Functional Expression of Multidrug-Resistance (MDR) Transporters in Developing Human Fetal Brain Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Isolation and Culture of Human Brain Endothelial Cells
2.3. Immunofluorescence
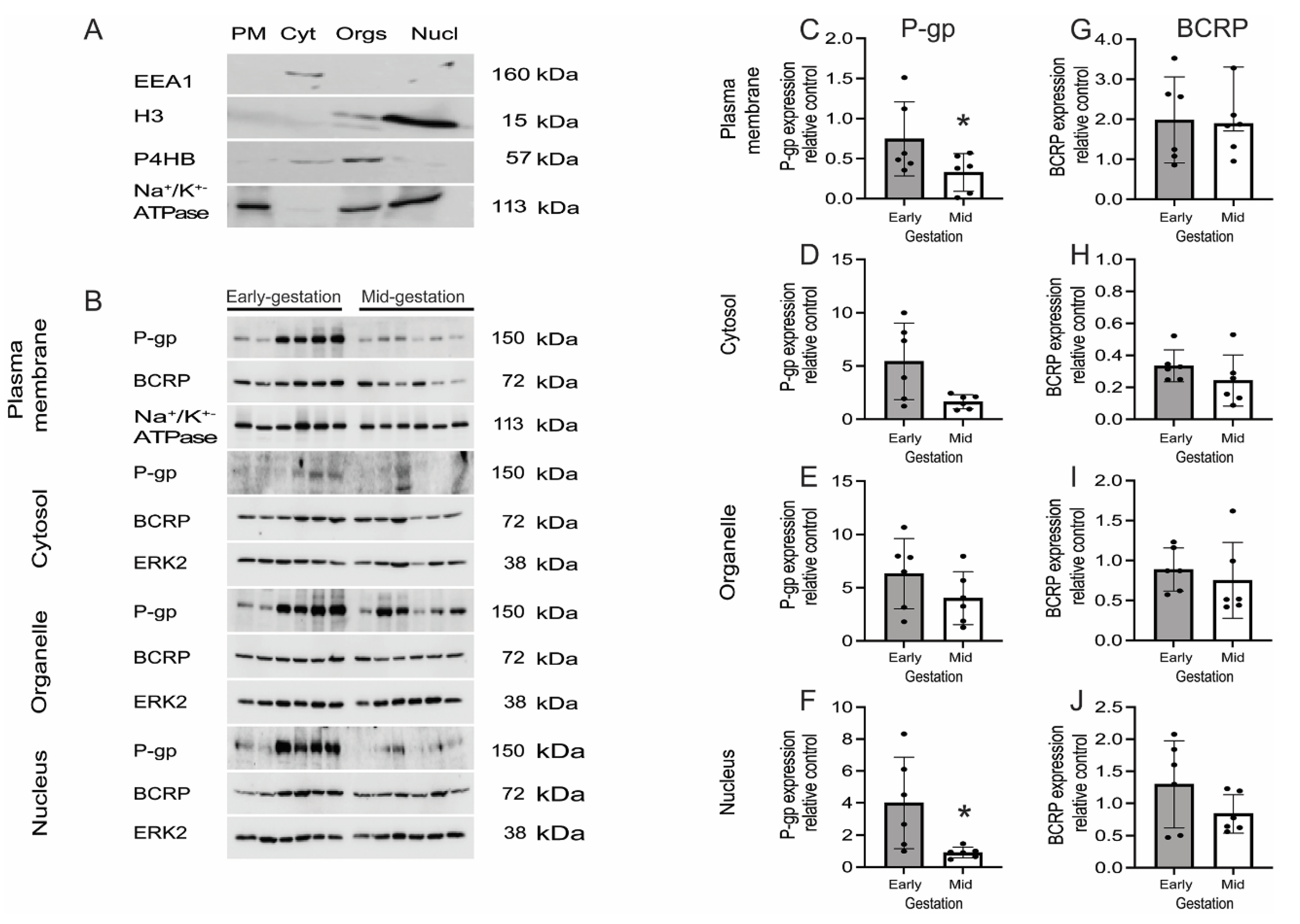
2.4. Cellular Fractionation
2.5. Immunoblotting
2.6. Quantitative Real Time PCR (qPCR)
2.7. P-gp and BCRP Activity Assay
2.8. Matrigel Tube Formation Assay
2.9. Cell Proliferation and Viability
2.10. Statistical Analyses
3. Results
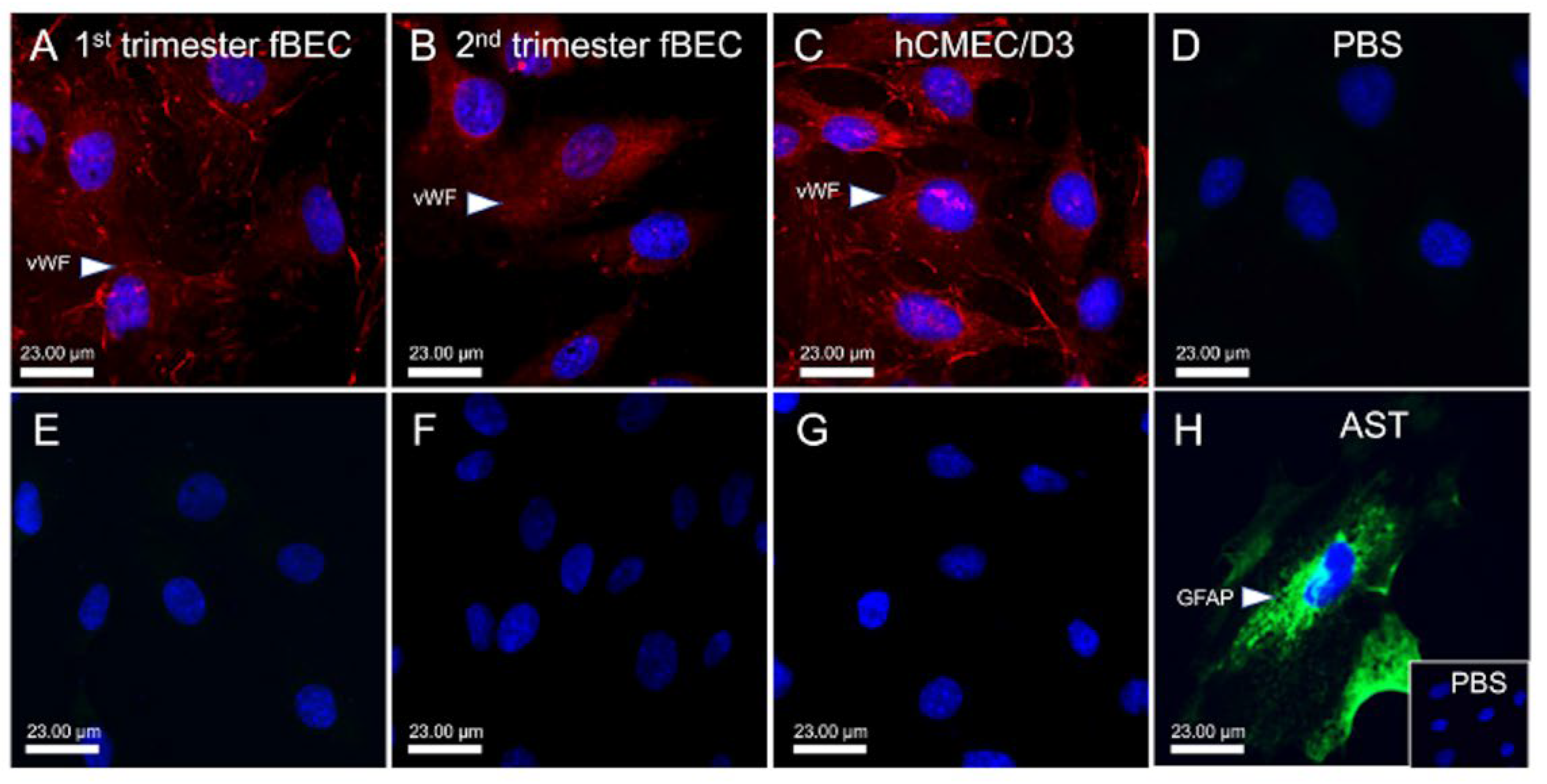
3.1. Human Fetal Brain Endothelial Cells (hfBECs) Derived from Early and Mid-Gestation Express the Endothelial Cell Maker von Willebrand Factor (vWF)
3.2. Expression of Tight Junction Proteins by hfBECs in Early and Mid-Gestation
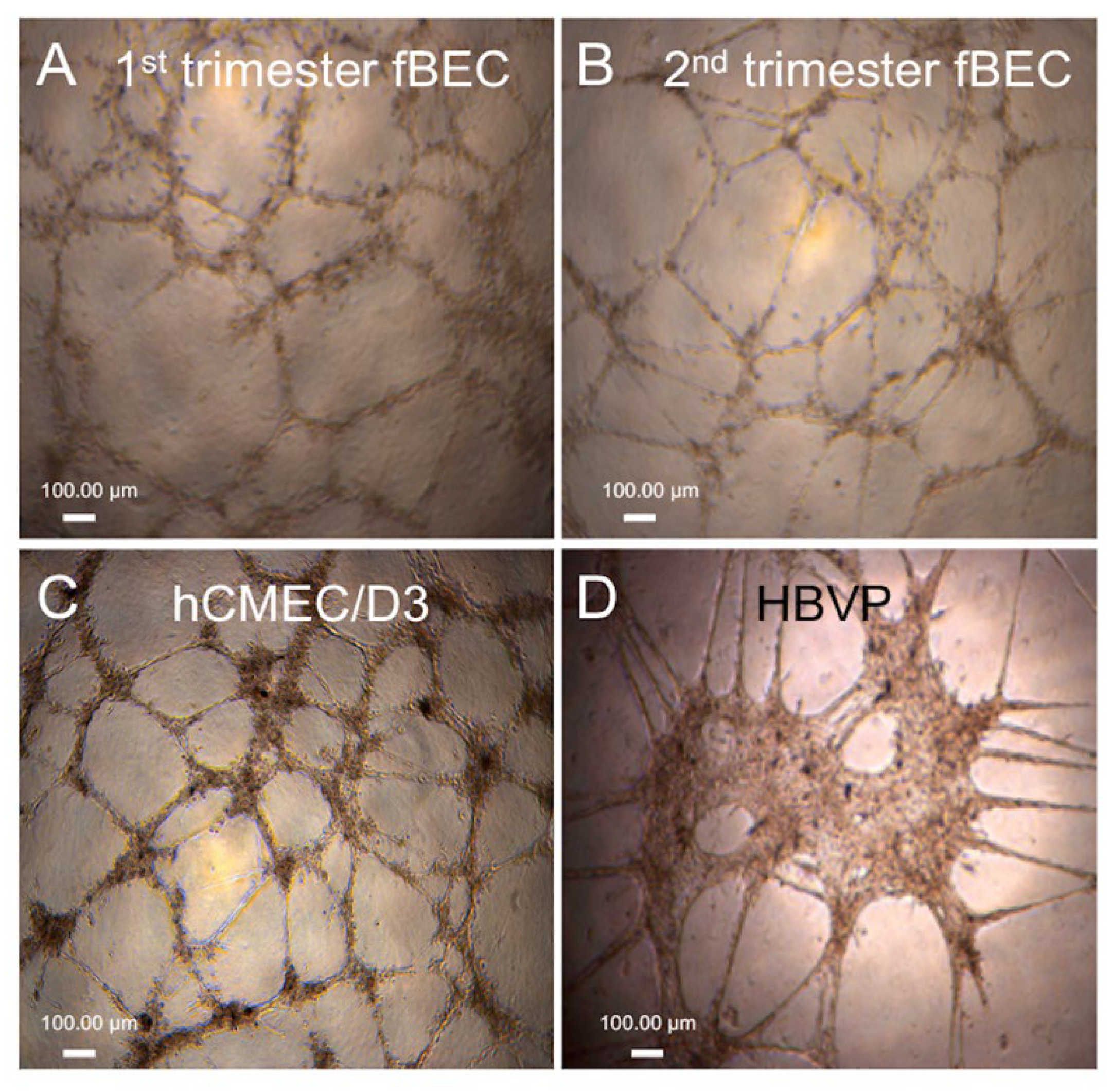
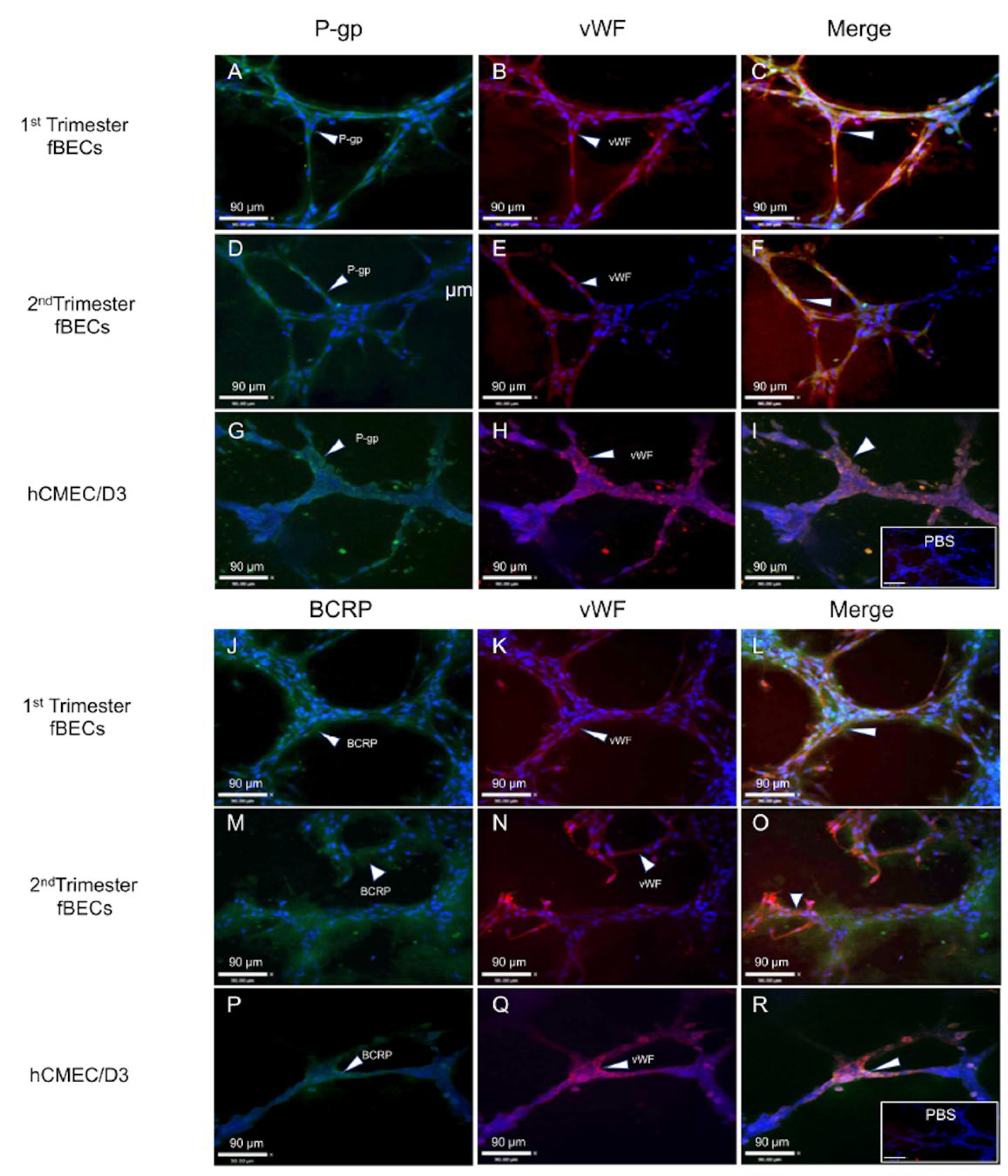
3.3. Tube Formation by Early and Mid-Gestation Derived hfBECs, and Expression of Endothelial Markers and Drug Transporters
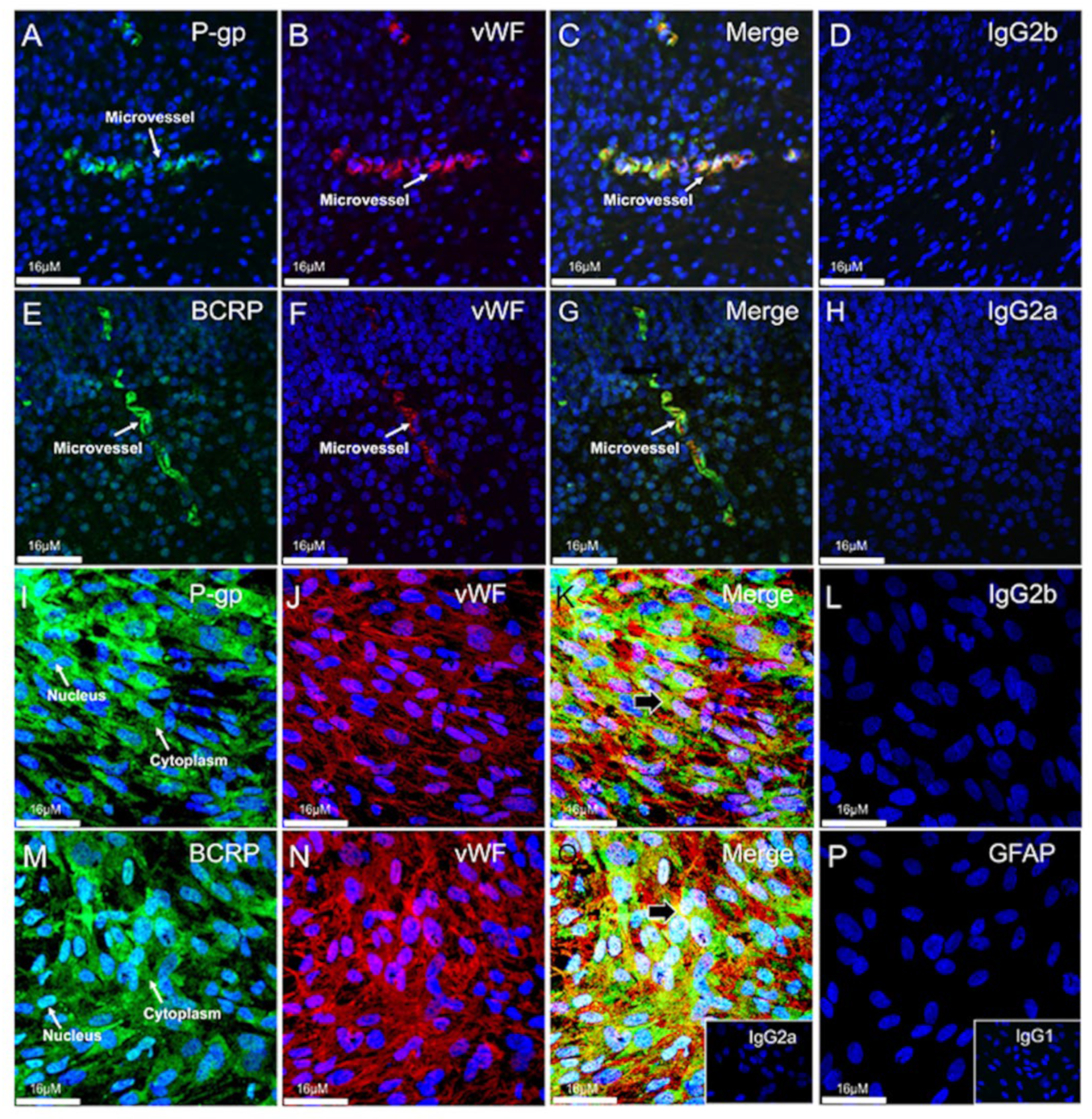
3.4. Expression and Localization of P-gp and BCRP in the Human Fetal Brain Tissue and in Primary Human Fetal Brain Endothelial Cells (hfBECs)
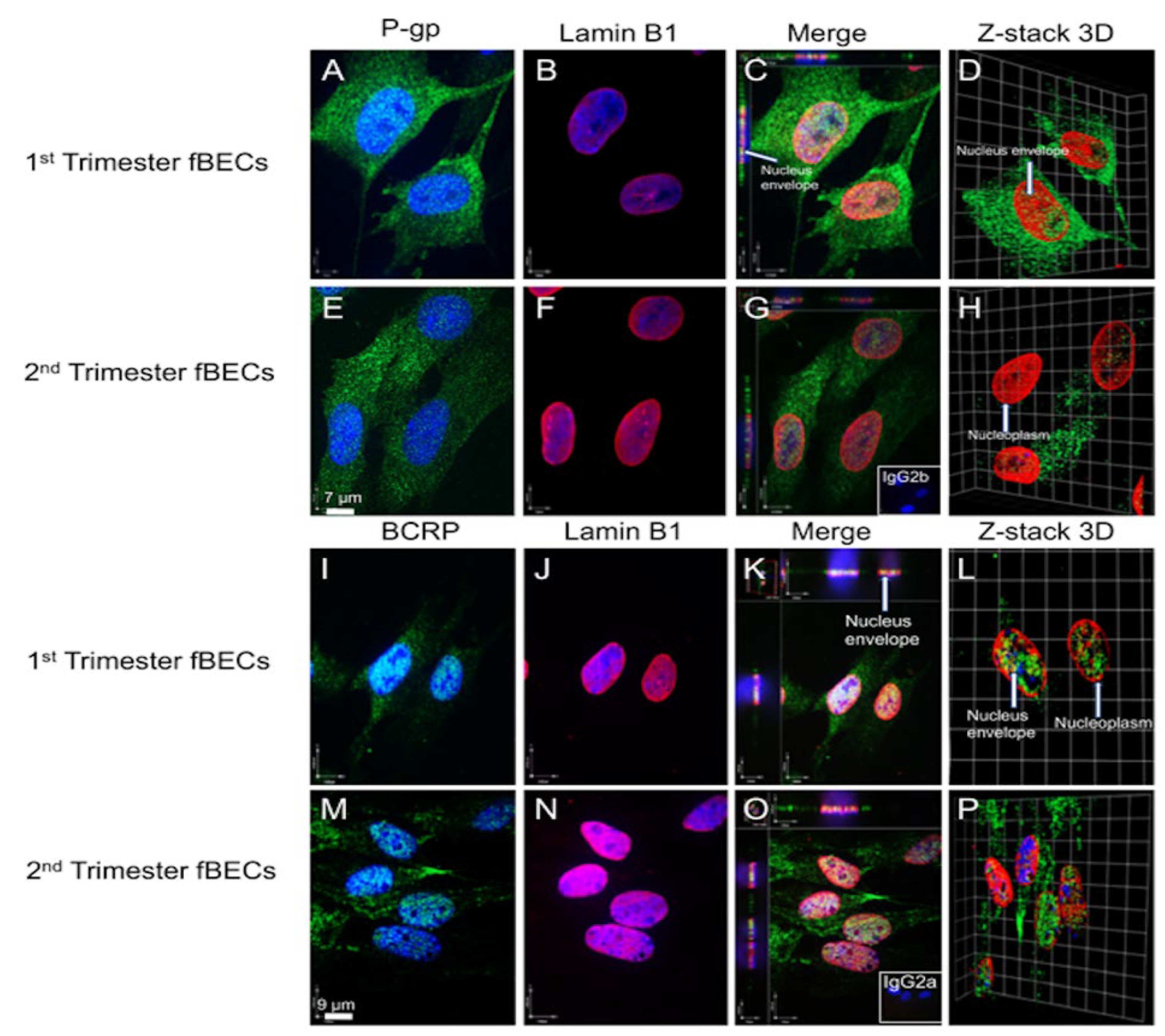
3.5. P-gp and BCRP Are Enriched in Cytoplasm and Nucleus of Primary Human Fetal Brain Endothelial Cells (hfBECs)
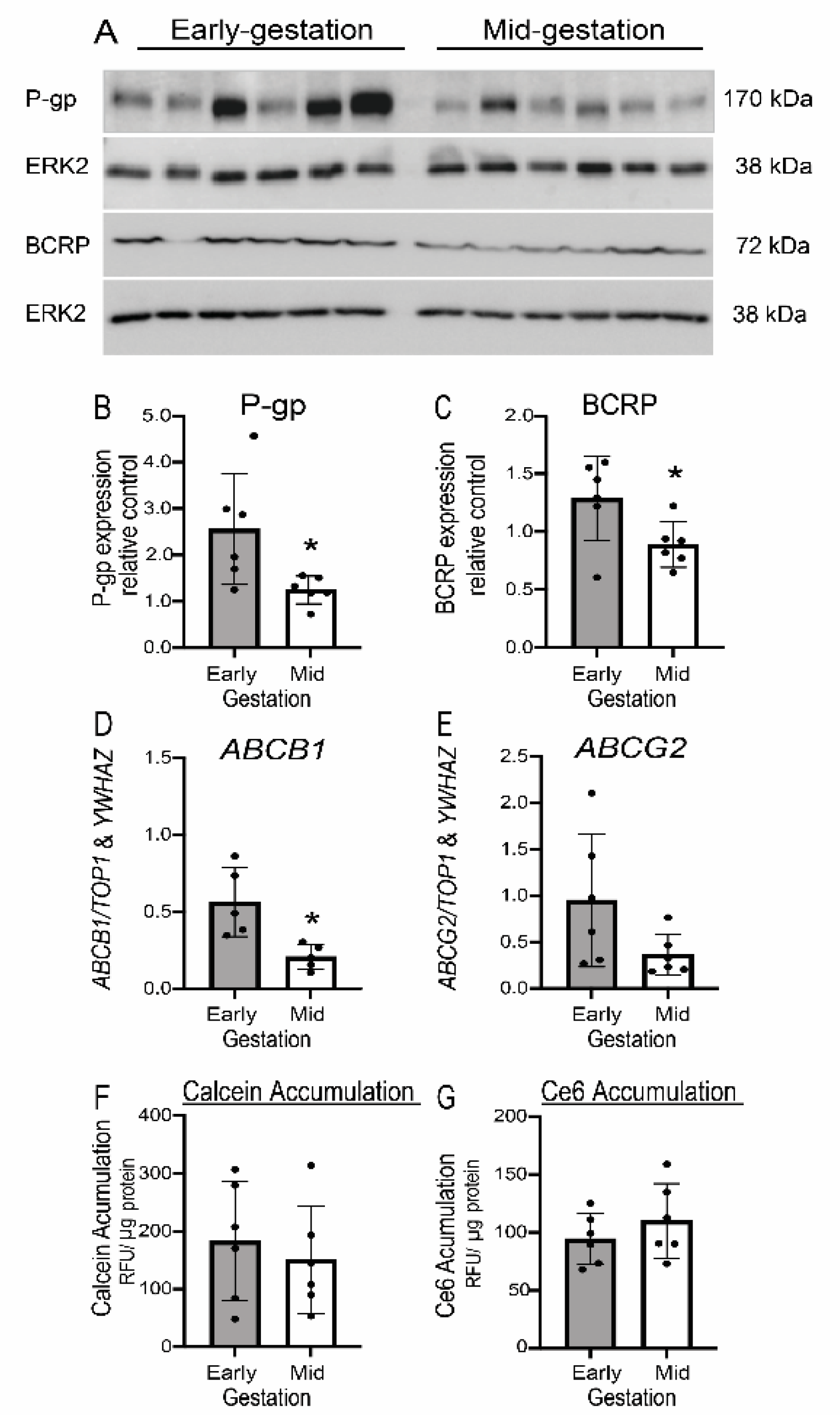
3.6. Expression but Not Function of P-gp/ABCB1 and BCRP/ABCG2 Is Developmentally Regulated in Human Fetal Brain Endothelial Cells (hfBECs)
3.7. Human Fetal Brain Endothelial Cells (hfBECs) Proliferation and Viability Does Not Change from Early to Mid-Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engelhardt, B.; Liebner, S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014, 355, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Eng, M.E.; Imperio, G.E.; Bloise, E.; Matthews, S.G. ATP-binding cassette (ABC) drug transporters in the developing blood-brain barrier: Role in fetal brain protection. Cell. Mol. Life Sci. CMLS 2022, 79, 415. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Hartz, A.M.; Bauer, B. ABC transporters in the CNS—An inventory. Curr. Pharm. Biotechnol. 2011, 12, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Matthews, S.G. Chapter 19—Multidrug Resistance P-Glycoprotein (P-gp), Glucocorticoids, and the Stress Response. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 227–241. [Google Scholar]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015, 1628, 298–316. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Friedman, A. Overview and introduction: The blood-brain barrier in health and disease. Epilepsia 2012, 53 (Suppl. S6), 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bloise, E.; Ortiga-Carvalho, T.M.; Reis, F.M.; Lye, S.J.; Gibb, W.; Matthews, S.G. ATP-binding cassette transporters in reproduction: A new frontier. Hum. Reprod. Update 2016, 22, 164–181. [Google Scholar] [CrossRef] [Green Version]
- Connor, K.L.; Bloise, E.; DeSantis, T.Z.; Lye, S.J. Adaptation of the gut holobiont to malnutrition during mouse pregnancy depends on the type of nutritional adversity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Li, M.; Anderson, G.D.; Wang, J. Drug-drug interactions involving membrane transporters in the human kidney. Expert Opin. Drug Metab. Toxicol. 2006, 2, 505–532. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, N.D.; Hardwick, R.N.; Brouwer, K.L. Role of hepatic efflux transporters in regulating systemic and hepatocyte exposure to xenobiotics. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 509–535. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Taskar, K.S.; Zamek-Gliszczynski, M.J. Importance of Hepatic Transporters in Clinical Disposition of Drugs and Their Metabolites. J. Clin. Pharmacol. 2016, 56 (Suppl. S7), S23–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, L.M.; Fontes, K.N.; Reginatto, M.W.; Andrade, C.B.V.; Monteiro, V.R.S.; Gomes, H.R.; Silva-Filho, J.L.; Pinheiro, A.A.S.; Vago, A.R.; Almeida, F.; et al. Malaria in pregnancy regulates P-glycoprotein (P-gp/Abcb1a) and ABCA1 efflux transporters in the Mouse Visceral Yolk Sac. J. Cell. Mol. Med. 2020, 24, 10636–10647. [Google Scholar] [CrossRef] [PubMed]
- Lye, P.; Bloise, E.; Dunk, C.; Javam, M.; Gibb, W.; Lye, S.J.; Matthews, S.G. Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta 2013, 34, 817–823. [Google Scholar] [CrossRef]
- Bloise, E.; Braga, J.R.S.; Andrade, C.B.V.; Imperio, G.E.; Martinelli, L.M.; Antunes, R.A.; Silva, K.R.; Nunes, C.B.; Cobellis, L.; Bloise, F.F.; et al. Altered Umbilical Cord Blood Nutrient Levels, Placental Cell Turnover and Transporter Expression in Human Term Pregnancies Conceived by Intracytoplasmic Sperm Injection (ICSI). Nutrients 2021, 13, 2587. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Baello, S.; Iqbal, M.; Kelly, L.E.; Shannon, P.T.; Chitayat, D.; Matthews, S.G.; Koren, G. The ontogeny of P-glycoprotein in the developing human blood-brain barrier: Implication for opioid toxicity in neonates. Pediatr. Res. 2015, 78, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Baello, S.; Iqbal, M.; Bloise, E.; Javam, M.; Gibb, W.; Matthews, S.G. TGF-β1 regulation of multidrug resistance P-glycoprotein in the developing male blood-brain barrier. Endocrinology 2014, 155, 475–484. [Google Scholar] [CrossRef]
- Iqbal, M.; Gibb, W.; Matthews, S.G. Corticosteroid regulation of P-glycoprotein in the developing blood-brain barrier. Endocrinology 2011, 152, 1067–1079. [Google Scholar] [CrossRef] [Green Version]
- Baello, S.; Iqbal, M.; Kearney, S.; Kuthiala, S.; Bloise, E.; Gibb, W.; Matthews, S.G. Glucocorticoids modify effects of TGF-β1 on multidrug resistance in the fetal blood-brain barrier. Growth Factors 2016, 34, 33–41. [Google Scholar] [CrossRef]
- Iqbal, M.; Ho, H.L.; Petropoulos, S.; Moisiadis, V.G.; Gibb, W.; Matthews, S.G. Pro-inflammatory cytokine regulation of P-glycoprotein in the developing blood-brain barrier. PLoS ONE 2012, 7, e43022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eustaquio Do Imperio, G.; Lye, P.; Bloise, E.; Matthews, S.G. Function of Multidrug Resistance Transporters is Disrupted by Infection Mimics in Human Brain Endothelial Cells. Tissue Barriers 2021, 9, 1860616. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Gibb, W.; Matthews, S.G. Developmental expression of multidrug resistance phosphoglycoprotein (P-gp) in the mouse fetal brain and glucocorticoid regulation. Brain Res. 2010, 1357, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Imperio, G.E.; Lye, P.; Mughis, H.; Hamada, H.; Bloise, E.; Lye, S.J.; Matthews, S.G. Hypoxia alters the expression of ACE2 and TMPRSS2 SARS-CoV-2 cell entry mediators in hCMEC/D3 brain endothelial cells. Microvasc. Res. 2021, 138, 104232. [Google Scholar] [CrossRef] [PubMed]
- Varanat, M.; Maggi, R.G.; Linder, K.E.; Breitschwerdt, E.B. Infection of human brain vascular pericytes (HBVPs) by Bartonella henselae. Med Microbiol Immunol 2013, 202, 143–151. [Google Scholar] [CrossRef]
- Baello, S.; Iqbal, M.; Gibb, W.; Matthews, S.G. Astrocyte-mediated regulation of multidrug resistance p-glycoprotein in fetal and neonatal brain endothelial cells: Age-dependent effects. Physiol. Rep. 2016, 4, e12853. [Google Scholar] [CrossRef] [Green Version]
- Farine, T.; Parsons, M.; Lye, S.; Shynlova, O. Isolation of Primary Human Decidual Cells from the Fetal Membranes of Term Placentae. J. Vis. Exp. 2018, 134, e57443. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, L.; Brkic, J.; Chen, Y.F.; Bui, T.; Munir, S.; Peng, C. Cytoplasmic mislocalization of p27 and CDK2 mediates the anti-migratory and anti-proliferative effects of Nodal in human trophoblast cells. J. Cell Sci. 2013, 126, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Lye, P.; Bloise, E.; Nadeem, L.; Gibb, W.; Lye, S.J.; Matthews, S.G. Glucocorticoids modulate multidrug resistance transporters in the first trimester human placenta. J. Cell. Mol. Med. 2018, 22, 3652–3660. [Google Scholar] [CrossRef] [Green Version]
- Lye, P.; Bloise, E.; Nadeem, L.; Peng, C.; Gibb, W.; Ortiga-Carvalho, T.M.; Lye, S.J.; Matthews, S.G. Breast Cancer Resistance Protein (BCRP/ABCG2) Inhibits Extra Villous Trophoblast Migration: The Impact of Bacterial and Viral Infection. Cells 2019, 8, 1150. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Drewlo, S.; Levytska, K.; Kingdom, J. Revisiting the housekeeping genes of human placental development and insufficiency syndromes. Placenta 2012, 33, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Drewe, J.; Krahenbuhl, S.; Huwyler, J.; Gutmann, H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell. Mol. Neurobiol. 2010, 30, 63–70. [Google Scholar] [CrossRef]
- Poller, B.; Gutmann, H.; Krahenbuhl, S.; Weksler, B.; Romero, I.; Couraud, P.O.; Tuffin, G.; Drewe, J.; Huwyler, J. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J. Neurochem. 2008, 107, 1358–1368. [Google Scholar] [CrossRef]
- Marquez-Curtis, L.A.; Bokenfohr, R.; McGann, L.E.; Elliott, J.A.W. Cryopreservation of human cerebral microvascular endothelial cells and astrocytes in suspension and monolayers. PLoS ONE 2021, 16, e0249814. [Google Scholar] [CrossRef]
- Shathasivam, P.; Kollara, A.; Spybey, T.; Park, S.; Clarke, B.; Ringuette, M.J.; Brown, T.J. VEPH1 expression decreases vascularisation in ovarian cancer xenografts and inhibits VEGFA and IL8 expression through inhibition of AKT activation. Br. J. Cancer 2017, 116, 1065–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, K.S.; Siegel, A.C. Cell Viability Analysis Using Trypan Blue: Manual and Automated Methods. Mamm. Cell Viability 2011, 740, 7–12. [Google Scholar] [CrossRef]
- Cooray, H.C.; Blackmore, C.G.; Maskell, L.; Barrand, M.A. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport 2002, 13, 2059–2063. [Google Scholar] [CrossRef]
- Zhang, W.; Mojsilovic-Petrovic, J.; Andrade, M.F.; Zhang, H.; Ball, M.; Stanimirovic, D.B. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003, 17, 2085–2087. [Google Scholar] [CrossRef] [Green Version]
- Aronica, E.; Gorter, J.A.; Redeker, S.; van Vliet, E.A.; Ramkema, M.; Scheffer, G.L.; Scheper, R.J.; van der Valk, P.; Leenstra, S.; Baayen, J.C.; et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia 2005, 46, 849–857. [Google Scholar] [CrossRef]
- Virgintino, D.; Robertson, D.; Errede, M.; Benagiano, V.; Girolamo, F.; Maiorano, E.; Roncali, L.; Bertossi, M. Expression of P-glycoprotein in human cerebral cortex microvessels. J. Histochem. Cytochem. 2002, 50, 1671–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollgard, K.; Dziegielewska, K.M.; Holst, C.B.; Habgood, M.D.; Saunders, N.R. Brain barriers and functional interfaces with sequential appearance of ABC efflux transporters during human development. Sci. Rep. 2017, 7, 11603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daood, M.; Tsai, C.; Ahdab-Barmada, M.; Watchko, J.F. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 2008, 39, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Podgrabinska, S.; Braun, P.; Velasco, P.; Kloos, B.; Pepper, M.S.; Jackson, D.G.; Skobe, M. Molecular characterization of lymphatic endothelial cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16069. [Google Scholar] [CrossRef] [Green Version]
- Kriehuber, E.; Breiteneder-Geleff, S.; Groeger, M.; Soleiman, A.; Schoppmann, S.F.; Stingl, G.; Kerjaschki, D.; Maurer, D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 2001, 194, 797–808. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Diaz, K.; Koster, K.P.; Tai, L.M. In Vitro Assays to Assess Blood-brain Barrier Mesh-like Vessel Formation and Disruption. J. Vis. Exp. 2017, 124, 55846. [Google Scholar] [CrossRef]
- Bernard-Patrzynski, F.; Lécuyer, M.-A.; Puscas, I.; Boukhatem, I.; Charabati, M.; Bourbonnière, L.; Ramassamy, C.; Leclair, G.; Prat, A.; Roullin, V.G. Isolation of endothelial cells, pericytes and astrocytes from mouse brain. PLoS ONE 2019, 14, e0226302. [Google Scholar] [CrossRef] [Green Version]
- Dömer, P.; Kayal, J.; Janssen-Bienhold, U.; Kewitz, B.; Kretschmer, T.; Heinen, C. Rapid and efficient immunomagnetic isolation of endothelial cells from human peripheral nerves. Sci. Rep. 2021, 11, 1951. [Google Scholar] [CrossRef]
- Ribatti, D. Angiogenesis. In Brenner’s Encyclopedia of Genetics (Second Edition); Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 130–132. [Google Scholar]
- Dunk, C.E.; Pappas, J.J.; Lye, P.; Kibschull, M.; Javam, M.; Bloise, E.; Lye, S.J.; Szyf, M.; Matthews, S.G. P-Glycoprotein (P-gp)/ABCB1 plays a functional role in extravillous trophoblast (EVT) invasion and is decreased in the pre-eclamptic placenta. J. Cell. Mol. Med. 2018, 22, 5378–5393. [Google Scholar] [CrossRef]
- Barakat, S.; Turcotte, S.; Demeule, M.; Lachambre, M.P.; Régina, A.; Baggetto, L.G.; Béliveau, R. Regulation of brain endothelial cells migration and angiogenesis by P-glycoprotein/caveolin-1 interaction. Biochem. Biophys. Res. Commun. 2008, 372, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Salaroli, R.; Parenti, F.; De Maria, R.; Zannoni, A.; Bernardini, C.; Gola, C.; Brocco, A.; Marangio, A.; Benazzi, C.; et al. Doxorubicin treatment modulates chemoresistance and affects the cell cycle in two canine mammary tumour cell lines. BMC Vet. Res. 2021, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Gibb, W.; Matthews, S.G. Breast cancer-resistance protein (BCRP1) in the fetal mouse brain: Development and glucocorticoid regulation. Biol. Reprod. 2011, 84, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, M.; Watanabe, M.; Hori, S.; Fukaya, M.; Ohtsuki, S.; Asashima, T.; Terasaki, T. Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J. Neurochem. 2005, 95, 294–304. [Google Scholar] [CrossRef]
- Orford, M.; Mean, R.; Lapathitis, G.; Genethliou, N.; Panayiotou, E.; Panayi, H.; Malas, S. Generation of an ABCG2(GFPn-puro) transgenic line--a tool to study ABCG2 expression in mice. Biochem. Biophys. Res. Commun. 2009, 384, 199–203. [Google Scholar] [CrossRef]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC Transporters at the Blood-Brain Interfaces, Their Study Models, and Drug Delivery Implications in Gliomas. Pharmaceutics 2019, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X.B. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. B 2019, 9, 659–674. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.F. Efflux transporters in blood-brain interfaces of the developing brain. Front. Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Bloise, E.; Petropoulos, S.; Iqbal, M.; Kostaki, A.; Ortiga-Carvalho, T.M.; Gibb, W.; Matthews, S.G. Acute Effects of Viral Exposure on P-Glycoprotein Function in the Mouse Fetal Blood-Brain Barrier. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 1044–1050. [Google Scholar] [CrossRef]
- Bloise, E.; Bhuiyan, M.; Audette, M.C.; Petropoulos, S.; Javam, M.; Gibb, W.; Matthews, S.G. Prenatal endotoxemia and placental drug transport in the mouse: Placental size-specific effects. PLoS ONE 2013, 8, e65728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, M.E.; Bloise, E.; Matthews, S.G. Fetal glucocorticoid exposure leads to sex-specific changes in drug-transporter function at the blood-brain barrier in juvenile guinea pigs. FASEB J. 2022, 36, e22245. [Google Scholar] [CrossRef] [PubMed]
- Szaflarski, W.; Sujka-Kordowska, P.; Januchowski, R.; Wojtowicz, K.; Andrzejewska, M.; Nowicki, M.; Zabel, M. Nuclear localization of P-glycoprotein is responsible for protection of the nucleus from doxorubicin in the resistant LoVo cell line. Biomed. Pharmacother. 2013, 67, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Tome, M.E.; Herndon, J.M.; Schaefer, C.P.; Jacobs, L.M.; Zhang, Y.; Jarvis, C.K.; Davis, T.P. P-glycoprotein traffics from the nucleus to the plasma membrane in rat brain endothelium during inflammatory pain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 1913–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendayan, R.; Ronaldson, P.; Gingras, D.; Bendayan, M. In Situ Localization of P-glycoprotein (ABCB1) in Human and Rat Brain. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2006, 54, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, P.; Bernier, M.; Sanghvi, M.; Moaddel, R.; Schwarting, R.; Ramamoorthy, A.; Wainer, I.W. Breast cancer resistance protein (BCRP/ABCG2) localises to the nucleus in glioblastoma multiforme cells. Xenobiotica 2012, 42, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Nickel, S.; Selo, M.A.; Fallack, J.; Clerkin, C.G.; Huwer, H.; Schneider-Daum, N.; Lehr, C.M.; Ehrhardt, C. Expression and Activity of Breast Cancer Resistance Protein (BCRP/ABCG2) in Human Distal Lung Epithelial Cells In Vitro. Pharm. Res. 2017, 34, 2477–2487. [Google Scholar] [CrossRef]
- Liang, S.C.; Yang, C.Y.; Tseng, J.Y.; Wang, H.L.; Tung, C.Y.; Liu, H.W.; Chen, C.Y.; Yeh, Y.C.; Chou, T.Y.; Yang, M.H.; et al. ABCG2 localizes to the nucleus and modulates CDH1 expression in lung cancer cells. Neoplasia 2015, 17, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Takada, T.; Suzuki, H.; Gotoh, Y.; Sugiyama, Y. Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab. Dispos. 2005, 33, 905–909. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Han, X.; Wang, Z.; Wang, Z.; Cui, Y.; Tian, R.; Zhu, Y.; Han, B.; Liu, H.; Zuo, X.; et al. Mitochondrial Breast Cancer Resistant Protein Sustains the Proliferation and Survival of Drug-Resistant Breast Cancer Cells by Regulating Intracellular Reactive Oxygen Species. Front. Cell Dev. Biol. 2021, 9, 719209. [Google Scholar] [CrossRef]
- Solazzo, M.; Fantappie, O.; D’Amico, M.; Sassoli, C.; Tani, A.; Cipriani, G.; Bogani, C.; Formigli, L.; Mazzanti, R. Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines. Cancer Res. 2009, 69, 7235–7242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsani, E.; Della Vedova, A.M.; Rezzani, R.; Rodella, L.F.; Cristini, C. Correlation between human nervous system development and acquisition of fetal skills: An overview. Brain Dev. 2019, 41, 225–233. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Sequence | Reference |
|---|---|---|
| ABCB1 | Forward: 5′GCCCTTGTTAGACAGCCTCA-3′ | [30] |
| Reverse: 5′GGCTTTGTCCAGGGCTTCTT-3′ | ||
| ABCG2 | Forward: 5′-TGGAATCCAGAACAGAGCTGGGGT-3′ | [30] |
| Reverse: 5′-AGAGTTCCACGGCTGAAACACTGC-3′ | ||
| YWHAZ | Forward: 5′-CCGCCAGGACAAACCAGTAT-3 | [33] |
| Reverse: 5′-CAC ATC ACA GCT CCC CAC CA-3′ | ||
| TOP1 | Forward: 5′-GATGAACCTGAAGATGATGGC-3′ | [33] |
| Reverse: 5′-TCAGCATCATCCTCATCTCG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lye, P.; Bloise, E.; Imperio, G.E.; Chitayat, D.; Matthews, S.G. Functional Expression of Multidrug-Resistance (MDR) Transporters in Developing Human Fetal Brain Endothelial Cells. Cells 2022, 11, 2259. https://doi.org/10.3390/cells11142259
Lye P, Bloise E, Imperio GE, Chitayat D, Matthews SG. Functional Expression of Multidrug-Resistance (MDR) Transporters in Developing Human Fetal Brain Endothelial Cells. Cells. 2022; 11(14):2259. https://doi.org/10.3390/cells11142259
Chicago/Turabian StyleLye, Phetcharawan, Enrrico Bloise, Guinever E. Imperio, David Chitayat, and Stephen G. Matthews. 2022. "Functional Expression of Multidrug-Resistance (MDR) Transporters in Developing Human Fetal Brain Endothelial Cells" Cells 11, no. 14: 2259. https://doi.org/10.3390/cells11142259
APA StyleLye, P., Bloise, E., Imperio, G. E., Chitayat, D., & Matthews, S. G. (2022). Functional Expression of Multidrug-Resistance (MDR) Transporters in Developing Human Fetal Brain Endothelial Cells. Cells, 11(14), 2259. https://doi.org/10.3390/cells11142259






