 (ERAP1) suppresses free heavy chain expression and Th17 responses in ankylosing spondylitis. Ann. Rheum. Dis. 2016, 75, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Harvey, D.; Pointon, J.J.; Evans, D.M.; Karaderi, T.; Farrar, C.; Appleton, L.H.; Sturrock, R.D.; Stone, M.A.; Oppermann, U.; Brown, M.A.; et al. Investigating the genetic association between ERAP1 and ankylosing spondylitis. Hum. Mol. Genet. 2009, 18, 4204–4212. [Google Scholar] [CrossRef] [Green Version]
- Cinar, M.; Akar, H.; Yilmaz, S.; Simsek, I.; Karkucak, M.; Sagkan, R.I.; Pekel, A.; Erdem, H.; Avci, I.Y.; Acikel, C.; et al. A polymorphism in ERAP1 is associated with susceptibility to ankylosing spondylitis in a Turkish population. Rheumatol. Int. 2013, 33, 2851–2858. [Google Scholar] [CrossRef]
- Reeves, E.; Colebatch-Bourn, A.; Elliott, T.; Edwards, C.J.; James, E. Functionally distinct ERAP1 allotype combinations distinguish individuals with Ankylosing Spondylitis. Proc. Natl. Acad. Sci. USA 2014, 111, 17594–17599. [Google Scholar] [CrossRef] [Green Version]
- Ombrello, M.J.; Kastner, D.L.; Remmers, E.F. Endoplasmic reticulum-associated amino-peptidase 1 and rheumatic disease, genetics. Curr. Opin. Rheumatol. 2015, 27, 349–356. [Google Scholar] [CrossRef]
- Roberts, A.R.; Appleton, L.H.; Cortes, A.; Vecellio, M.; Lau, J.; Watts, L.; Brown, M.A.; Wordsworth, P. ERAP1 association with ankylosing spondylitis is attributable to common genotypes rather than rare haplotype combinations. Proc. Natl. Acad. Sci. USA 2017, 114, 558–561. [Google Scholar] [CrossRef] [Green Version]
- Hanson, A.L.; Cuddihy, T.; Haynes, K.; Loo, D.; Morton, C.J.; Oppermann, U.; Leo, P.; Thomas, G.P.; Le Cao, K.A.; Kenna, T.J.; et al. Genetic Variants in ERAP1 and ERAP2 Associated With Immune-Mediated Diseases Influence Protein Expression and the Isoform Profile. Arthritis Rheumatol. 2018, 70, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Haroon, N.; Tsui, F.W.; Uchanska-Ziegler, B.; Ziegler, A.; Inman, R.D. Endoplasmic reticulum aminopeptidase 1 (ERAP1) exhibits functionally significant interaction with HLA-B27 and relates to subtype specificity in ankylosing spondylitis. Ann. Rheum. Dis. 2012, 71, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Padula, M.C.; Leccese, P.; Lascaro, N.; Carbone, T.; Limongi, A.R.; Radice, R.P.; Padula, A.A.; D’Angelo, S.; Martelli, G. From structure to function for the characterization of ERAP1 active site in Behçet syndrome. A novel polymorphism associated with known gene variations. Mol. Immunol. 2020, 117, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, H.; Koftori, D.; Sekine, T.; Nicastri, A.; Ternette, N.; Bowness, P. Identification of an Unconventional Subpeptidome Bound to the Behçet’s Disease-associated HLA-B*51:01 that is Regulated by Endoplasmic Reticulum Aminopeptidase 1 (ERAP1). Mol. Cell. Proteom. MCP 2020, 19, 871–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.C.; Sung-Ching, H.W.; Hsu, Y.W.; Wen, Y.F.; Wang, W.C.; Wong, R.H.; Lu, H.F.; Gaalen, F.A.; Chang, W.C. Interaction between HLA-B60 and HLA-B27 as a Better Predictor of Ankylosing Spondylitis in a Taiwanese Population. PLoS ONE 2015, 10, e0137189. [Google Scholar]
- Robinson, W.P.; van der Linden, S.M.; Khan, M.A.; Rentsch, H.U.; Cats, A.; Russell, A.; Thomson, G. HLA-Bw60 increases susceptibility to ankylosing spondylitis in HLA-B27+ patients. Arthritis Rheum. 1989, 32, 1135–1141. [Google Scholar] [CrossRef]
- Chipurupalli, S.; Samavedam, U.; Robinson, N. Crosstalk between ER Stress, Autophagy and Inflammation. Front. Med. 2021, 8, 758311. [Google Scholar] [CrossRef]
- Brown, M.A.; Kenna, T.; Wordsworth, B.P. Genetics of ankylosing spondylitis—Insights into pathogenesis. Nat. Rev. Rheumatol. 2016, 12, 81–91. [Google Scholar] [CrossRef]
- Rahmati, M.; Moosavi, M.A.; McDermott, M.F. ER Stress: A Therapeutic Target in Rheumatoid Arthritis? Trends Pharmacol. Sci. 2018, 39, 610–623. [Google Scholar] [CrossRef]
- DeLay, M.L.; Turner, M.J.; Klenk, E.I.; Smith, J.A.; Sowders, D.P.; Colbert, R.A. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009, 60, 2633–2643. [Google Scholar] [CrossRef]
- Becker, C.; Wirtz, S.; Blessing, M.; Pirhonen, J.; Strand, D.; Bechthold, O.; Frick, J.; Galle, P.R.; Autenrieth, I.; Neurath, M.F. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Investig. 2003, 112, 693–706. [Google Scholar] [CrossRef] [Green Version]
- Kinnebrew, M.A.; Buffie, C.G.; Diehl, G.E.; Zenewicz, L.A.; Leiner, I.; Hohl, T.M.; Flavell, R.A.; Littman, D.R.; Pamer, E.G. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 2012, 36, 276–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodall, J.C.; Wu, C.; Zhang, Y.; McNeill, L.; Ellis, L.; Saudek, V.; Gaston, J.S. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl. Acad. Sci. USA 2010, 107, 17698–17703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, Y.; Ogawa, K.; Hattori, A.; Tsujimoto, M. Secretion of endoplasmic reticulum aminopeptidase 1 is involved in the activation of macrophages induced by lipopolysaccharide and interferon-gamma. J. Biol. Chem. 2011, 286, 21906–21914. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.; Nakamura, T.J.; Ogawa, K.; Hattori, A.; Tsujimoto, M. Acute-phase protein-like properties of endoplasmic reticulum aminopeptidase 1. J. Biochem. 2019, 165, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ogawa, Y.; Tsumoto, H.; Miura, Y.; Nakamura, T.J.; Ogawa, K.; Akimoto, Y.; Kawakami, H.; Endo, T.; Yanoshita, R.; et al. Contribution of the exosome-associated form of secreted endoplasmic reticulum aminopeptidase 1 to exosome-mediated macrophage activation. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 874–888. [Google Scholar] [CrossRef] [PubMed]
- Babaie, F.; Mohammadi, H.; Hemmatzadeh, M.; Ebrazeh, M.; Torkamandi, S.; Yousefi, M.; Hajaliloo, M.; Rezaiemanesh, A.; Salimi, S.; Salimi, R.; et al. Evaluation of ERAP1 gene single nucleotide polymorphisms in immunomodulation of pro-inflammatory and anti-inflammatory cytokines profile in ankylosing spondylitis. Immunol. Lett. 2020, 217, 31–38. [Google Scholar] [CrossRef]
- Hemmatzadeh, M.; Babaie, F.; Ezzatifar, F.; Mohammadi, F.S.; Ebrazeh, M.; Golabi Aghdam, S.; Hajaliloo, M.; Azizi, G.; Gowhari Shabgah, A.; Shekari, N.; et al. Susceptibility to ERAP1 gene single nucleotide polymorphism modulates the inflammatory cytokine setting in ankylosing spondylitis. Int. J. Rheum. Dis. 2019, 22, 715–724. [Google Scholar] [CrossRef]
| ERAP1 Allelic Variant * | Estimated Frequency | Permutation ** | Logistic Regression | Logistic Regression Adjusted for Sex | ||||
|---|---|---|---|---|---|---|---|---|
| AS | Normal | All | p Value | p Value | OR (95% CI) | p Value | OR (95% CI) | |
| (2N = 1726) | (2N = 2876) | (2N = 4602) | ||||||
| 001 | 46.85% | 36.69% | 40.50% | <0.000001 | 1.32 × 10−11 | 1.53 (1.35–1.73) | 1.39 × 10−11 | 1.53 (1.35–1.73) |
| 002 | 21.91% | 23.41% | 22.85% | 0.23895 | 0.2390 | 0.92 (0.79–1.06) | 0.2419 | 0.92 (0.79–1.06) |
| 003 | 11.91% | 12.43% | 12.23% | 0.5833 | 0.5905 | 0.95 (0.79–1.15) | 0.5874 | 0.95 (0.79–1.15) |
| 004 | 7.79% | 6.96% | 7.27% | 0.2902 | 0.2938 | 1.13 (0.90–1.42) | 0.2952 | 1.13 (0.90–1.42) |
| 005 | 3.63% | 3.62% | 3.63% | 0.99285 | 0.9898 | 1.00 (0.72–1.39) | 0.9917 | 1.00 (0.72–1.39) |
| 006 | 2.08% | 2.41% | 2.29% | 0.4558 | 0.4541 | 0.85 (0.56–1.29) | 0.4531 | 0.85 (0.56–1.29) |
| ERAP1 Allelic Variant * | Estimated Frequency | Permutation ** | Logistic Regression | Logistic Regression Adjusted for Sex | ||||
|---|---|---|---|---|---|---|---|---|
| HLA-B27+ | HLA-B27− | All | p Value | p Value | OR (95% CI) | p Value | OR (95% CI) | |
| (2N = 1602) | (2N = 124) | (2N = 1726) | ||||||
| 001 | 47.77% | 36.80% | 46.93% | 0.0118 | 0.0132 | 1.62 (1.11–2.36) | 0.0147 | 1.61 (1.10–2.36) |
| 002 | 20.89% | 33.95% | 21.89% | 2.00 × 10−4 | 0.0005 | 0.50 (0.34–0.74) | 0.00064 | 0.50 (0.34–0.75) |
| 003 | 12.07% | 10.90% | 11.98% | 0.674 | 0.6776 | 1.13 (0.63–2.05) | 0.7637 | 1.10 (0.60–2.00) |
| 004 | 8.18% | 3.10% | 7.80% | 0.0293 | 0.0458 | 2.82 (1.02–7.79) | 0.0409 | 2.90 (1.04–8.04) |
| 005 | 3.54% | 5.12% | 3.66% | 0.3132 | 0.3408 | 0.66 (0.28–1.54) | 0.3741 | 0.68 (0.29–1.60) |
| 006 | 2.10% | 1.84% | 2.08% | 0.8378 | 0.8398 | 1.15 (0.30–4.40) | 0.8863 | 1.10 (0.29–4.27) |
| ERAP1 Allelic Variant * | Estimated Frequency | Permutation ** | Logistic Regression | Logistic Regression Adjusted for Sex | ||||
|---|---|---|---|---|---|---|---|---|
| AS B27− | Normal B27− | All | p Value | p Value | OR (95% CI) | p Value | OR (95% CI) | |
| (2N = 124) | (2N = 2686) | (2N = 2810) | ||||||
| 001 | 36.64% | 36.33% | 36.35% | 0.9453 | 0.9448 | 1.01 (0.70–1.47) | 0.9548 | 1.01 (0.70–1.47) |
| 002 | 34.53% | 23.65% | 24.13% | 0.0045 | 0.0058 | 1.72 (1.17–2.52) | 0.0080 | 1.69 (1.15–2.49) |
| 003 | 11.63% | 13.11% | 13.04% | 0.6147 | 0.6205 | 0.86 (0.48–1.54) | 0.6614 | 0.88 (0.49–1.57) |
| 004 | 5.84% | 5.40% | 5.42% | 0.8202 | 0.8245 | 1.10 (0.49–2.48) | 0.8515 | 1.08 (0.48–2.45) |
| 005 | 3.13% | 6.80% | 6.64% | 0.0966 | 0.1174 | 0.43 (0.15–1.24) | 0.1075 | 0.42 (0.15–1.21) |
| 006 | 2.75% | 1.98% | 2.01% | 0.5287 | 0.5242 | 1.46 (0.46–4.67) | 0.5207 | 1.46 (0.46–4.67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-M.; Liu, M.-K.; Jan Wu, Y.-J.; Lin, J.-C.; Zheng, J.-W.; Wu, J.; Chen, J.-Y. Functional ERAP1 Variants Distinctively Associate with Ankylosing Spondylitis Susceptibility under the Influence of HLA-B27 in Taiwanese. Cells 2022, 11, 2427. https://doi.org/10.3390/cells11152427
Wang C-M, Liu M-K, Jan Wu Y-J, Lin J-C, Zheng J-W, Wu J, Chen J-Y. Functional ERAP1 Variants Distinctively Associate with Ankylosing Spondylitis Susceptibility under the Influence of HLA-B27 in Taiwanese. Cells. 2022; 11(15):2427. https://doi.org/10.3390/cells11152427
Chicago/Turabian StyleWang, Chin-Man, Ming-Kun Liu, Yeong-Jian Jan Wu, Jing-Chi Lin, Jian-Wen Zheng, Jianming Wu, and Ji-Yih Chen. 2022. "Functional ERAP1 Variants Distinctively Associate with Ankylosing Spondylitis Susceptibility under the Influence of HLA-B27 in Taiwanese" Cells 11, no. 15: 2427. https://doi.org/10.3390/cells11152427
APA StyleWang, C.-M., Liu, M.-K., Jan Wu, Y.-J., Lin, J.-C., Zheng, J.-W., Wu, J., & Chen, J.-Y. (2022). Functional ERAP1 Variants Distinctively Associate with Ankylosing Spondylitis Susceptibility under the Influence of HLA-B27 in Taiwanese. Cells, 11(15), 2427. https://doi.org/10.3390/cells11152427






