Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections
Abstract
1. Introduction
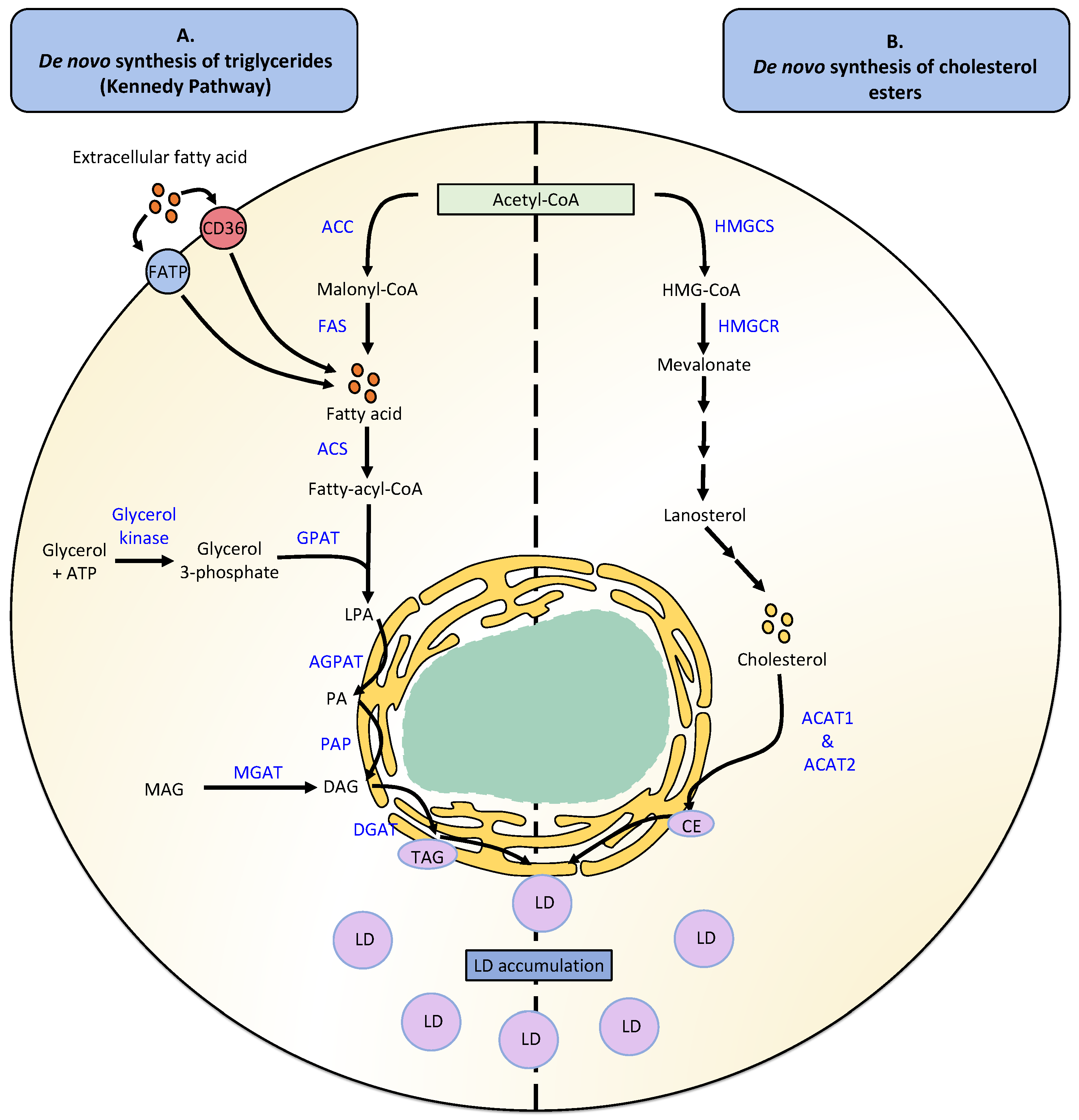
2. Lipid Droplet Metabolism
2.1. Lipid Droplet Formation
2.2. Neutral Lipid Degradation
2.3. Fatty Acid Uptake
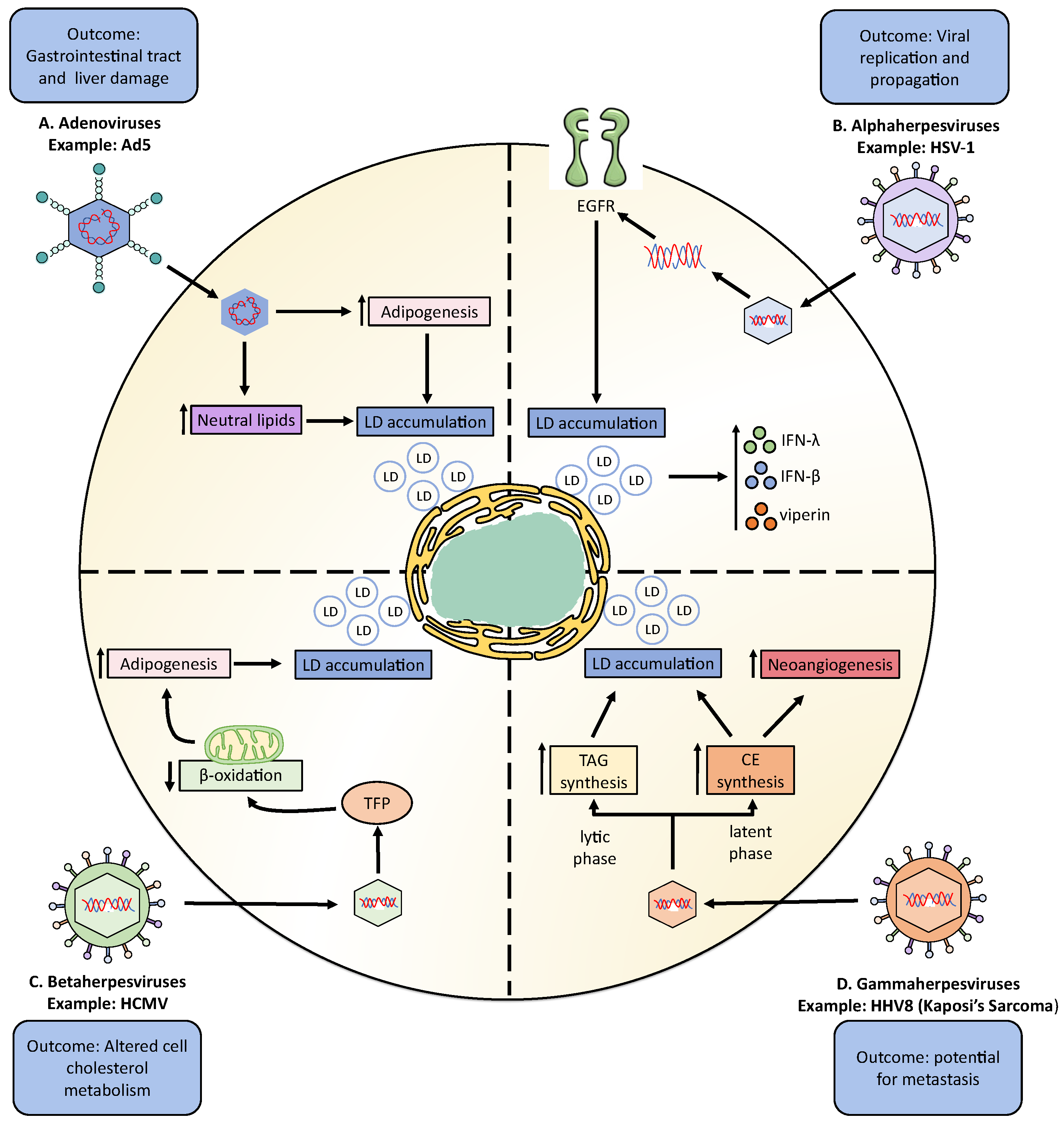
3. Modulation of Neutral Lipid Metabolism by Double-Stranded DNA Viruses
3.1. Adenoviruses
3.2. Herpesviruses
3.2.1. Alphaherpesviruses
Herpes Simplex Virus Type 1
Varicella-Zoster Virus
3.2.2. Betaherpesviruses
Cytomegalovirus
3.2.3. Gammaherpesviruses
Epstein–Barr Virus
Human Herpesvirus 8: Kaposi’s Sarcoma
3.3. Hepadnaviridae
Hepatitis B Virus
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Pol, A.; Gross, S.P.; Parton, R.G. Biogenesis of the multifunctional lipid droplet: Lipids, proteins, and sites. J. Cell Biol. 2014, 204, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Tauchi-Sato, K.; Ozeki, S.; Houjou, T.; Taguchi, R.; Fujimoto, T. The Surface of Lipid Droplets Is a Phospholipid Monolayer with a Unique Fatty Acid Composition. J. Biol. Chem. 2002, 277, 44507–44512. [Google Scholar] [CrossRef]
- Caillon, L.; Nieto, V.; Gehan, P.; Omrane, M.; Rodriguez, N.; Monticelli, L.; Thiam, A.R. Triacylglycerols sequester monotopic membrane proteins to lipid droplets. Nat. Commun. 2020, 11, 3944. [Google Scholar] [CrossRef]
- Guo, Y.; Cordes, K.R.; Farese, R.V., Jr.; Walther, T.C. Lipid droplets at a glance. J. Cell Sci. 2009, 122, 749–752. [Google Scholar] [CrossRef]
- Cui, L.; Liu, P. Two Types of Contact Between Lipid Droplets and Mitochondria. Front. Cell Dev. Biol. 2020, 8, 618322. [Google Scholar] [CrossRef]
- Romanauska, A.; Köhler, A. The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets. Cell 2018, 174, 700–715.e18. [Google Scholar] [CrossRef] [PubMed]
- Sołtysik, K.; Ohsaki, Y.; Tatematsu, T.; Cheng, J.; Maeda, A.; Morita, S.-Y.; Fujimoto, T. Nuclear lipid droplets form in the inner nuclear membrane in a seipin-independent manner. J. Cell Biol. 2020, 220, e202005026. [Google Scholar] [CrossRef]
- Benador, I.Y.; Veliova, M.; Mahdaviani, K.; Petcherski, A.; Wikstrom, J.D.; Assali, E.A.; Acín-Pérez, R.; Shum, M.; Oliveira, M.F.; Cinti, S.; et al. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018, 27, 869–885.e6. [Google Scholar] [CrossRef]
- Anastasia, I.; Ilacqua, N.; Raimondi, A.; Lemieux, P.; Ghandehari-Alavijeh, R.; Faure, G.; Mekhedov, S.L.; Williams, K.J.; Caicci, F.; Valle, G.; et al. Mitochondria-rough-ER contacts in the liver regulate systemic lipid homeostasis. Cell Rep. 2021, 34, 108873. [Google Scholar] [CrossRef]
- Kong, J.; Ji, Y.; Jeon, Y.G.; Han, J.S.; Han, K.H.; Lee, J.H.; Lee, G.; Jang, H.; Choe, S.S.; Baes, M.; et al. Spatiotemporal contact between peroxisomes and lipid droplets regulates fasting-induced lipolysis via PEX5. Nat. Commun. 2020, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Zoni, V.; Khaddaj, R.; Campomanes, P.; Thiam, A.R.; Schneiter, R.; Vanni, S. Pre-existing bilayer stresses modulate triglyceride accumulation in the ER versus lipid droplets. eLife 2021, 10, e62886. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Mendez, R.; Heng, H.H.; Yang, Z.-Q.; Zhang, K. Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation. Am. J. Transl. Res. 2012, 4, 102–113. [Google Scholar] [PubMed]
- Mailler, E.; Guardia, C.M.; Bai, X.; Jarnik, M.; Williamson, C.D.; Li, Y.; Maio, N.; Golden, A.; Bonifacino, J.S. The autophagy protein ATG9A enables lipid mobilization from lipid droplets. Nat. Commun. 2021, 12, 6750. [Google Scholar] [CrossRef]
- Saka, H.A.; Valdivia, R. Emerging Roles for Lipid Droplets in Immunity and Host-Pathogen Interactions. Annu. Rev. Cell Dev. Biol. 2012, 28, 411–437. [Google Scholar] [CrossRef]
- Melo, R.C.; Weller, P.F. Lipid droplets in leukocytes: Organelles linked to inflammatory responses. Exp. Cell Res. 2015, 340, 193–197. [Google Scholar] [CrossRef]
- Castoldi, A.; Monteiro, L.B.; Bakker, N.V.T.; Sanin, D.E.; Rana, N.; Corrado, M.; Cameron, A.M.; Hässler, F.; Matsushita, M.; Caputa, G.; et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat. Commun. 2020, 11, 4107. [Google Scholar] [CrossRef]
- Wu, H.; Han, Y.; Sillke, Y.R.; Deng, H.; Siddiqui, S.; Treese, C.; Schmidt, F.; Friedrich, M.; Keye, J.; Wan, J.; et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol. Med. 2019, 11, e10698. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Silberman, P.C.; Rutkowski, M.R.; Chopra, S.; Perales-Puchalt, A.; Song, M.; Zhang, S.; Bettigole, S.E.; Gupta, D.; Holcomb, K.; et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015, 161, 1527–1538. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.; Mohammadyani, D.; Blasi, M.; Duperret, E.K.; Donthireddy, L.; Hashimoto, A.; Kapralov, O.; Amoscato, A.; Angelini, R.; et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat. Commun. 2017, 8, 2122. [Google Scholar] [CrossRef]
- Shi, R.-Z.; Pan, Y.-Q.; Xing, L. RNA Helicase A Regulates the Replication of RNA Viruses. Viruses 2021, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Lucic, B.; de Castro, I.J.; Lusic, M. Viruses in the Nucleus. Cold Spring Harb. Perspect. Biol. 2021, 13, a039446. [Google Scholar] [CrossRef]
- Iarovaia, O.; Ioudinkova, E.; Velichko, A.; Razin, S. Manipulation of Cellular Processes via Nucleolus Hijaking in the Course of Viral Infection in Mammals. Cells 2021, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-C.; Garcia-Blanco, M. Role of Alternative Splicing in Regulating Host Response to Viral Infection. Cells 2021, 10, 1720. [Google Scholar] [CrossRef] [PubMed]
- Imbert, F.; Langford, D. Viruses, SUMO, and immunity: The interplay between viruses and the host SUMOylation system. J. NeuroVirology 2021, 27, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Naghavi, M.H. Exploitation of Cytoskeletal Networks during Early Viral Infection. Trends Microbiol. 2018, 27, 39–50. [Google Scholar] [CrossRef]
- Robinson, M.; Schor, S.; Barouch-Bentov, R.; Einav, S. Viral journeys on the intracellular highways. Experientia 2018, 75, 3693–3714. [Google Scholar] [CrossRef] [PubMed]
- Beziau, A.; Brand, D.; Piver, E. The Role of Phosphatidylinositol Phosphate Kinases during Viral Infection. Viruses 2020, 12, 1124. [Google Scholar] [CrossRef]
- Chander, Y.; Kumar, R.; Khandelwal, N.; Singh, N.; Shringi, B.N.; Barua, S.; Kumar, N. Role of p38 mitogen-activated protein kinase signalling in virus replication and potential for developing broad spectrum antiviral drugs. Rev. Med Virol. 2021, 31, 1–16. [Google Scholar] [CrossRef]
- Brezgin, S.; Kostyusheva, A.; Bayurova, E.; Volchkova, E.; Gegechkori, V.; Gordeychuk, I.; Glebe, D.; Kostyushev, D.; Chulanov, V. Immunity and Viral Infections: Modulating Antiviral Response via CRISPR–Cas Systems. Viruses 2021, 13, 1373. [Google Scholar] [CrossRef]
- Jheng, J.-R.; Ho, J.-Y.; Horng, J.-T. ER stress, autophagy, and RNA viruses. Front. Microbiol. 2014, 5, 388. [Google Scholar] [CrossRef] [PubMed]
- Quarleri, J.; Cevallos, C.; Delpino, M.V. Apoptosis in infectious diseases as a mechanism of immune evasion and survival. Adv. Protein Chem. Struct. Biol. 2021, 125, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, K.; Zeng, S.; Zhao, F.; Fan, J.; Li, Z.; Yi, L.; Ding, H.; Zhao, M.; Fan, S.; et al. Viral Infection Modulates Mitochondrial Function. Int. J. Mol. Sci. 2021, 22, 4260. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Navarro, A.J.; Diethelm-Varela, B.; Kalergis, A.M.; González, P.A. Is there a role for HSF1 in viral infections? FEBS Open Bio 2022, 12, 1112–1124. [Google Scholar] [CrossRef]
- Reyes, A.; Duarte, L.; Farías, M.; Tognarelli, E.; Kalergis, A.; Bueno, S.; González, P. Impact of Hypoxia over Human Viral Infections and Key Cellular Processes. Int. J. Mol. Sci. 2021, 22, 7954. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, S.K.; Rouse, B.T. How host metabolism impacts on virus pathogenesis. Curr. Opin. Virol. 2018, 28, 37–42. [Google Scholar] [CrossRef]
- Bosch, M.; Sweet, M.J.; Parton, R.G.; Pol, A. Lipid droplets and the host–pathogen dynamic: FATal attraction? J. Cell Biol. 2021, 220, e202104005. [Google Scholar] [CrossRef]
- Moessinger, C.; Klizaite, K.; Steinhagen, A.; Philippou-Massier, J.; Shevchenko, A.; Hoch, M.; Ejsing, C.S.; Thiele, C. Two different pathways of phosphatidylcholine synthesis, the Kennedy Pathway and the Lands Cycle, differentially regulate cellular triacylglycerol storage. BMC Cell Biol. 2014, 15, 43. [Google Scholar] [CrossRef]
- Ellis, J.M.; Frahm, J.L.; O Li, L.; A Coleman, R. Acyl-coenzyme A synthetases in metabolic control. Curr. Opin. Lipidol. 2010, 21, 212–217. [Google Scholar] [CrossRef]
- Krahmer, N.; Guo, Y.; Wilfling, F.; Hilger, M.; Lingrell, S.; Heger, K.; Newman, H.W.; Schmidt-Supprian, M.; Vance, D.E.; Mann, M.; et al. Phosphatidylcholine Synthesis for Lipid Droplet Expansion Is Mediated by Localized Activation of CTP:Phosphocholine Cytidylyltransferase. Cell Metab. 2011, 14, 504–515. [Google Scholar] [CrossRef]
- Coleman, R.A.; Haynes, E.B. Subcellular location and topography of rat hepatic monoacylglycerol acyltransferase activity. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1985, 834, 180–187. [Google Scholar] [CrossRef]
- Cases, S.; Smith, S.J.; Zheng, Y.-W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef] [PubMed]
- Lardizabal, K.D.; Mai, J.T.; Wagner, N.W.; Wyrick, A.; Voelker, T.; Hawkins, D.J. DGAT2 Is a New Diacylglycerol Acyltransferase Gene Family. J. Biol. Chem. 2001, 276, 38862–38869. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Alphonse, P.A.S.; Jones, P.J.H. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and its Key Regulators. Lipids 2016, 51, 519–536. [Google Scholar] [CrossRef]
- Sharpe, L.; Brown, A.J. Controlling Cholesterol Synthesis beyond 3-Hydroxy-3-methylglutaryl-CoA Reductase (HMGCR). J. Biol. Chem. 2013, 288, 18707–18715. [Google Scholar] [CrossRef]
- Buhman, K.; Chen, H.C.; Farese, R.V. The Enzymes of Neutral Lipid Synthesis. J. Biol. Chem. 2001, 276, 40369–40372. [Google Scholar] [CrossRef]
- Kassan, A.; Herms, A.; Fernández-Vidal, A.; Bosch, M.; Schieber, N.L.; Reddy, B.J.N.; Fajardo, A.; Gelabert-Baldrich, M.; Tebar, F.; Enrich, C.; et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J. Cell Biol. 2013, 203, 985–1001. [Google Scholar] [CrossRef]
- Hashemi, H.F.; Goodman, J.M. The life cycle of lipid droplets. Curr. Opin. Cell Biol. 2015, 33, 119–124. [Google Scholar] [CrossRef]
- Choudhary, V.; Golani, G.; Joshi, A.S.; Cottier, S.; Schneiter, R.; Prinz, W.A.; Kozlov, M.M. Architecture of Lipid Droplets in Endoplasmic Reticulum Is Determined by Phospholipid Intrinsic Curvature. Curr. Biol. 2018, 28, 915–926.e9. [Google Scholar] [CrossRef]
- Choudhary, V.; Ojha, N.; Golden, A.; Prinz, W.A. A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J. Cell Biol. 2015, 211, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Becuwe, M.; E Housden, B.; Chitraju, C.; Porras, A.J.; Graham, M.M.; Liu, X.N.; Thiam, A.R.; Savage, D.B.; Agarwal, A.K.; et al. Seipin is required for converting nascent to mature lipid droplets. eLife 2016, 5, e16582. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.A.; Zhan, C.; Silver, D.L. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. Proc. Natl. Acad. Sci. USA 2011, 108, 19581–19586. [Google Scholar] [CrossRef] [PubMed]
- Bulankina, A.V.; Deggerich, A.; Wenzel, D.; Mutenda, K.; Wittmann, J.G.; Rudolph, M.G.; Burger, K.N.; Höning, S. TIP47 functions in the biogenesis of lipid droplets. J. Cell Biol. 2009, 185, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, Y.; Honda, M.; Wada, Y.; Yazaki, Y.; Suzuki, T.; Ohba, Y.; Nabata, H.; Endo, A.; Matsumoto, A.; Itakura, H.; et al. Sterol-Mediated Regulation of SREBP-1a,1b,1c and SREBP-2 in Cultured Human Cells. Biochem. Biophys. Res. Commun. 1994, 202, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Miyazaki, M.; Man, W.C.; Ntambi, J.M. Sterol Regulatory Element-Binding Proteins (SREBPs) as Regulators of Lipid Metabolism. Ann. N. Y. Acad. Sci. 2006, 967, 34–42. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Zhang, X.; Li, J.; Bionaz, M. Activation of liver X receptor promotes fatty acid synthesis in goat mammary epithelial cells via modulation of SREBP1 expression. J. Dairy Sci. 2019, 102, 3544–3555. [Google Scholar] [CrossRef]
- Chawla, A.; Schwarz, E.J.; Dimaculangan, D.D.; A Lazar, M. Peroxisome proliferator-activated receptor (PPAR) gamma: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 1994, 135, 798–800. [Google Scholar] [CrossRef]
- Bardot, O.; Aldridge, T.; Latruffe, N.; Green, S. PPAR-RXR Heterodimer Activates a Peroxisome Proliferator Response Element Upstream of the Bifunctional Enzyme Gene. Biochem. Biophys. Res. Commun. 1993, 192, 37–45. [Google Scholar] [CrossRef]
- Cai, D.; Li, Y.; Zhang, K.; Zhou, B.; Guo, F.; Holm, L.; Liu, H. Co-option of PPARα in the regulation of lipogenesis and fatty acid oxidation in CLA-induced hepatic steatosis. J. Cell. Physiol. 2020, 236, 4387–4402. [Google Scholar] [CrossRef]
- Derecka, K.; Sheldrick, E.; Wathes, D.; Abayasekara, D.; Flint, A. A PPAR-independent pathway to PUFA-induced COX-2 expression. Mol. Cell. Endocrinol. 2008, 287, 65–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gan, Z.; Burkart-Hartman, E.M.; Han, D.-H.; Finck, B.; Leone, T.C.; Smith, E.Y.; Ayala, J.E.; Holloszy, J.; Kelly, D.P. The nuclear receptor PPARβ/δ programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 2011, 25, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Quintela, A.M.; Jiménez, R.; Piqueras, L.; Gómez-Guzmán, M.; Haro, J.; Zarzuelo, M.J.; Cogolludo, A.; Sanz, M.J.; Toral, M.; Romero, M.; et al. PPARβ activation restores the high glucose-induced impairment of insulin signalling in endothelial cells. J. Cereb. Blood Flow Metab. 2014, 171, 3089–3102. [Google Scholar] [CrossRef] [PubMed]
- Arimura, N.; Horiba, T.; Imagawa, M.; Shimizu, M.; Sato, R. The Peroxisome Proliferator-activated Receptor γ Regulates Expression of the Perilipin Gene in Adipocytes. J. Biol. Chem. 2004, 279, 10070–10076. [Google Scholar] [CrossRef]
- Dalen, K.T.; Schoonjans, K.; Ulven, S.M.; Weedon-Fekjaer, M.S.; Bentzen, T.G.; Koutnikova, H.; Auwerx, J.; Nebb, H.I. Adipose Tissue Expression of the Lipid Droplet–Associating Proteins S3-12 and Perilipin Is Controlled by Peroxisome Proliferator–Activated Receptor-γ. Diabetes 2004, 53, 1243–1252. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Marshall, P.J.; Kulmacz, R.J. Prostaglandin H synthase: Distinct binding sites for cyclooxygenase and peroxidase substrates. Arch. Biochem. Biophys. 1988, 266, 162–170. [Google Scholar] [CrossRef]
- Sales, K.; Jabbour, H. Cyclooxygenase enzymes and prostaglandins in reproductive tract physiology and pathology. Prostaglandins Other Lipid Mediat. 2003, 71, 97–117. [Google Scholar] [CrossRef]
- D’Avila, H.; Melo, R.C.N.; Parreira, G.G.; Werneck-Barroso, E.; Castro-Faria-Neto, H.C.; Bozza, P.T. Mycobacterium bovis Bacillus Calmette-Guérin Induces TLR2-Mediated Formation of Lipid Bodies: Intracellular Domains for Eicosanoid Synthesis In Vivo. J. Immunol. 2006, 176, 3087–3097. [Google Scholar] [CrossRef]
- Moreira, L.S.; Piva, B.; Gentile, L.B.; Mesquita-Santos, F.P.; D’Avila, H.; Maya-Monteiro, C.M.; Bozza, P.T.; Bandeira-Melo, C.; Diaz, B.L. Cytosolic phospholipase A2-driven PGE2 synthesis within unsaturated fatty acids-induced lipid bodies of epithelial cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2009, 1791, 156–165. [Google Scholar] [CrossRef]
- Bozza, P.; Yu, W.; Penrose, J.F.; Morgan, E.S.; Dvorak, A.M.; Weller, P.F. Eosinophil Lipid Bodies: Specific, Inducible Intracellular Sites for Enhanced Eicosanoid Formation. J. Exp. Med. 1997, 186, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Accioly, M.T.; Pacheco, P.; Maya-Monteiro, C.M.; Carrossini, N.; Robbs, B.K.; Oliveira, S.S.; Kaufmann, C.; Morgado-Diaz, J.A.; Bozza, P.T.; Viola, J.P. Lipid Bodies Are Reservoirs of Cyclooxygenase-2 and Sites of Prostaglandin-E2 Synthesis in Colon Cancer Cells. Cancer Res. 2008, 68, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Carrossini, N.; Da Costa, N.M.; Andrade-Barreto, E.; Sousa, V.P.L.; Nicolau-Neto, P.; Souza-Santos, P.T.; Mansur, G.R.; Wernersbach, L.; Bozza, P.T.; Viola, J.P.B.; et al. Lipid droplet biogenesis and COX-2 pathway activation are triggered by Barrett’s esophagus and adenocarcinoma, but not esophageal squamous cell carcinoma risk factors. Sci. Rep. 2021, 11, 981. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.; Silva, R.J.; Milián, I.C.B.; Rosini, A.M.; de Araújo, T.E.; Teixeira, S.C.; Oliveira, M.C.; Franco, P.S.; da Silva, C.V.; Mineo, J.R.; et al. Cyclooxygenase (COX)-2 modulates Toxoplasma gondii infection, immune response and lipid droplets formation in human trophoblast cells and villous explants. Sci. Rep. 2021, 11, 12709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Fisher, B.J.; Clair, R.W.S.; Rudel, L.L.; Ghosh, S. Redistribution of macrophage cholesteryl ester hydrolase from cytoplasm to lipid droplets upon lipid loading. J. Lipid Res. 2005, 46, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Athenstaedt, K.; Daum, G. The life cycle of neutral lipids: Synthesis, storage and degradation. Experientia 2006, 63, 1355–1369. [Google Scholar] [CrossRef]
- Meyers, A.; Weiskittel, T.M.; Dalhaimer, P. Lipid Droplets: Formation to Breakdown. Lipids 2017, 52, 465–475. [Google Scholar] [CrossRef]
- Bolsoni-Lopes, A.; Alonso-Vale, M.I.C. Lipolysis and lipases in white adipose tissue—An update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [Google Scholar] [CrossRef]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty Acid Trafficking in Starved Cells: Regulation by Lipid Droplet Lipolysis, Autophagy, and Mitochondrial Fusion Dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar] [CrossRef]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS - Lipases and Lipolysis in Lipid Metabolism and Signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef]
- Quiroga, A.D.; Lehner, R. Pharmacological intervention of liver triacylglycerol lipolysis: The good, the bad and the ugly. Biochem. Pharmacol. 2018, 155, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Schulze, R.J.; Sathyanarayan, A.; Mashek, D.G. Breaking fat: The regulation and mechanisms of lipophagy. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Schulze, R.J.; McNiven, M.A. Lipid Droplet Formation and Lipophagy in Fatty Liver Disease. Semin. Liver Dis. 2019, 39, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Czaja, M.J. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2012, 20, 3–11. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Singh, R. Autophagy and Lipid Droplets in the Liver. Annu. Rev. Nutr. 2015, 35, 215–237. [Google Scholar] [CrossRef]
- Cui, W.; Sathyanarayan, A.; Lopresti, M.; Aghajan, M.; Chen, C.; Mashek, D.G. Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis. Autophagy 2020, 17, 690–705. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Chen, E.; Li, L.; Saha, P.; Lee, H.-J.; Huang, L.-S.; Shelness, G.S.; Chan, L.; Chang, B.H.-J. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 2017, 13, 1130–1144. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. Membrane Fatty Acid Transporters as Regulators of Lipid Metabolism: Implications for Metabolic Disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.-L.; et al. Fatty Acid Uptake and Lipid Storage Induced by HIF-1α Contribute to Cell Growth and Survival after Hypoxia-Reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef]
- Brink, D.V.D.; Cubizolle, A.; Chatelain, G.; Davoust, N.; Girard, V.; Johansen, S.; Napoletano, F.; Dourlen, P.; Guillou, L.; Angebault-Prouteau, C.; et al. Physiological and pathological roles of FATP-mediated lipid droplets in Drosophila and mice retina. PLoS Genet. 2018, 14, e1007627. [Google Scholar] [CrossRef]
- Ahowesso, C.; Black, P.N.; Saini, N.; Montefusco, D.; Chekal, J.; Malosh, C.; Lindsley, C.W.; Stauffer, S.R.; DiRusso, C.C. Chemical inhibition of fatty acid absorption and cellular uptake limits lipotoxic cell death. Biochem. Pharmacol. 2015, 98, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Khnykin, D. Fatty acid transporters in skin development, function and disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1841, 362–368. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, R.; Shi, D. The role of FATP1 in lipid accumulation: A review. Mol. Cell. Biochem. 2021, 476, 1897–1903. [Google Scholar] [CrossRef]
- Melton, E.M.; Cerny, R.L.; Watkins, P.A.; DiRusso, C.C.; Black, P.N. Human Fatty Acid Transport Protein 2a/Very Long Chain Acyl-CoA Synthetase 1 (FATP2a/Acsvl1) Has a Preference in Mediating the Channeling of Exogenous n-3 Fatty Acids into Phosphatidylinositol. J. Biol. Chem. 2011, 286, 30670–30679. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.C.; Luiken, J.J.F.P. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jay, A.; Brunaldi, K.; Huang, N.; Hamilton, J.A. CD36 Enhances Fatty Acid Uptake by Increasing the Rate of Intracellular Esterification but Not Transport across the Plasma Membrane. Biochemistry 2013, 52, 7254–7261. [Google Scholar] [CrossRef]
- Jay, A.G.; Chen, A.N.; Paz, M.A.; Hung, J.P.; Hamilton, J.A. CD36 Binds Oxidized Low Density Lipoprotein (LDL) in a Mechanism Dependent upon Fatty Acid Binding. J. Biol. Chem. 2015, 290, 4590–4603. [Google Scholar] [CrossRef]
- Kolleck, I.; Guthmann, F.; Ladhoff, A.-M.; Tandon, N.N.; Schlame, M.; Rüstow, B. Cellular Cholesterol Stimulates Acute Uptake of Palmitate by Redistribution of Fatty Acid Translocase in Type II Pneumocytes. Biochemistry 2002, 41, 6369–6375. [Google Scholar] [CrossRef]
- Samovski, D.; Sun, J.; Pietka, T.; Gross, R.W.; Eckel, R.H.; Su, X.; Stahl, P.D.; Abumrad, N.A. Regulation of AMPK Activation by CD36 Links Fatty Acid Uptake to β-Oxidation. Diabetes 2014, 64, 353–359. [Google Scholar] [CrossRef]
- Luiken, J.J.; Chanda, D.; Nabben, M.; Neumann, D.; Glatz, J.F. Post-translational modifications of CD36 (SR-B2): Implications for regulation of myocellular fatty acid uptake. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 2253–2258. [Google Scholar] [CrossRef]
- Wang, W.; Yan, Z.; Hu, J.; Shen, W.-J.; Azhar, S.; Kraemer, F.B. Scavenger receptor class B, type 1 facilitates cellular fatty acid uptake. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158554. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.; Nachliel, E.; Gutman, M. Fatty Acid Binding Proteins: Same Structure but Different Binding Mechanisms? Molecular Dynamics Simulations of Intestinal Fatty Acid Binding Protein. Biophys. J. 2006, 90, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Abumrad, N.A. Cellular fatty acid uptake: A pathway under construction. Trends Endocrinol. Metab. 2009, 20, 72–77. [Google Scholar] [CrossRef]
- Schwenk, R.W.; Luiken, J.J.; Bonen, A.; Glatz, J.F. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc. Res. 2008, 79, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Briand, N.; Prado, C.; Mabilleau, G.; Lasnier, F.; Le Lièpvre, X.; Covington, J.D.; Ravussin, E.; Le Lay, S.; Dugail, I. Caveolin-1 Expression and Cavin Stability Regulate Caveolae Dynamics in Adipocyte Lipid Store Fluctuation. Diabetes 2014, 63, 4032–4044. [Google Scholar] [CrossRef]
- Razani, B.; Combs, T.P.; Wang, X.B.; Frank, P.G.; Park, D.S.; Russell, R.G.; Li, M.; Tang, B.; Jelicks, L.A.; Scherer, P.E.; et al. Caveolin-1-deficient Mice Are Lean, Resistant to Diet-induced Obesity, and Show Hypertriglyceridemia with Adipocyte Abnormalities. J. Biol. Chem. 2002, 277, 8635–8647. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Lee, M.-J.; Summer, R.; Liu, L.; Fried, S.K.; Pilch, P.F. Pleiotropic Effects of Cavin-1 Deficiency on Lipid Metabolism. J. Biol. Chem. 2014, 289, 8473–8483. [Google Scholar] [CrossRef]
- Matthaeus, C.; Lahmann, I.; Kunz, S.; Jonas, W.; Melo, A.A.; Lehmann, M.; Larsson, E.; Lundmark, R.; Kern, M.; Blüher, M.; et al. EHD2-mediated restriction of caveolar dynamics regulates cellular fatty acid uptake. Proc. Natl. Acad. Sci. USA 2020, 117, 7471–7481. [Google Scholar] [CrossRef]
- Kuo, A.; Lee, M.Y.; Yang, K.; Gross, R.W.; Sessa, W.C. Caveolin-1 regulates lipid droplet metabolism in endothelial cells via autocrine prostacyclin–stimulated, cAMP-mediated lipolysis. J. Biol. Chem. 2018, 293, 973–983. [Google Scholar] [CrossRef]
- Monson, E.A.; Trenerry, A.M.; Laws, J.L.; Mackenzie, J.M.; Helbig, K.J. Lipid droplets and lipid mediators in viral infection and immunity. FEMS Microbiol. Rev. 2021, 45, fuaa066. [Google Scholar] [CrossRef]
- Piodi, A.; Chouteau, P.; Lerat, H.; Hezode, C.; Pawlotsky, J. Morphological changes in intracellular lipid droplets induced by different hepatitis C virus genotype core sequences and relationship with steatosis. Hepatology 2008, 48, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, Y.; Li, M.Y.; Lamers, M.M.; Fusade-Boyer, M.; Klemm, E.; Thiele, C.; Ashour, J.; Sanyal, S. Flaviviruses Exploit the Lipid Droplet Protein AUP1 to Trigger Lipophagy and Drive Virus Production. Cell Host Microbe 2018, 23, 819–831.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Cortese, M.; Haselmann, U.; Tabata, K.; Romero-Brey, I.; Funaya, C.; Schieber, N.L.; Qiang, Y.; Bartenschlager, M.; Kallis, S.; et al. Spatiotemporal Coupling of the Hepatitis C Virus Replication Cycle by Creating a Lipid Droplet- Proximal Membranous Replication Compartment. Cell Rep. 2019, 27, 3602–3617.e5. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Tay, E.; Shahidi, M.; George, J.; Douglas, M.W. Hepatitis C virus infection mediates cholesteryl ester synthesis to facilitate infectious particle production. J. Gen. Virol. 2014, 95, 1900–1910. [Google Scholar] [CrossRef]
- Galli, A.; Scheel, T.; Prentoe, J.; Mikkelsen, L.S.; Gottwein, J.; Bukh, J. Analysis of hepatitis C virus core/NS5A protein co-localization using novel cell culture systems expressing core–NS2 and NS5A of genotypes 1–7. J. Gen. Virol. 2013, 94, 2221–2235. [Google Scholar] [CrossRef]
- Boulant, S.; Targett-Adams, P.; Mclauchlan, J. Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J. Gen. Virol. 2007, 88, 2204–2213. [Google Scholar] [CrossRef]
- Dias, S.S.G.; Soares, V.C.; Ferreira, A.C.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Teixeira, L.; da Silva, M.A.N.; Barreto, E.; Mattos, M.; et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLOS Pathog. 2020, 16, e1009127. [Google Scholar] [CrossRef]
- Ison, M.G.; Hayden, R.T. Adenovirus. Microbiol. Spectr. 2016, 4, 217–232. [Google Scholar] [CrossRef]
- Rocha, N.; Kuijl, C.; van der Kant, R.; Janssen, L.; Houben, D.; Janssen, H.; Zwart, W.; Neefjes, J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7–RILP–p150Glued and late endosome positioning. J. Cell Biol. 2009, 185, 1209–1225. [Google Scholar] [CrossRef]
- Cianciola, N.L.; Chung, S.; Manor, D.; Carlin, C.R. Adenovirus Modulates Toll-Like Receptor 4 Signaling by Reprogramming ORP1L-VAP Protein Contacts for Cholesterol Transport from Endosomes to the Endoplasmic Reticulum. J. Virol. 2017, 91, e01904-16. [Google Scholar] [CrossRef]
- Li, P.; Feng, F.; Pan, E.; Fan, X.; Yang, Q.; Guan, M.; Chen, L.; Sun, C. Scavenger receptor-mediated Ad5 entry and acLDL accumulation in monocytes/macrophages synergistically trigger innate responses against viral infection. Virology 2018, 519, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Bil-Lula, I.; Krzywonos-Zawadzka, A.; Sawicki, G.; Woźniak, M. An infection of human adenovirus 31 affects the differentiation of preadipocytes into fat cells, its metabolic profile and fat accumulation. J. Med Virol. 2015, 88, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Yu, Y.; Zhang, X.H.; Floyd, E.Z.; Cefalu, W.T. Human adenovirus 36 decreases fatty acid oxidation and increases de novo lipogenesis in primary cultured human skeletal muscle cells by promoting Cidec/FSP27 expression. Int. J. Obes. 2010, 34, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Liang, X.; Hou, J.; Aisa, Y.; Wu, H.; Zhang, Z.; Nuermaimaiti, N.; Zhao, Y.; Jiang, S.; Guan, Y. Adenovirus type 36 regulates adipose stem cell differentiation and glucolipid metabolism through the PI3K/Akt/FoxO1/PPARγ signaling pathway. Lipids Heal. Dis. 2019, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Hou, L.; Wei, L.; Quan, R.; Wang, J.; Liu, H.; Liu, J. Fowl Adenovirus Serotype 4 Induces Hepatic Steatosis via Activation of Liver X Receptor-α. J. Virol. 2021, 95, e01938-20. [Google Scholar] [CrossRef]
- Monson, E.A.; Crosse, K.M.; Duan, M.; Chen, W.; O’Shea, R.D.; Wakim, L.M.; Carr, J.M.; Whelan, D.R.; Helbig, K.J. Intracellular lipid droplet accumulation occurs early following viral infection and is required for an efficient interferon response. Nat. Commun. 2021, 12, 4303. [Google Scholar] [CrossRef]
- Li, M.; Liao, Z.; Xu, Z.; Zou, X.; Wang, Y.; Peng, H.; Li, Y.; Ou, X.; Deng, Y.; Guo, Y.; et al. The Interaction Mechanism Between Herpes Simplex Virus 1 Glycoprotein D and Host Antiviral Protein Viperin. Front. Immunol. 2019, 10, 2810. [Google Scholar] [CrossRef]
- Jerkofsky, M.; De Siervo, A.J. Differentiation of strains of varicella-zoster virus by changes in neutral lipid metabolism in infected cells. J. Virol. 1986, 57, 809–815. [Google Scholar] [CrossRef]
- Abrahamsen, L.H.; Clay, M.J.; Lyle, J.M.; Zink, J.M.; Fredrikson, L.J.; DeSiervo, A.J.; Jerkofsky, M. The Effects of Cytomegalovirus Infection on Polar Lipids and Neutral Lipids in Cultured Human Cells. Intervirology 1996, 39, 223–229. [Google Scholar] [CrossRef]
- Li, Y.; Webster-Cyriaque, J.; Tomlinson, C.C.; Yohe, M.; Kenney, S. Fatty Acid Synthase Expression Is Induced by the Epstein-Barr Virus Immediate-Early Protein BRLF1 and Is Required for Lytic Viral Gene Expression. J. Virol. 2004, 78, 4197–4206. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, Z.; Ersing, I.; Nobre, L.; Guo, R.; Jiang, S.; Trudeau, S.; Zhao, B.; Weekes, M.P.; Gewurz, B.E. Epstein-Barr virus subverts mevalonate and fatty acid pathways to promote infected B-cell proliferation and survival. PLOS Pathog. 2019, 15, e1008030. [Google Scholar] [CrossRef] [PubMed]
- Savard, M.; Bélanger, C.; Tremblay, M.J.; Dumais, N.; Flamand, L.; Borgeat, P.; Gosselin, J. EBV Suppresses Prostaglandin E2Biosynthesis in Human Monocytes. J. Immunol. 2000, 164, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.K.-F.; Lung, R.W.-M.; Dawson, C.W.; Young, L.S.; Ko, C.-W.; Yeung, W.W.; Kang, W.; To, K.-F.; Lo, K.-W. Activation of sterol regulatory element-binding protein 1 (SREBP1)-mediated lipogenesis by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) promotes cell proliferation and progression of nasopharyngeal carcinoma. J. Pathol. 2018, 246, 180–190. [Google Scholar] [CrossRef]
- Angius, F.; Uda, S.; Piras, E.; Spolitu, S.; Ingianni, A.; Batetta, B.; Pompei, R. Neutral lipid alterations in Human Herpesvirus 8-infected HUVEC cells and their possible involvement in neo-angiogenesis. BMC Microbiol. 2015, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Walia, N.; Paul, A.; Patel, K.; Chandran, K.; Ahmad, W.; Chandran, B. NFAT and CREB Regulate Kaposi’s Sarcoma-Associated Herpesvirus-Induced Cyclooxygenase 2 (COX-2). J. Virol. 2010, 84, 12733–12753. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Song, E.H.; Lee, H.J.; Oh, Y.K.; Choi, K.-H.; Yu, D.-Y.; Park, S.I.; Seong, J.-K.; Kim, W.-H. HBx-Induced Hepatic Steatosis and Apoptosis Are Regulated by TNFR1- and NF-κB-Dependent Pathways. J. Mol. Biol. 2010, 397, 917–931. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Fang, H.; Zhou, H. Hepatitis B virus infection is not associated with fatty liver disease: Evidence from a cohort study and functional analysis. Mol. Med. Rep. 2018, 19, 320–326. [Google Scholar] [CrossRef]
- Hwang, K.B.; Kyaw, Y.Y.; Kang, H.R.; Seong, M.S.; Cheong, J. Mitochondrial dysfunction stimulates HBV gene expression through lipogenic transcription factor activation. Virus Res. 2019, 277, 197842. [Google Scholar] [CrossRef]
- Joven, J.; Espinel, E.; Rull, A.; Beltrán-Debón, R.; Aragonès, G.; Rodríguez-Gallego, E.; Camps, J.; Pedro-Botet, J.; Sans, T.; Menéndez, J.A.; et al. Serum fatty acid synthase concentration is increased in patients with hepatitis viral infection and may assist in the prediction of liver steatosis. J. Clin. Virol. 2011, 51, 199–201. [Google Scholar] [CrossRef]
- Yasumoto, J.; Kasai, H.; Yoshimura, K.; Otoguro, T.; Watashi, K.; Wakita, T.; Yamashita, A.; Tanaka, T.; Takeda, S.; Moriishi, K. Hepatitis B virus prevents excessive viral production via reduction of cell death-inducing DFF45-like effectors. J. Gen. Virol. 2017, 98, 1762–1773. [Google Scholar] [CrossRef]
- Fernandes, J.D.S.; Schuelter-Trevisol, F.; Cancelier, A.C.L.; e Silva, H.C.G.; de Sousa, D.G.; Atkinson, R.L.; Trevisol, D.J. Adenovirus 36 prevalence and association with human obesity: A systematic review. Int. J. Obes. 2021, 45, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Namazue, J.; Kato, T.; Okuno, T.; Shiraki, K.; Yamanishi, K. Evidence for Attachment of Fatty Acid to Varicella-Zoster Virus Glycoproteins and Effect of Cerulenin on the Maturation of Varicella-Zoster Virus Glycoproteins. Intervirology 1989, 30, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Ammer, E.; Nietzsche, S.; Rien, C.; Kühnl, A.; Mader, T.; Heller, R.; Sauerbrei, A.; Henke, A. The anti-obesity drug orlistat reveals anti-viral activity. Med. Microbiol. Immunol. 2015, 204, 635–645. [Google Scholar] [CrossRef]
- Okamura, H.; Nio, Y.; Akahori, Y.; Kim, S.; Watashi, K.; Wakita, T.; Hijikata, M. Fatty acid biosynthesis is involved in the production of hepatitis B virus particles. Biochem. Biophys. Res. Commun. 2016, 475, 87–92. [Google Scholar] [CrossRef]
- Jhuang, H.-J.; Hsu, W.-H.; Lin, K.-T.; Hsu, S.-L.; Wang, F.-S.; Chou, C.-K.; Lee, K.-H.; Tsou, A.-P.; Lai, J.-M.; Yeh, S.-F.; et al. Gluconeogenesis, lipogenesis, and HBV replication are commonly regulated by PGC-1α-dependent pathway. Oncotarget 2015, 6, 7788–7803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Esser, K.; Lucifora, J.; Wettengel, J.; Singethan, K.; Glinzer, A.; Zernecke, A.; Protzer, U. Lipase inhibitor orlistat prevents hepatitis B virus infection by targeting an early step in the virus life cycle. Antivir. Res. 2018, 151, 4–7. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Wing, P.A.C.; Diniz, M.O.; Pallett, L.J.; Swadling, L.; Harris, J.M.; Burton, A.R.; Jeffery-Smith, A.; Zakeri, N.; Amin, O.E.; et al. Targeting human Acyl-CoA:cholesterol acyltransferase as a dual viral and T cell metabolic checkpoint. Nat. Commun. 2021, 12, 2814. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Sharma-Walia, N. Targeting Host Cellular Factors as a Strategy of Therapeutic Intervention for Herpesvirus Infections. Front. Cell. Infect. Microbiol. 2021, 11, 603309. [Google Scholar] [CrossRef]
- Cole, S. Herpes Simplex Virus. Nurs. Clin. N. Am. 2020, 55, 337–345. [Google Scholar] [CrossRef]
- Ibarra, C.; Parada, M.; Retamal-Díaz, A.R.; A Suazo, P.; Garrido, I.; Kalergis, A.M.; A González, P. Producción de Penicilina en Chile entre 1944 y 1954. Rev. Chil. Infectol. 2015, 32, 88–96. [Google Scholar] [CrossRef][Green Version]
- Langeland, N.; Haarr, L.; Holmsen, H. Polyphosphoinositide metabolism in baby-hamster kidney cells infected with herpes simplex virus type 1. Biochem. J. 1986, 237, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.; de Oliveira, A.P.; Tobler, K.; Schraner, E.M.; Sonda, S.; Kaech, A.; Lucas, M.S.; Ackermann, M.; Wild, P. Herpes simplex virus 1 induces de novo phospholipid synthesis. Virology 2012, 429, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Asher, Y.; Heller, M.; Becker, Y. Incorporation of Lipids into Herpes Simplex Virus Particles. J. Gen. Virol. 1969, 4, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Kamble, N.; Kaufer, B.B.; Behboudi, S. Replication of Marek’s Disease Virus Is Dependent on Synthesis of De Novo Fatty Acid and Prostaglandin E 2. J. Virol. 2019, 93, e00352-19. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, H.L.; Nogalski, M.T.; Collins-McMillen, D.; Yurochko, A.D. Overview of Human Cytomegalovirus Pathogenesis. Hum. Cytomegaloviruses 2021, 2244, 1–18. [Google Scholar] [CrossRef]
- Low, H.; Mukhamedova, N.; Cui, H.L.; McSharry, B.P.; Avdic, S.; Hoang, A.; Ditiatkovski, M.; Liu, Y.; Fu, Y.; Meikle, P.J.; et al. Cytomegalovirus Restructures Lipid Rafts via a US28/CDC42-Mediated Pathway, Enhancing Cholesterol Efflux from Host Cells. Cell Rep. 2016, 16, 186–200. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Cresswell, P. Viperin Regulates Cellular Lipid Metabolism during Human Cytomegalovirus Infection. PLOS Pathog. 2013, 9, e1003497. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, N.; Yan, L.; Cao, X.; Lv, F.; Wang, J.; Wang, Y.; Cong, H. Down-regulation of single-stranded DNA-binding protein 1 expression induced by HCMV infection promotes lipid accumulation in cells. Braz. J. Med Biol. Res. 2017, 50. [Google Scholar] [CrossRef]
- Wong, C.S.Y.; Robinson, I.; Ochsenkühn, M.A.; Arlt, J.; Hossack, W.J.; Crain, J. Changes to lipid droplet configuration in mCMV-infected fibroblasts: Live cell imaging with simultaneous CARS and two-photon fluorescence microscopy. Biomed. Opt. Express 2011, 2, 2504–2516. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Wang, Y.; Qian, D.; Wang, Z.; Qin, Z.; Liu, X.; Liu, T.; Wang, B. HCMV-encoded IE2 promotes NAFLD pro-gression by up-regulation of SREBP1c expression in UL122 genetically modified mice. Int. J. Clin. Exp. Pathol. 2018, 11, 4213–4220. [Google Scholar]
- Rosemarie, Q.; Sugden, B. Epstein–Barr Virus: How Its Lytic Phase Contributes to Oncogenesis. Microorganisms 2020, 8, 1824. [Google Scholar] [CrossRef] [PubMed]
- Murono, S.; Inoue, H.; Tanabe, T.; Joab, I.; Yoshizaki, T.; Furukawa, M.; Pagano, J.S. Induction of cyclooxygenase-2 by Epstein–Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 2001, 98, 6905–6910. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Gaur, N.; Khera, L.; Kaul, R.; Robertson, E.S. COX-2 induces lytic reactivation of EBV through PGE2 by modulating the EP receptor signaling pathway. Virology 2015, 484, 1–14. [Google Scholar] [CrossRef]
- Hulse, M.; Johnson, S.M.; Boyle, S.; Caruso, L.B.; Tempera, I. Epstein-Barr Virus-Encoded Latent Membrane Protein 1 and B-Cell Growth Transformation Induce Lipogenesis through Fatty Acid Synthase. J. Virol. 2021, 95, e01857-20. [Google Scholar] [CrossRef] [PubMed]
- Rewane, A.; Tadi, P. Herpes Virus Type 8. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zhang, J.; Ling, N.; Lei, Y.; Peng, M.; Hu, P.; Chen, M. Multifaceted Interaction Between Hepatitis B Virus Infection and Lipid Metabolism in Hepatocytes: A Potential Target of Antiviral Therapy for Chronic Hepatitis B. Front. Microbiol. 2021, 12, 636897. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Shin, H.; Kim, K.; Choi, H.M.; Rhee, S.H.; Moon, H.; Kim, H.H.; Yang, U.S.; Yu, D.; Cheong, J. Hepatitis B Virus X Protein Induces Hepatic Steatosis Via Transcriptional Activation of SREBP1 and PPARγ. Gastroenterology 2007, 132, 1955–1967. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.H.; Kim, H.H.; Cheong, J. Hepatitis B virus X protein induces lipogenic transcription factor SREBP1 and fatty acid synthase through the activation of nuclear receptor LXRα. Biochem. J. 2008, 416, 219–230. [Google Scholar] [CrossRef]
- Na, T.-Y.; Shin, Y.K.; Roh, K.J.; Kang, S.-A.; Hong, I.; Oh, S.J.; Seong, J.K.; Park, C.K.; La Choi, Y.; Lee, M.-O. Liver X receptor mediates hepatitis B virus X protein-induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2008, 49, 1122–1131. [Google Scholar] [CrossRef]
- Qiao, L.; Wu, Q.; Lu, X.; Zhou, Y.; Fernández-Alvarez, A.; Ye, L.; Zhang, X.; Han, J.; Casado, M.; Liu, Q. SREBP-1a activation by HBx and the effect on hepatitis B virus enhancer II/core promoter. Biochem. Biophys. Res. Commun. 2013, 432, 643–649. [Google Scholar] [CrossRef][Green Version]
- Wu, Q.; Qiao, L.; Yang, J.; Zhou, Y.; Liu, Q. Stronger activation of SREBP-1a by nucleus-localized HBx. Biochem. Biophys. Res. Commun. 2015, 460, 561–565. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Peng, X.-E.; Zhu, Y.-B.; Yan, X.-L.; Chen, W.-N.; Lin, X. Hepatitis B Virus X Protein Induces Hepatic Steatosis by Enhancing the Expression of Liver Fatty Acid Binding Protein. J. Virol. 2016, 90, 1729–1740. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Yang, P.; Chen, Z.; Tang, N.; Ruan, X.Z.; Chen, Y. Genome-Wide Transcriptome Analysis of CD36 Overexpression in HepG2.2.15 Cells to Explore Its Regulatory Role in Metabolism and the Hepatitis B Virus Life Cycle. PLoS ONE 2016, 11, e0164787. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Yang, P.; Chen, Z.; Ruan, X.Z.; Huang, A.; Tang, N.; Chen, Y. Fatty acid translocase promoted hepatitis B virus replication by upregulating the levels of hepatic cytosolic calcium. Exp. Cell Res. 2017, 358, 360–368. [Google Scholar] [CrossRef]
- Cho, H.K.; Kim, S.Y.; Yoo, S.K.; Choi, Y.H.; Cheong, J. Fatty acids increase hepatitis B virus X protein stabilization and HBx-induced inflammatory gene expression. FEBS J. 2014, 281, 2228–2239. [Google Scholar] [CrossRef]
| Virus Family | Virus | LDs Outcome | Cellular Type | Reference |
|---|---|---|---|---|
| Adenoviridae | Human adenovirus serotype 2 (Ad2) | The infection induces LD formation associated with a cholesterol ester accumulation. | Human alveolar basal epithelial (A549) cells | [120] |
| The infection does not induce LD accumulation and FA oxidation. | Human adipose-derived stem cells (hADSCs) | [123,124] | ||
| Adenovirus serotype 5 (Ad5) | The infection induces the neutral lipid accumulation associated with high levels of cholesterol, but not TAG. | Human monocytes and PBMCs | [121] | |
| Human adenovirus serotype 31 (Ad31) | The infection upregulates lipid accumulation by the activation of C/EBP-b and PPARγ genes. It is unknown if the infection regulates LDs accumulation. | Mouse embryonic fibroblast adipose-like cell line (3T3L1) | [122] | |
| Human adenovirus serotype 36 (Ad36) | The infection induces LD accumulation. | Human skeletal muscle cell | [123] | |
| Promotes FA and TAG synthesis, producing LD accumulation by the modulation of PI3K/Akt/FoxO1/ PPARγ signaling pathway. | Human adipose-derived stem cells (hADSCs) | [124] | ||
| Fowl adenovirus serotype 4 (FadV-4) | The infection induces TAG accumulation shaped similar to LDs by inducing LXR-α, PPARγ, and SREBP-1c adipose-related genes. | Chicken hepatocytes | [125] | |
| Herpesviridae | Herpes simplex virus type 1 (HSV-1) | The infection induces LD accumulation at early times of infection by activating the epidermal growth factor receptor (EGFR). | Astrocytes and HeLa cells | [126] |
| Glycoprotein D (gD) interacts with LDs through their interaction with viperin protein. | COS-7 cells | [127] | ||
| Varicella-zoster virus (VZV) | The infection induces the synthesis of TAG, but not cholesterol ester. | Human embryonic lung cells | [128] | |
| Cytomegalovirus (CMV) | The infection increases neutral lipids synthesis by the accumulation of cholesterol. | Human embryonic lung cells, human saphenous veins smooth muscle cells, and human fibroblast | [129] | |
| Epstein-Barr virus (EBV) | The viral protein BRLF1 activates the FAS expression. | Human epithelial tongue cells | [130] | |
| The infection induces the cholesterol and FA synthesis pathways, a process modulated by the EBNA2, SREBP, and MYP proteins. | Human B cells | [131] | ||
| The infection inhibits PGE2 expression due to the downregulation of COX-2. | Monocytes | [132] | ||
| The viral LMP1 protein induces de novo lipid synthesis and LD formation. | Epithelial cells | [133] | ||
| Human herpesvirus 8: Kaposi’sarcoma | The infection induces the accumulation of LDs. A TAG accumulation in the lytic phase of infection was produced, meaning in the latent phase of infection, CE are increased. | Endothelial cells | [134] | |
| The infection increases the expression of COX-2/PGE2 signaling. | mECK36 cells | [135] | ||
| Hepadnaviridae | Hepatitis B virus (HBV) | HBx viral protein induces SREBP, PPARγ, and FAS expression by the interaction of HBx with LXR-α. | HepG2 cells | [136] |
| The infection induces LD formation. | HBx-expressing cells | [137,138] | ||
| The infection increases the FAS concentration in HBV chronic patients. | Human serum | [139] | ||
| The infection reduces intracellular TAG accumulation, reducing LD size. | Epithelial cells | [140] |
| Virus | Drug | Drug Target | Viral Outcome | Cellular Type | Reference |
|---|---|---|---|---|---|
| Fowl adenovirus serotype 4 (FAdV-4) | SR9243 | LXR-α antagonist | Decreases the virus production and the LD number. | Hepatocytes. | [125] |
| Herpes simplex virus type 1 (HSV-1) | AG-1478 | Inhibitor of epidermal growth factor receptor | Increases viral replication and decreases LD accumulation. | Astrocytes | [126] |
| Varicella-zoster virus (VZV) | Cerulenin | FAS inhibitor | Inhibits viral growth without affecting VZV protein synthesis. | Human embryo fibroblast cells | [142] |
| Orlistat | Inhibitor of LPL and FAS | Inhibits VZV replication. | Human embryonic lung fibroblast cells (HELF) | [143] | |
| Epstein-Barr virus (EBV) | Cerulenin | FAS inhibitor | Inhibits the activity of BRLF1 viral protein and BMRF1 lytic expression. | Human epithelial tongue cells | [130] |
| C75 | FAS inhibitor | Inhibits the activity of BRLF1 viral protein and BMRF1 lytic expression. | Human epithelial tongue cells | [130] | |
| Hepatitis B virus (HBV) | CP64018 | ACC1 inhibitor | Decreases extracellular HBV DNA. | HepG2-2-15-7 cells | [144] |
| GSK1995010 | FAS inhibitor | Decreases extracellular HBV DNA. | HepG2-2-15-7 cells. | [144] | |
| MK8245 | SCD1 inhibitor | No effect. | HepG2-2-15-7 cells. | [144] | |
| Graptopelatum paraguayense (HH-F3) | Inhibit PGC-1α and FASN expression | Inhibits HBV core promoter activity. | Huh-7, HepG2, and Hep3B/T2 cells | [145] | |
| Orlistat | LPL and FAS inhibitor | Does not affect HBV gene expression. | Differentiated HepaRG cells | [146] | |
| Avasimibe | ACAT inhibitor | Inhibits HBV replication. | HepG2 cells | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farías, M.A.; Diethelm-Varela, B.; Navarro, A.J.; Kalergis, A.M.; González, P.A. Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections. Cells 2022, 11, 2224. https://doi.org/10.3390/cells11142224
Farías MA, Diethelm-Varela B, Navarro AJ, Kalergis AM, González PA. Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections. Cells. 2022; 11(14):2224. https://doi.org/10.3390/cells11142224
Chicago/Turabian StyleFarías, Mónica A., Benjamín Diethelm-Varela, Areli J. Navarro, Alexis M. Kalergis, and Pablo A. González. 2022. "Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections" Cells 11, no. 14: 2224. https://doi.org/10.3390/cells11142224
APA StyleFarías, M. A., Diethelm-Varela, B., Navarro, A. J., Kalergis, A. M., & González, P. A. (2022). Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections. Cells, 11(14), 2224. https://doi.org/10.3390/cells11142224







