Mining the Mesenchymal Stromal Cell Secretome in Patients with Chronic Left Ventricular Dysfunction
Abstract
:Introduction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Bolli, R.; Garry, D.J.; Marban, E.; Menasche, P.; Zimmermann, W.H.; Kamp, T.J.; Wu, J.C.; Dzau, V.J. Basic and Translational Research in Cardiac Repair and Regeneration: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 2092–2105. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, D.; Bernardini, C.; Zannoni, A.; Salaroli, R.; Wang, C.; Bencivenni, S.; Forni, M. Efficacy of Stem Cell Therapy in Large Animal Models of Ischemic Cardiomyopathies: A Systematic Review and Meta-Analysis. Animals 2022, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.N.; Bolli, R.; Hare, J.M. Clinical Studies of Cell Therapy in Cardiovascular Medicine: Recent Developments and Future Directions. Circ. Res. 2018, 123, 266–287. [Google Scholar] [CrossRef] [PubMed]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regen. Ther. 2020, 14, 136–153. [Google Scholar] [CrossRef]
- Taylor, D.A.; Perin, E.C.; Willerson, J.T.; Zierold, C.; Resende, M.; Carlson, M.; Nestor, B.; Wise, E.; Orozco, A.; Pepine, C.J.; et al. Cardiovascular Cell Therapy Research N. Identification of Bone Marrow Cell Subpopulations Associated with Improved Functional Outcomes in Patients with Chronic Left Ventricular Dysfunction: An Embedded Cohort Evaluation of the FOCUS-CCTRN Trial. Cell Transplant. 2016, 25, 1675–1687. [Google Scholar] [CrossRef] [Green Version]
- Alberty, C.L.; Perin, E.C.; Willerson, J.T.; Gahremanpour, A.; Bolli, R.; Yang, P.C.; Traverse, J.H.; Lai, D.; Pepine, C.J.; Taylor, D.A. Peripheral Blood Biomarkers Associated with Improved Functional Outcome in Patients with Chronic Left Ventricular Dysfunction: A Biorepository Evaluation of the FOCUS-CCTRN Trial. Front. Cardiovasc. Med. 2021, 8, 698088. [Google Scholar] [CrossRef]
- Bolli, R.; Mitrani, R.D.; Hare, J.M.; Pepine, C.J.; Perin, E.C.; Willerson, J.T.; Traverse, J.H.; Henry, T.D.; Yang, P.C.; Murphy, M.P.; et al. Cardiovascular Cell Therapy Research N. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: The CCTRN CONCERT-HF trial. Eur. J. Heart Fail. 2021, 23, 661–674. [Google Scholar] [CrossRef]
- Perin, E.C.; Willerson, J.T.; Pepine, C.J.; Henry, T.D.; Ellis, S.G.; Zhao, D.X.; Silva, G.V.; Lai, D.; Thomas, J.D.; Kronenberg, M.W.; et al. Cardiovascular Cell Therapy Research N. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA 2012, 307, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Willerson, J.T.; Perin, E.C.; Ellis, S.G.; Pepine, C.J.; Henry, T.D.; Zhao, D.X.; Lai, D.; Penn, M.S.; Byrne, B.J.; Silva, G.; et al. Cardiovascular Cell Therapy Research N. Intramyocardial injection of autologous bone marrow mononuclear cells for patients with chronic ischemic heart disease and left ventricular dysfunction (First Mononuclear Cells injected in the US [FOCUS]): Rationale and design. Am. Heart J. 2010, 160, 215–223. [Google Scholar]
- Nigro, P.; Bassetti, B.; Cavallotti, L.; Catto, V.; Carbucicchio, C.; Pompilio, G. Cell therapy for heart disease after 15 years: Unmet expectations. Pharmacol. Res. 2018, 127, 77–91. [Google Scholar] [CrossRef]
- Liu, C.; Han, D.; Liang, P.; Li, Y.; Cao, F. The Current Dilemma and Breakthrough of Stem Cell Therapy in Ischemic Heart Disease. Front. Cell Dev. Biol. 2021, 9, 636136. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Vahidy, F.S.; Rahbar, M.H.; Zhu, H.; Rowan, P.J.; Bambhroliya, A.B.; Savitz, S.I. Systematic Review and Meta-Analysis of Bone Marrow-Derived Mononuclear Cells in Animal Models of Ischemic Stroke. Stroke 2016, 47, 1632–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Viejo, M.; Menendez-Menendez, Y.; Blanco-Gelaz, M.A.; Ferrero-Gutierrez, A.; Fernandez-Rodriguez, M.A.; Gala, J.; Otero-Hernandez, J. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant. Proc. 2013, 45, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Hui, J.H.; Ling, L.; Nurcombe, V.; Cool, S.M. Telomere length analysis of human mesenchymal stem cells by quantitative PCR. Gene 2013, 519, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. Recent Trends in Multipotent Human Mesenchymal Stem/Stromal Cells: Learning from History and Advancing Clinical Applications. OMICS 2021, 25, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem. Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef]
- Meng, H.; Cheng, W.; Wang, L.; Chen, S.; Teng, Y.; Lu, Z.; Li, Y.; Zhao, M. Mesenchymal Stem Cell Exosomes in the Treatment of Myocardial Infarction: A Systematic Review of Preclinical In Vivo Studies. J. Cardiovasc. Transl. Res. 2022, 15, 317–339. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Chang, S.; Xu, R.; Chen, L.; Song, X.; Wu, J.; Qian, J.; Zou, Y.; Ma, J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem. Cell Res. Ther. 2020, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, R.; Gomez-Ferrer, M.; Reinal, I.; Buigues, M.; Villanueva-Badenas, E.; Ontoria-Oviedo, I.; Hernandiz, A.; Gonzalez-King, H.; Peiro-Molina, E.; Dorronsoro, A.; et al. miR-4732-3p in Extracellular Vesicles From Mesenchymal Stromal Cells Is Cardioprotective During Myocardial Ischemia. Front. Cell Dev. Biol. 2021, 9, 734143. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Agarwal, U.; George, A.; Bhutani, S.; Ghosh-Choudhary, S.; Maxwell, J.T.; Brown, M.E.; Mehta, Y.; Platt, M.O.; Liang, Y.; Sahoo, S.; et al. Experimental, Systems, and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell-Derived Exosomes From Pediatric Patients. Circ. Res. 2017, 120, 701–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Fattore, A.; Luciano, R.; Saracino, R.; Battafarano, G.; Rizzo, C.; Pascucci, L.; Alessandri, G.; Pessina, A.; Perrotta, A.; Fierabracci, A.; et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert. Opin. Biol. Ther. 2015, 15, 495–504. [Google Scholar] [CrossRef]
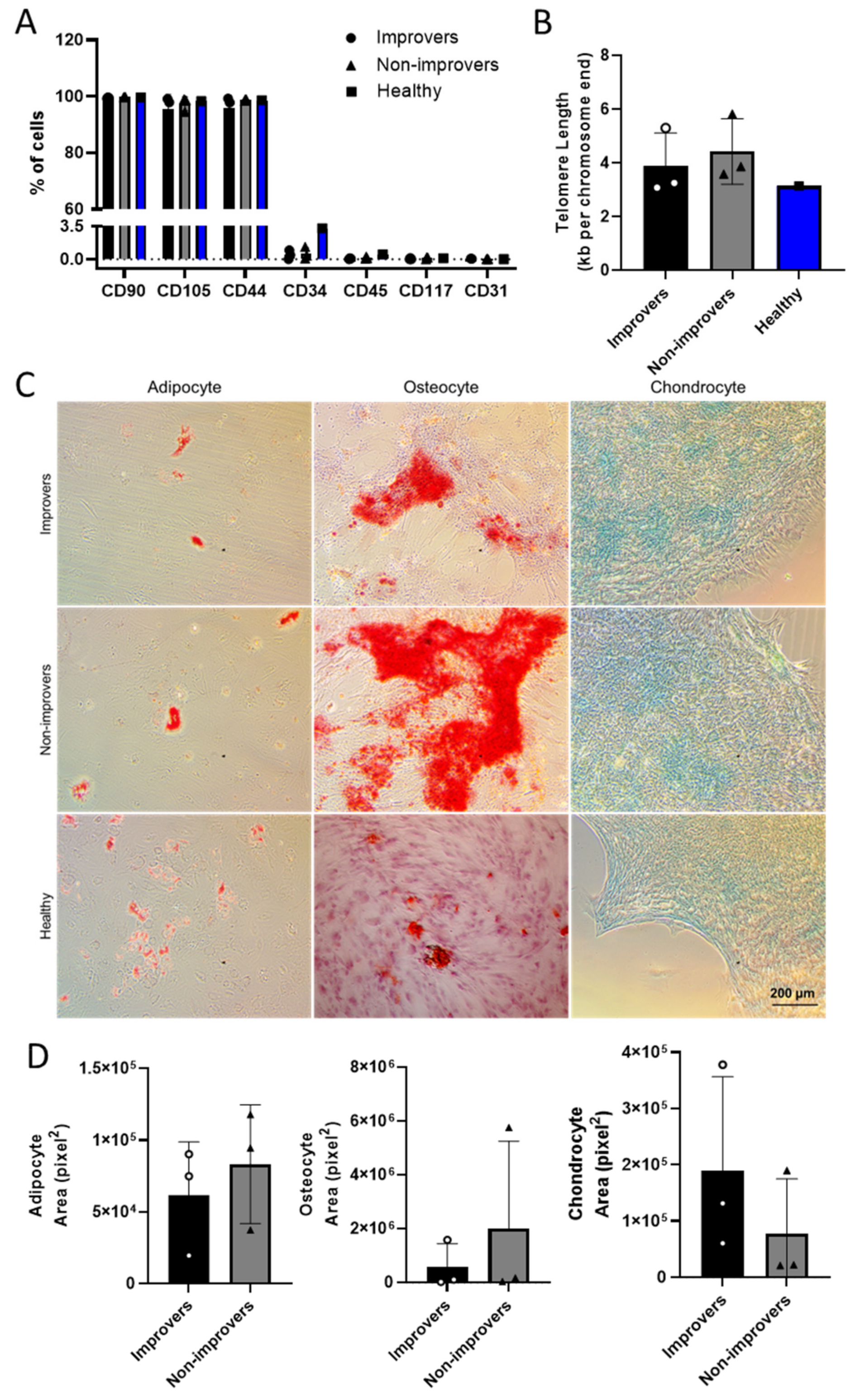
| Reference | Clinical Improvers (n = 17) | Improver BMMNC Characteristics |
|---|---|---|
| [5] | Decreased LVESV, increased VO2 max, and increased LVEF | Higher CD19+ B cells, CD11b+ monocytes, CD31dim cells, and CXCR4+ cells; lower CD31bright cells |
| Reference | Category | Characteristic |
|---|---|---|
| [8,9] | Patient demographics (enrollment) | Age: ≥18 years old with CAD, LVEF ≤ 45% and limiting angina and/or symptomatic CHF |
| Study size | 153 patients consented; 92 randomized to receive treatment, 6 excluded from treatment | |
| Study design | Randomized 2:1 for treatment (100 × 106 BMMNCs) vs. control (5% human albumin) | |
| BMMNC isolation technique | Sepax (Ficoll method) | |
| Release criteria | CD34+/CD133+; CFU-GM colony growth | |
| Product delivery | Within 12 h of bone marrow aspiration | |
| Follow-up | 6 months after delivery | |
| Primary endpoints | Left ventricular end-systolic volume, maximal oxygen consumption, infarct size | |
| Exploratory outcomes | Increased left ventricular ejection fraction |
| Category | Characteristics | Improvers (n = 3) | Non-Improvers (n = 3) |
|---|---|---|---|
| Patient demographics and primary endpoints | Age | 60.03 ± 5.92 | 67.71 ± 7.96 |
| Diabetes | No | No | |
| Hypertension | Yes | Yes | |
| Hyperlipidemia | Yes | Yes | |
| Smoking | Yes | Yes | |
| LVEF at baseline (%) | 35.17 ± 13.2 | 38.3 ± 7.52 | |
| LVEF at endpoint (%) | 37.67 ± 14.32 | 32.73 ± 8.68 | |
| LVESV at baseline (%) | 103.1 ± 32.1 | 154.4 ± 58.11 | |
| LVESV at endpoint (%) | 128.4 ± 46.32 | 137.4 ± 61.58 | |
| BMMNC characteristics (at baseline) | Viability (%) | 99 ± 1 | 97.67 ± 0.57 |
| CD34/CD133 (Mean %, p = 0.49) | 2.29 | 1.48 | |
| CD19 (Mean %, p = 0.79) | 11.51 | 13.11 | |
| CD11b (Mean %, p = 0.98) | 62.62 | 62.43 | |
| CXCR4 (Mean %, p = 0.86) | 63.12 | 61.9 | |
| CD31dim (Mean %, p = 0.75) | 9.36 | 8.57 | |
| CD31bright (Mean %, p = 0.38) | 0.12 | 0.25 |
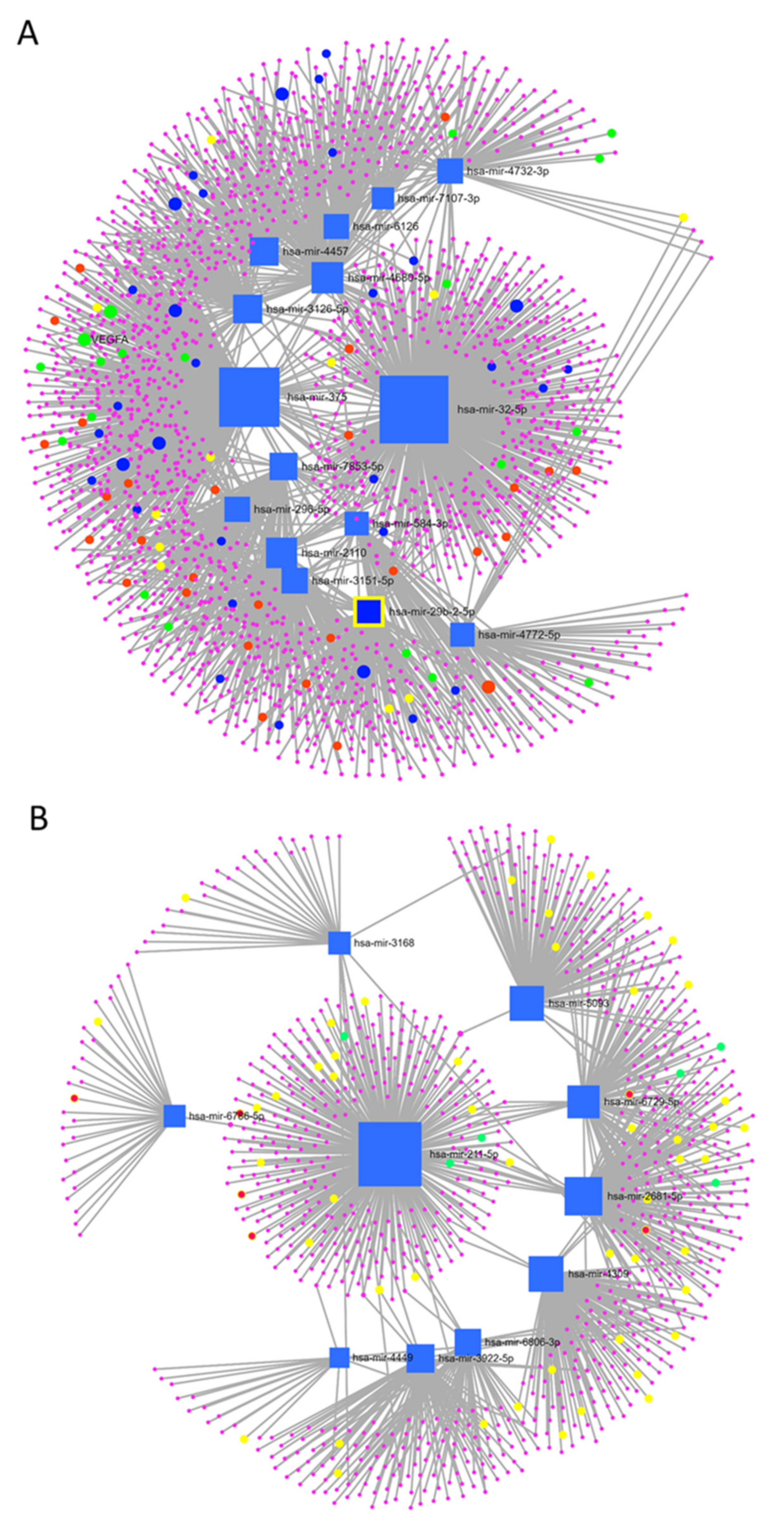
| Over-Expressed Improver miRNAs | Under-Expressed Improver miRNAs | ||
|---|---|---|---|
| hsa-miR-4680-5p | hsa-miR-584-3p | hsa-miR-2681-5p | hsa-miR-211-5p |
| hsa-miR-375 | hsa-miR-7107-3p | hsa-miR-5093 | hsa-miR-6729-5p |
| hsa-miR-32-5p | hsa-miR-296-5p | hsa-miR-3168 | hsa-miR-4449 |
| hsa-miR-4457 | hsa-miR-6126 | hsa-miR-6806-3p | hsa-miR-3922-5p |
| hsa-miR-3126-5p | hsa-miR-3151-5p | hsa-miR-4309 | hsa-miR-6786-5p |
| hsa-miR-2110 | hsa-miR-4732-3p | has-miR-019914 | has-miR-004153 |
| hsa-miR-29b-2-5p | hsa-miR-7853-5p | ||
| hsa-miR-105-3p | hsa-miR-4772-5p | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrissey, J.; Mesquita, F.C.P.; Chacon-Alberty, L.; Hochman-Mendez, C. Mining the Mesenchymal Stromal Cell Secretome in Patients with Chronic Left Ventricular Dysfunction. Cells 2022, 11, 2092. https://doi.org/10.3390/cells11132092
Morrissey J, Mesquita FCP, Chacon-Alberty L, Hochman-Mendez C. Mining the Mesenchymal Stromal Cell Secretome in Patients with Chronic Left Ventricular Dysfunction. Cells. 2022; 11(13):2092. https://doi.org/10.3390/cells11132092
Chicago/Turabian StyleMorrissey, Jacquelynn, Fernanda C. P. Mesquita, Lourdes Chacon-Alberty, and Camila Hochman-Mendez. 2022. "Mining the Mesenchymal Stromal Cell Secretome in Patients with Chronic Left Ventricular Dysfunction" Cells 11, no. 13: 2092. https://doi.org/10.3390/cells11132092
APA StyleMorrissey, J., Mesquita, F. C. P., Chacon-Alberty, L., & Hochman-Mendez, C. (2022). Mining the Mesenchymal Stromal Cell Secretome in Patients with Chronic Left Ventricular Dysfunction. Cells, 11(13), 2092. https://doi.org/10.3390/cells11132092






