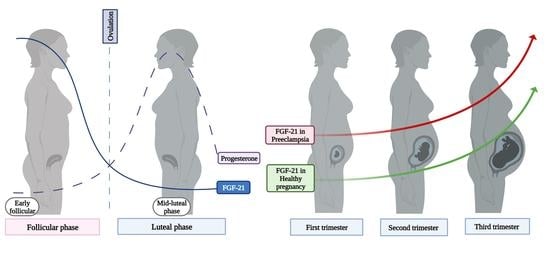
Maternal Fibroblast Growth Factor 21 Levels Decrease during Early Pregnancy in Normotensive Pregnant Women but Are Higher in Preeclamptic Women—A Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Study Population
2.3. Clinical Evaluation
2.4. Biochemical and Hormonal Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Serum Levels of Progesterone in Non-Pregnant Women
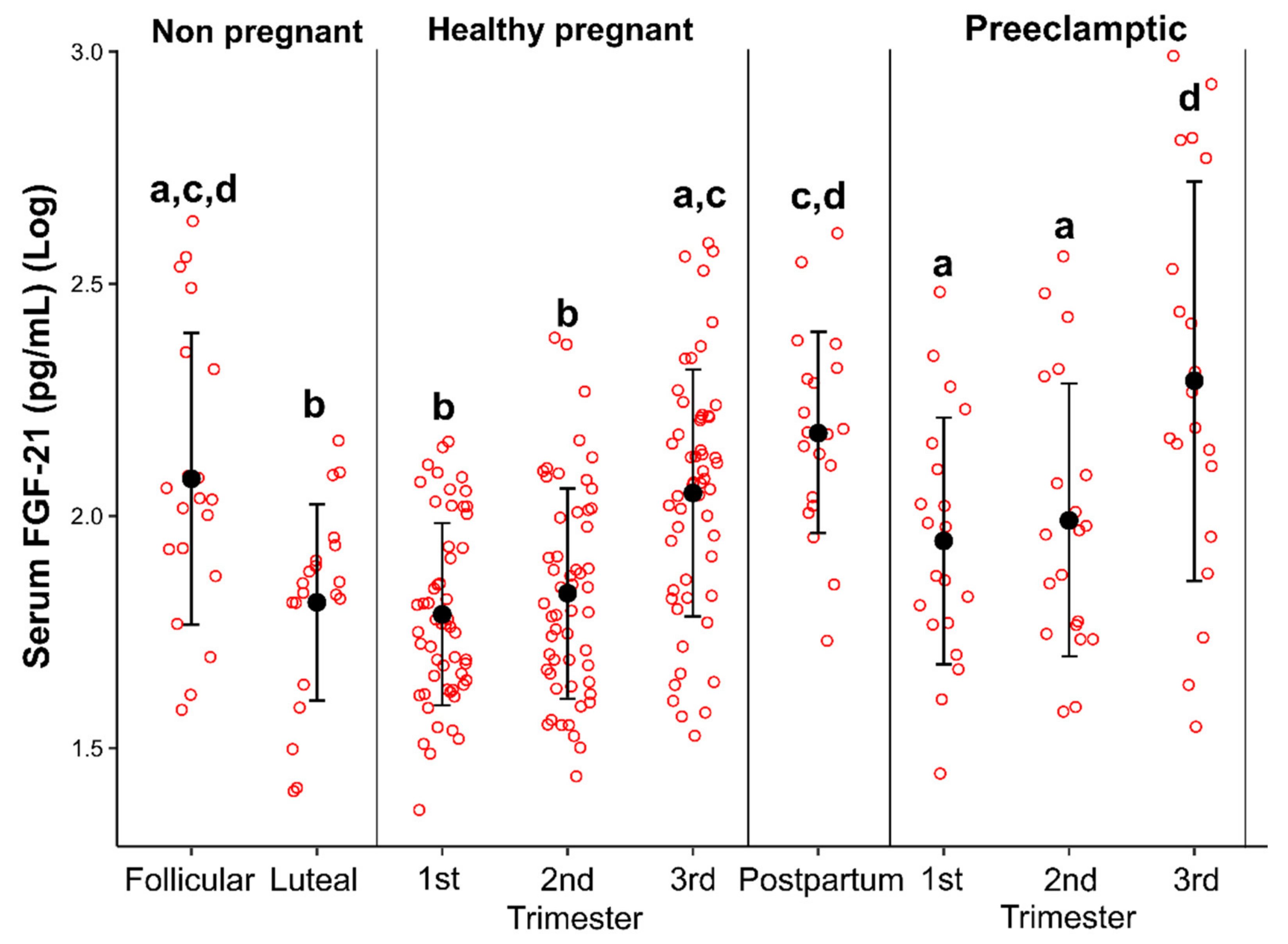
3.3. Serum Levels of FGF-21 in Eumenorrheic Women
3.4. Serum Levels of FGF-21 in Healthy Pregnant Women
3.5. Serum Levels of FGF-21 in Postpartum Period
3.6. Serum Levels of FGF-21 in Pregnant Women with Preeclampsia
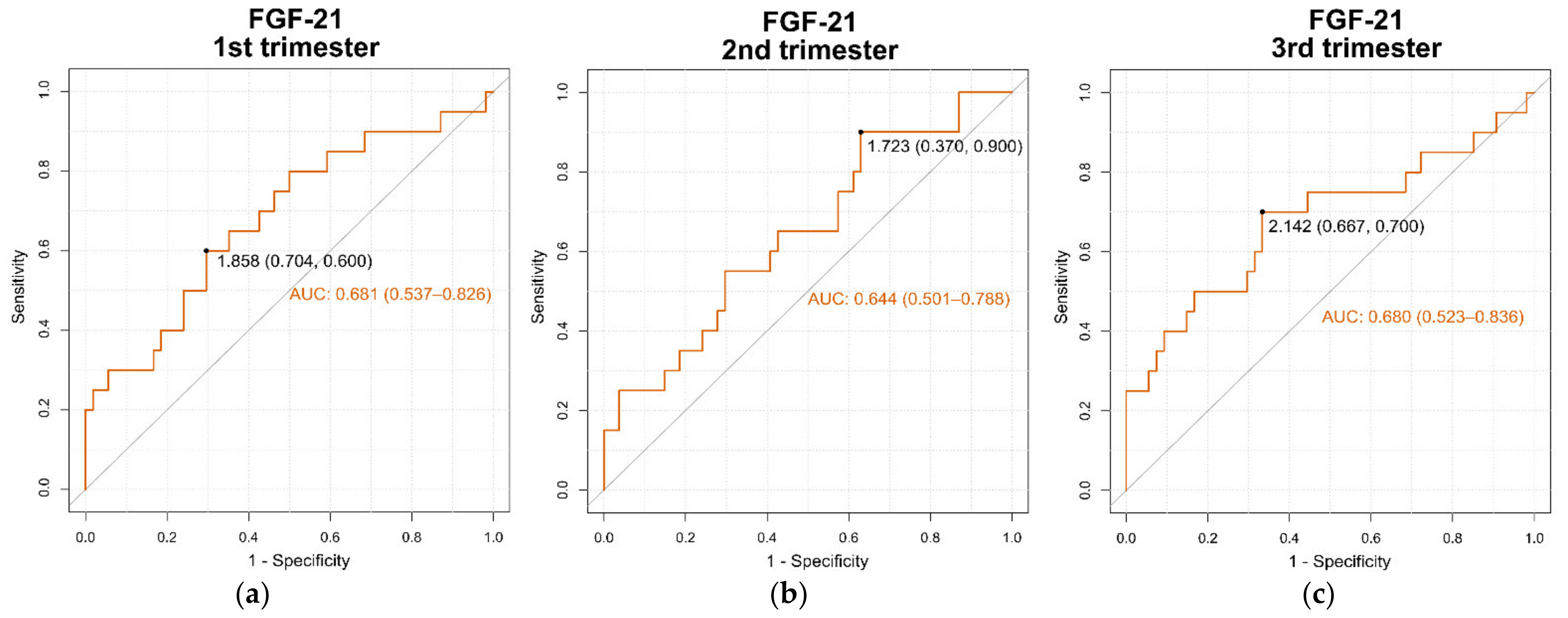
3.7. Area under the ROC Curve (AUC) for Serum FGF-21 Levels
3.8. Evaluation of sFlt-1/PlGF Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allard, C.; Bonnet, F.; Xu, B.; Coons, L.; Albarado, D.; Hill, C.; Fagherazzi, G.; Korach, K.S.; Levin, E.R.; Lefante, J.; et al. Activation of Hepatic Estrogen Receptor-α Increases Energy Expenditure by Stimulating the Production of Fibroblast Growth Factor 21 in Female Mice. Mol. Metab. 2019, 22, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake during Refeeding and Overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARα and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine Regulation of the Fasting Response by PPARalpha-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, L.S.; Giesy, S.L.; Krumm, C.S.; Perfield, J.W.; Butterfield, A.; Schoenberg, K.M.; Beitz, D.C.; Boisclair, Y.R. Effect of Circulating Glucagon and Free Fatty Acids on Hepatic FGF21 Production in Dairy Cows. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R526–R534. [Google Scholar] [CrossRef]
- Wang, J.G.; Guo, Y.Z.; Kong, Y.Z.; Dai, S.; Zhao, B.Y. High Non-Esterified Fatty Acid Concentrations Promote Expression and Secretion of Fibroblast Growth Factor 21 in Calf Hepatocytes Cultured in Vitro. J. Anim. Physiol. Anim. Nutr. 2018, 102, e476–e481. [Google Scholar] [CrossRef]
- Rosales-Soto, G.; Diaz-Vegas, A.; Casas, M.; Contreras-Ferrat, A.; Jaimovich, E. Fibroblast Growth Factor-21 Potentiates Glucose Transport in Skeletal Muscle Fibers. J. Mol. Endocrinol. 2020, 65, 85–95. [Google Scholar] [CrossRef]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The Effects of LY2405319, an FGF21 Analog, in Obese Human Subjects with Type 2 Diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef]
- Chavez, A.O.; Molina-Carrion, M.; Abdul-Ghani, M.A.; Folli, F.; Defronzo, R.A.; Tripathy, D. Circulating Fibroblast Growth Factor-21 Is Elevated in Impaired Glucose Tolerance and Type 2 Diabetes and Correlates with Muscle and Hepatic Insulin Resistance. Diabetes Care 2009, 32, 1542–1546. [Google Scholar] [CrossRef]
- Zhang, X.; Yeung, D.C.Y.; Karpisek, M.; Stejskal, D.; Zhou, Z.-G.; Liu, F.; Wong, R.L.C.; Chow, W.-S.; Tso, A.W.K.; Lam, K.S.L.; et al. Serum FGF21 Levels Are Increased in Obesity and Are Independently Associated with the Metabolic Syndrome in Humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef]
- Staiger, H.; Keuper, M.; Berti, L.; Hrabe de Angelis, M.; Häring, H.-U. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Martin, R.C.; Shi, X.; Pandit, H.; Yu, Y.; Liu, X.; Guo, W.; Tan, M.; Bai, O.; Meng, X.; et al. Lack of FGF21 Promotes NASH-HCC Transition via Hepatocyte-TLR4-IL-17A Signaling. Theranostics 2020, 10, 9923–9936. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Horvath, T.L.; Mangelsdorf, D.J.; Kliewer, S.A.; Dixit, V.D. Prolongevity Hormone FGF21 Protects against Immune Senescence by Delaying Age-Related Thymic Involution. Proc. Natl. Acad. Sci. USA 2016, 113, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Stepan, H.; Kratzsch, J.; Verlohren, M.; Verlohren, H.-J.; Drynda, K.; Lössner, U.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Serum Fibroblast Growth Factor 21 Levels in Gestational Diabetes Mellitus in Relation to Insulin Resistance and Dyslipidemia. Metabolism 2010, 59, 33–37. [Google Scholar] [CrossRef]
- Yuan, D.; Wu, B.J.; Henry, A.; Rye, K.-A.; Ong, K.L. Role of Fibroblast Growth Factor 21 in Gestational Diabetes Mellitus: A Mini-Review. Clin. Endocrinol. 2019, 90, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bakrania, B.A.; Spradley, F.T.; Drummond, H.A.; LaMarca, B.; Ryan, M.J.; Granger, J.P. Preeclampsia: Linking Placental Ischemia with Maternal Endothelial and Vascular Dysfunction. Compr. Physiol. 2020, 11, 1315–1349. [Google Scholar] [CrossRef]
- Chen, C.W.; Jaffe, I.Z.; Karumanchi, S.A. Pre-Eclampsia and Cardiovascular Disease. Cardiovasc. Res. 2014, 101, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Kley, K.; Hindricks, J.; Kralisch, S.; Jank, A.; Schaarschmidt, W.; Schrey, S.; Ebert, T.; Lössner, U.; Kratzsch, J.; et al. Serum Levels of the Adipokine Fibroblast Growth Factor-21 Are Increased in Preeclampsia. Cytokine 2013, 62, 322–326. [Google Scholar] [CrossRef]
- Dekker Nitert, M.; Scholz-Romero, K.; Kubala, M.H.; McIntyre, H.D.; Callaway, L.K.; Barrett, H.L. Placental Fibroblast Growth Factor 21 Is Not Altered in Late-Onset Preeclampsia. Reprod. Biol. Endocrinol. 2015, 13, 14. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Y.; Huang, Q. Serum Fibroblast Growth Factor 21 Level Is Increased in Pre-eclampsia Patients: Association with Blood Pressure and Lipid Profile. J. Obstet. Gynaecol. Res. 2021, 47, 375–381. [Google Scholar] [CrossRef]
- Dalamaga, M.; Srinivas, S.K.; Elovitz, M.A.; Chamberland, J.; Mantzoros, C.S. Serum Adiponectin and Leptin in Relation to Risk for Preeclampsia: Results from a Large Case-Control Study. Metabolism 2011, 60, 1539–1544. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Hypertension in Pregnancy: Executive Summary. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Y.; Zeng, H.; Li, L.; Zhao, J.; Zhao, J.; Liu, F.; Bao, Y.; Jia, W. Serum Fibroblast Growth Factor 21 Levels Is Associated with Lower Extremity Atherosclerotic Disease in Chinese Female Diabetic Patients. Cardiovasc. Diabetol. 2015, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a Novel Metabolic Regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Alvino, G.; Cozzi, V.; Radaelli, T.; Ortega, H.; Herrera, E.; Cetin, I. Maternal and Fetal Fatty Acid Profile in Normal and Intrauterine Growth Restriction Pregnancies with and Without Preeclampsia. Pediatr. Res. 2008, 64, 615–620. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, F.; Li, S. Metabolic Adaptations in Pregnancy: A Review. Ann. Nutr. Metab. 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Herrera, E.; Desoye, G. Maternal and Fetal Lipid Metabolism under Normal and Gestational Diabetic Conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of Preeclampsia: Risk Factors and Outcomes Associated with Early- versus Late-Onset Disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Steyn, W.; Zeeman, G.G.; Brown, M.A. The Classification, Diagnosis and Management of the Hypertensive Disorders of Pregnancy: A Revised Statement from the ISSHP. Pregnancy Hypertens. 2014, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.; Zembala-Szczerba, M.; Babczyk, D.; Kołodziejczyk-Pietruszka, M.; Lewaczyńska, O.; Huras, H. Early- and Late-Onset Preeclampsia: A Comprehensive Cohort Study of Laboratory and Clinical Findings According to the New ISHHP Criteria. Int. J. Hypertens. 2019, 2019, e4108271. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. The Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis & Management Recommendations for International Practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- El Beltagy, N.S.; El Deen Sadek, S.S.; Zidan, M.A.; Abd El Naby, R.E. Can Serum Free Fatty Acids Assessment Predict Severe Preeclampsia? Alex. J. Med. 2011, 47, 277–281. [Google Scholar] [CrossRef][Green Version]
- Robinson, N.J.; Minchell, L.J.; Myers, J.E.; Hubel, C.A.; Crocker, I.P. A Potential Role for Free Fatty Acids in the Pathogenesis of Preeclampsia. J. Hypertens. 2009, 27, 1293–1302. [Google Scholar] [CrossRef]
- Villa, P.M.; Laivuori, H.; Kajantie, E.; Kaaja, R. Free Fatty Acid Profiles in Preeclampsia. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-W.; Jiang, X.; Zhang, Y.; Wang, J.; Xie, J.; Wang, Y.-Q.; Li, Y.-H. FGF21 Protects Against Hypoxia Injury Through Inducing HSP72 in Cerebral Microvascular Endothelial Cells. Front. Pharmacol. 2019, 10, 101. [Google Scholar] [CrossRef]
- Bohorquez-Villamizar, L.; Garces, M.; Poveda, N.; Sanchez, E.; Alvarado-Quintero, H.; Sandoval-Alzate, H.; Rueda, E.; Dieguez, C.; Nogueiras, R.; Caminos, J. Changes in Serum Fatty Acids Levels during Pregnancy and After Delivery in a Longitudinal Study. J. Nutr. Biol. 2018, 4, 222–231. [Google Scholar] [CrossRef]
- Lorentzen, B.; Drevon, C.A.; Endresen, M.J.; Henriksen, T. Fatty Acid Pattern of Esterified and Free Fatty Acids in Sera of Women with Normal and Pre-Eclamptic Pregnancy. Br. J. Obstet. Gynaecol. 1995, 102, 530–537. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Fatty Acids as Modulators of the Cellular Production of Reactive Oxygen Species. Free Radic. Biol. Med. 2008, 45, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Ma, J.; Feng, B.; Zhang, H.; Diehl, J.A.; Chin, Y.E.; Yan, W.; Xu, H. FFA-Induced Adipocyte Inflammation and Insulin Resistance: Involvement of ER Stress and IKKβ Pathways. Obesity 2011, 19, 483–491. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Li, J.; Wang, G.; Li, L. Non-Esterified Fatty Acid-Induced Reactive Oxygen Species Mediated Granulosa Cells Apoptosis Is Regulated by Nrf2/P53 Signaling Pathway. Antioxidants 2020, 9, 523. [Google Scholar] [CrossRef]
- Gómez-Sámano, M.Á.; Grajales-Gómez, M.; Zuarth-Vázquez, J.M.; Navarro-Flores, M.F.; Martínez-Saavedra, M.; Juárez-León, Ó.A.; Morales-García, M.G.; Enríquez-Estrada, V.M.; Gómez-Pérez, F.J.; Cuevas-Ramos, D. Fibroblast Growth Factor 21 and Its Novel Association with Oxidative Stress. Redox Biol. 2017, 11, 335–341. [Google Scholar] [CrossRef]
- Tanajak, P.; Chattipakorn, S.C.; Chattipakorn, N. Effects of Fibroblast Growth Factor 21 on the Heart. J. Endocrinol. 2015, 227, R13–R30. [Google Scholar] [CrossRef]
- Xie, T.; So, W.Y.; Li, X.Y.; Leung, P.S. Fibroblast Growth Factor 21 Protects against Lipotoxicity-Induced Pancreatic β-Cell Dysfunction via Regulation of AMPK Signaling and Lipid Metabolism. Clin. Sci. 1979 2019, 133, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity Is a Fibroblast Growth Factor 21 (FGF21)-Resistant State. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef]
- Sivan, E.; Boden, G. Free Fatty Acids, Insulin Resistance, and Pregnancy. Curr. Diabetes Rep. 2003, 3, 319–322. [Google Scholar] [CrossRef]
- Diderholm, B.; Stridsberg, M.; Ewald, U.; Lindeberg-Nordén, S.; Gustafsson, J. Increased Lipolysis in Non-Obese Pregnant Women Studied in the Third Trimester. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 713–718. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Lipid Metabolism during Pregnancy and Its Implications for Fetal Growth. Curr. Pharm. Biotechnol. 2014, 15, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome Proliferator-Activated Receptors and Their Ligands: Nutritional and Clinical Implications—A Review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Huston Presley, L.P.; Locascio, J.J.; Catalano, P.M. Augmented Insulin Secretory Response in Early Pregnancy. Diabetologia 2019, 62, 1445–1452. [Google Scholar] [CrossRef]
- Phillips, M.I.; Kagiyama, S. Angiotensin II as a Pro-Inflammatory Mediator. Curr. Opin. Investig. Drugs 2002, 3, 569–577. [Google Scholar] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Mallick, R.; Duttaroy, A.K. Modulation of Endothelium Function by Fatty Acids. Mol. Cell. Biochem. 2022, 477, 15–38. [Google Scholar] [CrossRef]
- Pan, X.; Shao, Y.; Wu, F.; Wang, Y.; Xiong, R.; Zheng, J.; Tian, H.; Wang, B.; Wang, Y.; Zhang, Y.; et al. FGF21 Prevents Angiotensin II-Induced Hypertension and Vascular Dysfunction by Activation of ACE2/Angiotensin-(1-7) Axis in Mice. Cell Metab. 2018, 27, 1323–1337.e5. [Google Scholar] [CrossRef]
- Vigne, J.L.; Murai, J.T.; Arbogast, B.W.; Jia, W.; Fisher, S.J.; Taylor, R.N. Elevated Nonesterified Fatty Acid Concentrations in Severe Preeclampsia Shift the Isoelectric Characteristics of Plasma Albumin. J. Clin. Endocrinol. Metab. 1997, 82, 3786–3792. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Cantero, I.; Abete, I.; Bullón-Vela, V.; Crujeiras, A.B.; Casanueva, F.F.; Zulet, M.A.; Martinez, J.A. Fibroblast Growth Factor 21 Levels and Liver Inflammatory Biomarkers in Obese Subjects after Weight Loss. Arch. Med. Sci. AMS 2022, 18, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Brennecke, S.P.; Galindo, A.; Karumanchi, S.A.; Mirkovic, L.B.; Schlembach, D.; Stepan, H.; Vatish, M.; Zeisler, H.; Rana, S. Clinical Interpretation and Implementation of the SFlt-1/PlGF Ratio in the Prediction, Diagnosis and Management of Preeclampsia. Pregnancy Hypertens. 2022, 27, 42–50. [Google Scholar] [CrossRef] [PubMed]
| Variables | 1st Trimester | 2nd Trimester | 3rd Trimester | p Value (One-Way ANOVA Test) |
|---|---|---|---|---|
| Maternal Age (years) | 25.6 ± 6.3 (17.0–38.0) | - | - | |
| Gestational Age (weeks) | 12.13 ± 0.64 (11.0–13.6) | 24.46 ± 0.69 (23.4–27.3) | 354.7 ± 0.95 (33.4–38.6) | |
| BMI (kg/m2) | 22.49 ± 2.34 (18.50–27.60) | 24.31 ± 2.42 (19.20–30.20) | 26.22 ± 2.49 (20.60–31.60) | 0.0000 |
| SBP (mmHg) | 95.34 ± 8.69 (80.00–118.00) | 92.97 ± 9.62 (70.00–111.00) | 97.34 ± 9.46 (80.00–130.00) | 0.0285 |
| DBP (mmHg) | 61.60± 6.28 (50.00–80.00) | 60.45 ± 6.14 (50.00–90.00) | 62.89 ± 8.51 (50.00–90.00) | 0.1965 |
| MBP (mmHg) | 72.84 ± 6.33 (60.00–92.67) | 72.29 ± 6.10 (56.67–94.00) | 74.37 ± 7.96 (60.00–96.67) | 0.0520 |
| Blood Glucose (mg/dL) | 77.91 ± 6.20 (64.00–92.00) | 73.80 ± 5.27 (63.00–84.70) | 74.15 ± 6.20 (63.00–88.00) | 0.0001 |
| Insulin (µUI/mL) | 9.30 ± 4.25 (2.70–19.80) | 11.12 ± 4.61 (3.20–24.10) | 12.85 ± 5.82 (2.20–24.70) | 0.0025 |
| HOMA Index | 1.78 ± 0.88 (0.45–4.01) | 2.00 ± 0.83 (0.61–4.22) | 2.44 ± 1.14 (0.76–4.52) | 0.0266 |
| Total Cholesterol (mg/dL) | 166.39 ± 30.54 (111.02–251.60) | 218.30 ± 39.43 (133.50–310.00) | 246.12 ± 48.33 (152.20–362.80) | 0.0000 |
| HDL-C (mg/dL) | 56.27 ± 10.01 (38.00–80.00) | 67.39 ± 12.01 (42.97–90.27) | 66.51 ± 11.94 (39.94–93.37) | 0.0000 |
| LDL (mg/dL) | 116.96 ± 32.99 (53.70–197.12) | 145.12 ± 45.53 (72.22–260.60) | 157.60 ± 44.47 (75.00–257.97) | 0.0000 |
| VLDL (mg/dL) | 22.10 ± 6.78 (10.56–42.06) | 35.50 ± 10.26 (16.80–62.94) | 48.64 ± 14.58 (21.40–83.04) | 0.0000 |
| Triglycerides (mg/dL) | 116.65 ± 37.84 (69.80– 68.40) | 183.42 ± 60.04 (84.00–380.90) | 246.81 ± 77.26 (107.00–459.20) | 0.0000 |
| C-Reactive Protein | 5.26 ± 2.98 (0.60–15.22) | 4.73 ± 2.39 (0.69–10.00) | 5.40 ± 3.12 (0.90–13.80) | 0.6550 |
| Leptin (ng/mL) | 20.41 ± 7.24 (7.27–51.89) | 25.40 ± 11.86 (6.02–70.28) | 32.87 ± 13.40 (9.96–67.32) | 0.0000 |
| FGF-21 (pg/mL) | 67.86 ± 31.76 (23.27–144.57) | 78.37 ± 46.67 (27.49–241.98) | 133.97 ± 84.56 (33.62–387.0) | 0.0000 |
| Variables | 1st Trimester | 2nd Trimester | 3rd Trimester | p Value (One-Way ANOVA Test) |
|---|---|---|---|---|
| Age (years) | 23.6 ± 5.3 (17.0–34.0) | - | - | |
| Gestational Age (weeks) | 12.2 ± 0.70 (11.2–13.4) | 24.4 ± 0.57 (24.0–26.0) | 35.0 ± 0.86 (34.0–37.0) | |
| BMI (kg/m2) | 24.44 ± 3.11 (20.30–31.20) | 26.83 ± 3.17 (22.40–33.04) | 29.74 ± 2.97 (24.30–35.22) | 0.0000 |
| SBP (mmHg) | 103.57 ± 7.74 (90.00–120.00) | 103.39 ± 8.64 (88.00–126.00) | 108.74 ± 12.07 (90.00–145.00) | 0.1262 |
| DBP (mmHg) | 65.48 ± 7.27 (50.00–80.00) | 64.74 ± 7.28 (56.00–82.00) | 66.13 ± 6.92 (58.00–80.00) | 0.7895 |
| MBP (mmHg) | 78.18 ± 6.80 (63.33–93.33) | 77.62 ± 6.92 (67.33–91.33) | 80.33 ± 7.45 (70.00–101.67) | 0.3892 |
| Blood Glucose (mg/dL) | 80.50 ± 6.38 (72.00–99.00) | 76.65 ± 7.60 (65.00–91.00) | 73.53 ± 6.57 (64.00–90.00) | 0.0293 |
| Insulin (µUI/mL) | 12.06 ± 3.94 (4.00–24.50) | 14.90 ± 4.48 (8.40–24.40) | 15.23 ± 6.16 (4.10–32.50) | 0.0732 |
| HOMA Index | 2.39 ± 0.82 (0.71–4.78) | 2.94 ± 1.06 (1.59–5.80) | 2.83 ± 1.27 (0.66–6.50) | 0.7825 |
| Total Cholesterol (mg/dL) | 173.04 ± 27.83 (103.30–233.40) | 218.44 ± 41.84 (156.00–355.00) | 233.84 ± 46.26 (149.10–344.30) | 0.0000 |
| HDL-C (mg/dL) | 53.24 ± 10.76 (38.73–81.91) | 64.13 ± 14.36 (42.68–98.08) | 61.76 ± 14.40 (42.05–99.35) | 0.0207 |
| LDL (mg/dL) | 118.85 ± 38.41 (52.30–207.49) | 145.46 ± 57.24 (78.87–329.50) | 151.35 ± 67.40 (50.89–308.00) | 0.0776 |
| VLDL (mg/dL) | 23.68 ± 9.55 (7.92–43.20) | 35.33 ± 14.25 (5.90–69.90) | 51.01 ± 19.87 (11.40–87.40) | 0.0000 |
| Triglycerides (mg/dL) | 124.88 ± 44.89 (71.60–216.00) | 197.02 ± 88.32 (106.70–474.00) | 263.55 ± 89.45 (135.40–437.00) | 0.0000 |
| C-Reactive Protein | 6.10 ± 3.79 (0.65–13.74) | 7.43 ± 2.86 (1.96–11.13) | 7.02 ± 3.24 (1.57–16.24) | 0.1526 |
| Leptin (ng/mL) | 34.97 ± 12.57 (14.24–70.84) | 68.62 ± 32.49 (22.78–136.75) | 89.98 ± 42.16 (24.27–184.53) | 0.0000 |
| FGF-21 (pg/mL) | 105.84 ± 66.32 (27.87–303.59) | 119.65 ± 91.96 (18.78–362.20) | 303.24 ± 279.325 (35.1524–1052.7) | 0.0036 |
| Log(sFlt-1)/Log(PlGF) Healthy Pregnant Women | Log(sFlt-1)/Log(PlGF) Preeclamptic Women | p Value t-Test | |
|---|---|---|---|
| 1st trimester | 2.02488 ± 0.322863 | 1.809 ± 0.240961 | 0.01038 |
| 2nd trimester | 1.31829 ± 0.104664 | 1.23789 ± 0.0747276 | 0.00391 |
| 3rd trimester | 1.4239 ± 0.161476 | 1.58632 ± 0.330844 | 0.01284 |
| p value (ANOVA test) | 0.0000 | 0.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buell-Acosta, J.D.; Garces, M.F.; Parada-Baños, A.J.; Angel-Muller, E.; Paez, M.C.; Eslava-Schmalbach, J.; Escobar-Cordoba, F.; Caminos-Cepeda, S.A.; Lacunza, E.; Castaño, J.P.; et al. Maternal Fibroblast Growth Factor 21 Levels Decrease during Early Pregnancy in Normotensive Pregnant Women but Are Higher in Preeclamptic Women—A Longitudinal Study. Cells 2022, 11, 2251. https://doi.org/10.3390/cells11142251
Buell-Acosta JD, Garces MF, Parada-Baños AJ, Angel-Muller E, Paez MC, Eslava-Schmalbach J, Escobar-Cordoba F, Caminos-Cepeda SA, Lacunza E, Castaño JP, et al. Maternal Fibroblast Growth Factor 21 Levels Decrease during Early Pregnancy in Normotensive Pregnant Women but Are Higher in Preeclamptic Women—A Longitudinal Study. Cells. 2022; 11(14):2251. https://doi.org/10.3390/cells11142251
Chicago/Turabian StyleBuell-Acosta, Julieth Daniela, Maria Fernanda Garces, Arturo José Parada-Baños, Edith Angel-Muller, Maria Carolina Paez, Javier Eslava-Schmalbach, Franklin Escobar-Cordoba, Sofia Alexandra Caminos-Cepeda, Ezequiel Lacunza, Justo P. Castaño, and et al. 2022. "Maternal Fibroblast Growth Factor 21 Levels Decrease during Early Pregnancy in Normotensive Pregnant Women but Are Higher in Preeclamptic Women—A Longitudinal Study" Cells 11, no. 14: 2251. https://doi.org/10.3390/cells11142251
APA StyleBuell-Acosta, J. D., Garces, M. F., Parada-Baños, A. J., Angel-Muller, E., Paez, M. C., Eslava-Schmalbach, J., Escobar-Cordoba, F., Caminos-Cepeda, S. A., Lacunza, E., Castaño, J. P., Nogueiras, R., Dieguez, C., Ruiz-Parra, A. I., & Caminos, J. E. (2022). Maternal Fibroblast Growth Factor 21 Levels Decrease during Early Pregnancy in Normotensive Pregnant Women but Are Higher in Preeclamptic Women—A Longitudinal Study. Cells, 11(14), 2251. https://doi.org/10.3390/cells11142251