A TRPC3/6 Channel Inhibitor Promotes Arteriogenesis after Hind-Limb Ischemia
Abstract
:1. Introduction
2. Materials and Methods
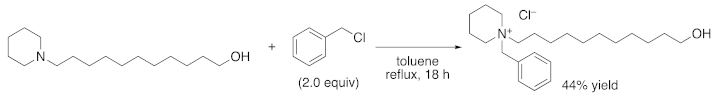
2.1. Synthesis of 1-BP (1-Benzyl-1-(11-hydroxyundecyl)piperidin-1-ium chloride)
2.2. Cell Culture and Transfection
2.3. Measurement of Ca2+ Responses in 96-Well Plates
2.4. Fluorescent Ca2+ Imaging
2.5. Immunofluorescent Staining
2.6. Mouse Models
2.7. Histological Analysis
2.8. Analysis of Motor Activities
2.9. Western Blotting
2.10. Measuring Sphk-1 mRNA Expression in Gastrocnemius Muscles
2.11. Fluorescent Measurement of Nitric Oxide (NO)
2.12. The Tension Measurement with Arterial Rings Isolated from Mice
2.13. Electrophysiology
2.14. Statistics
3. Results
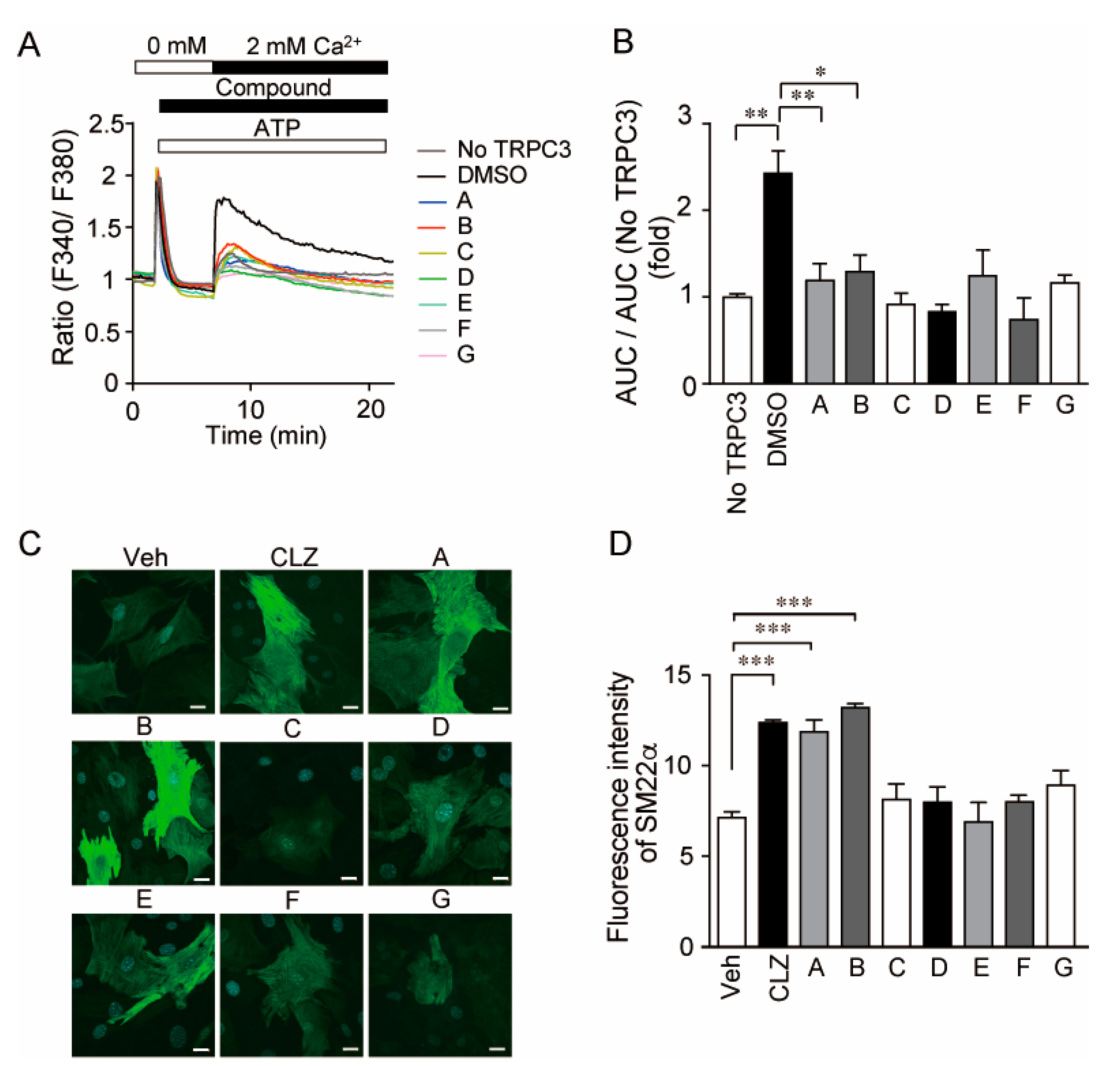
3.1. Identification of Selective TRPC3/6 Inhibitors
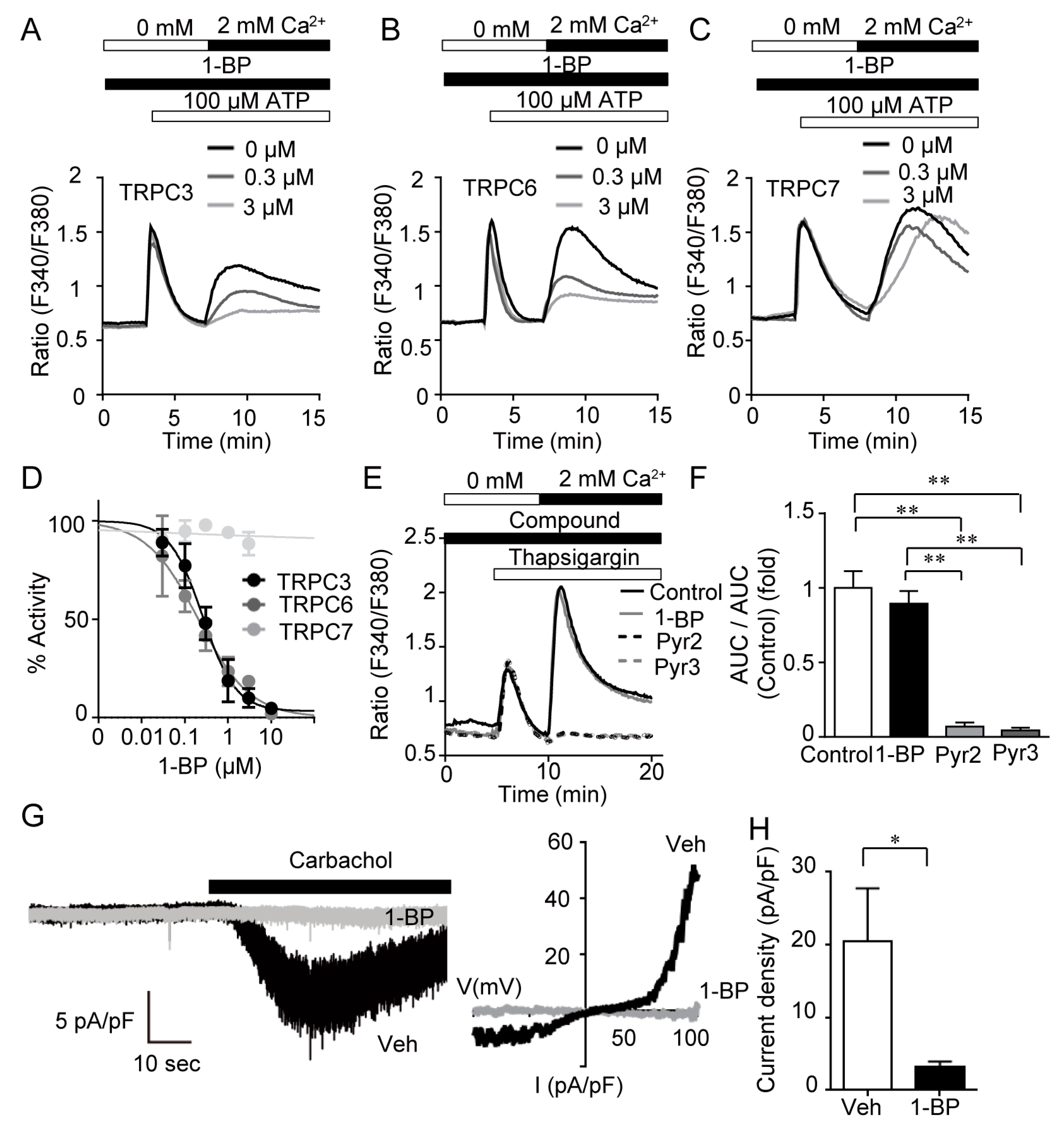
3.2. 1-BP Specifically Suppresses TRPC3/6 Channels
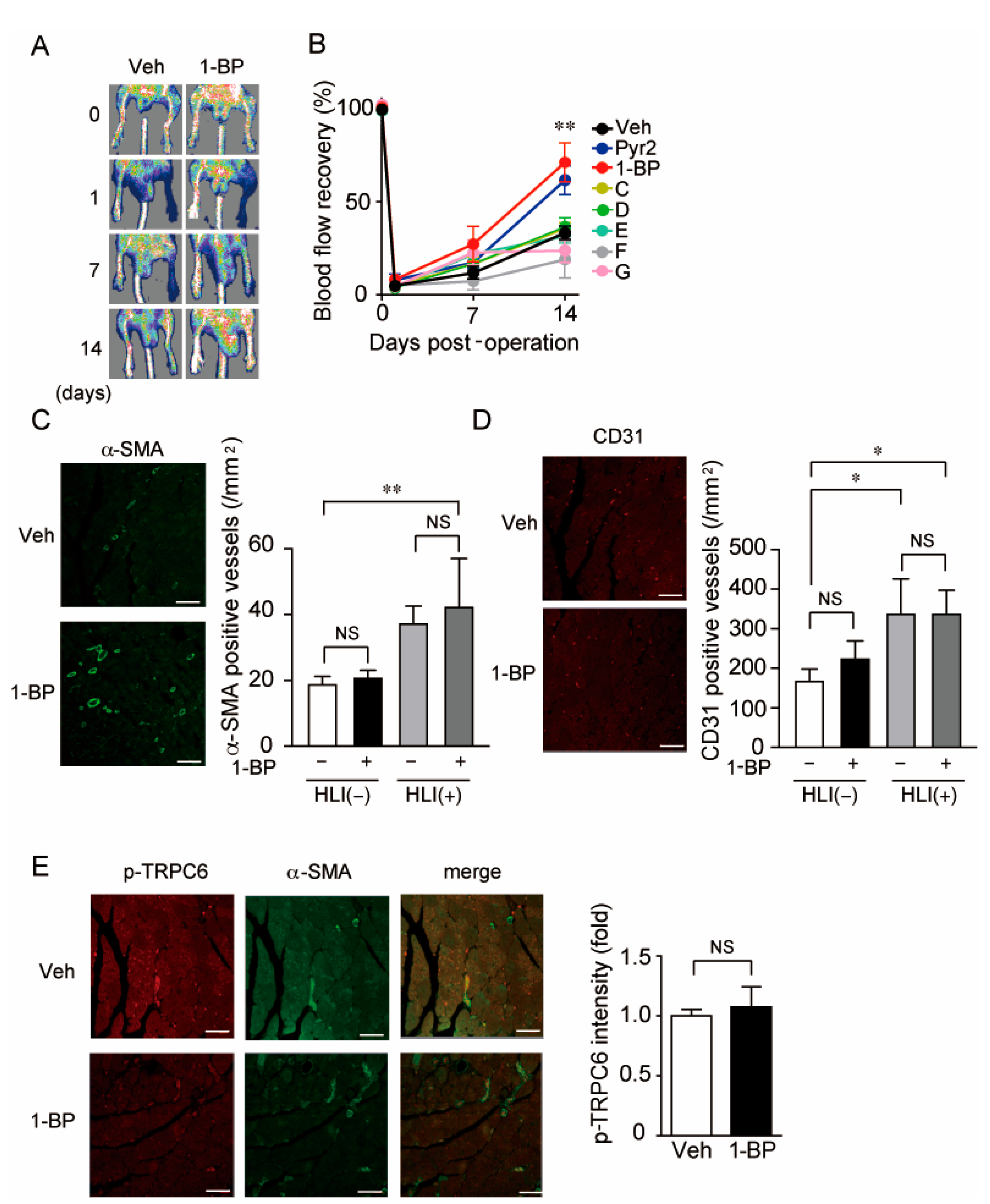
3.3. A TRPC3/6 Inhibitor 1-BP Improves Blood Flow Recovery after HLI
3.4. 1-BP Preserves Myoglobin Expression in Skeletal Muscle and Walking Abilities after HLI
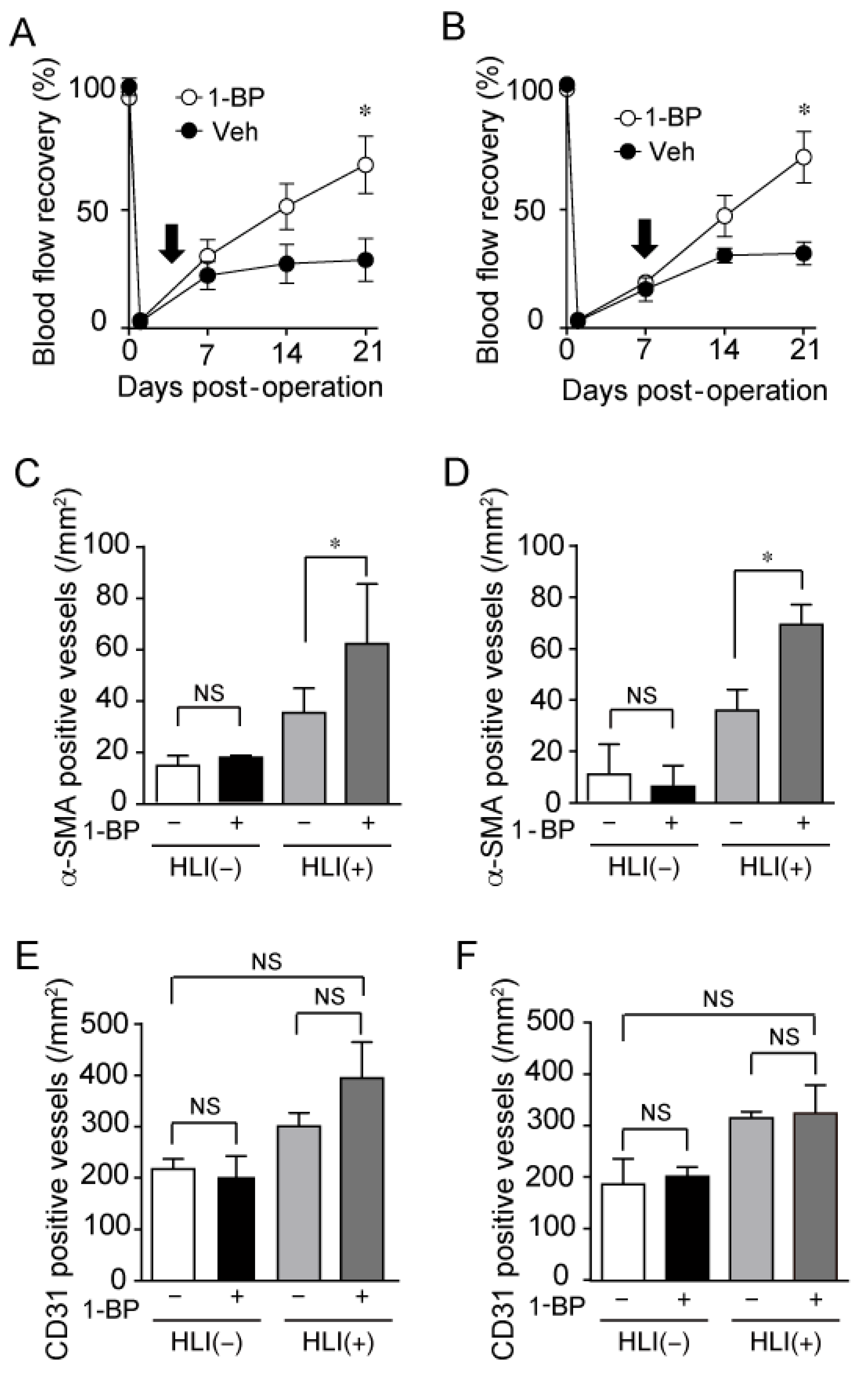
3.5. 1-BP Improves Blood Flow via TRPC6 Inhibition
3.6. 1-BP Promotes Arteriogenesis Independently of Hypoxia and Inflammation
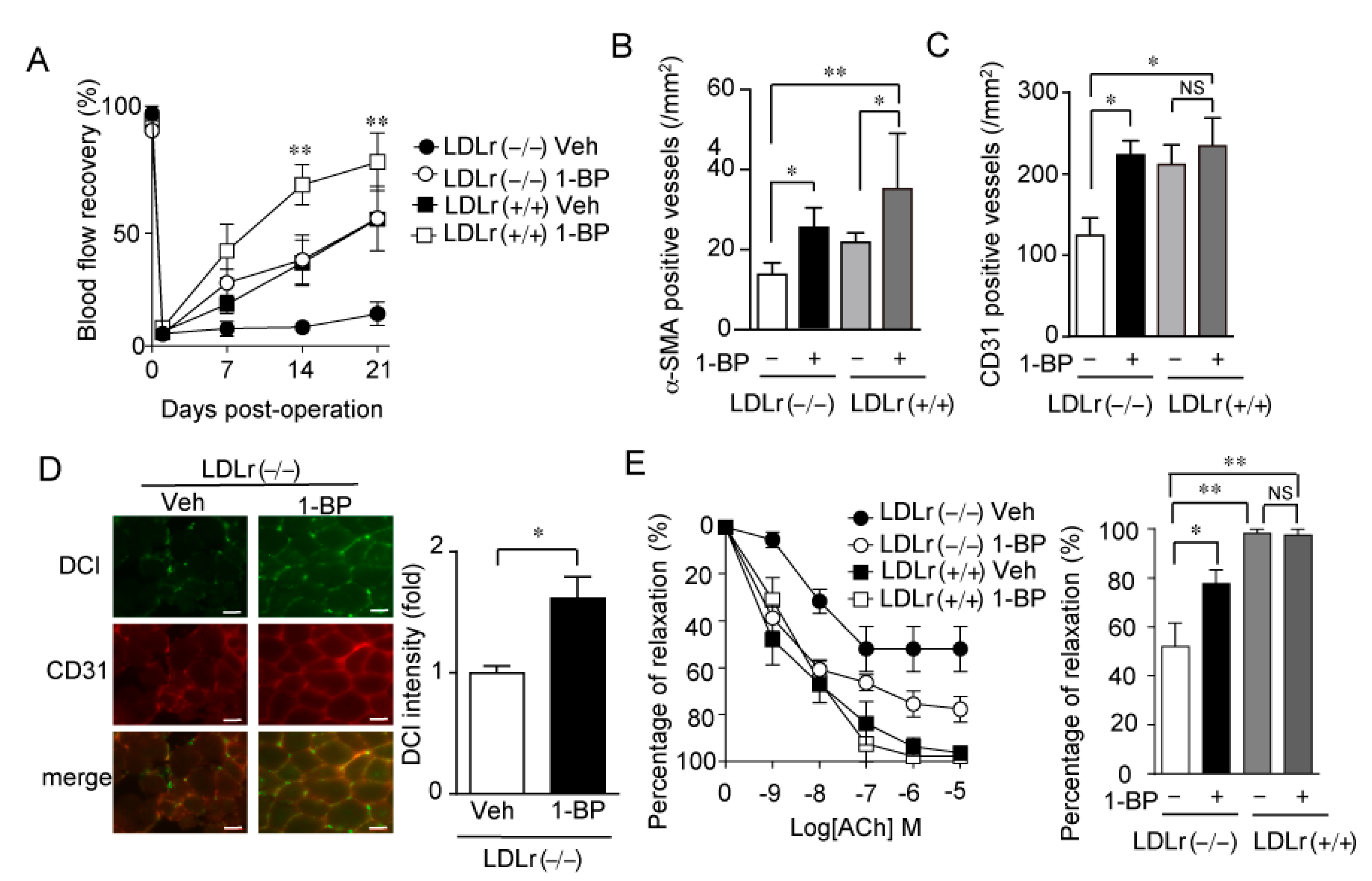
3.7. 1-BP Promotes Arteriogenesis Independently of Endothelium and Preserves Endothelial Function
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regensteiner, J.G.; Wolfel, E.E.; Brass, E.P.; Carry, M.R.; Ringel, S.P.; Hargarten, M.E.; Stamm, E.R.; Hiatt, W.R. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation 1993, 87, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Group, T.I.W. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiattarella, G.G.; Perrino, C.; Magliulo, F.; Carbone, A.; Bruno, A.G.; De Paulis, M.; Sorropago, A.; Corrado, R.V.; Bottino, R.; Menafra, G.; et al. Physical activity in the prevention of peripheral artery disease in the elderly. Front. Physiol. 2014, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Criqui, M.H.; Langer, R.D.; Fronek, A.; Feigelson, H.S.; Klauber, M.R.; McCann, T.J.; Browner, D. Mortality over a period of 10 years in patients with peripheral arterial disease. N. Engl. J. Med. 1992, 326, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [Green Version]
- Muluk, S.C.; Muluk, V.S.; Kelley, M.E.; Whittle, J.C.; Tierney, J.A.; Webster, M.W.; Makaroun, M.S. Outcome events in patients with claudication: A 15-year study in 2777 patients. J. Vasc. Surg. 2001, 33, 251–257; discussion 257–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinsomboon, J.; Ruas, J.; Gupta, R.K.; Thom, R.; Shoag, J.; Rowe, G.C.; Sawada, N.; Raghuram, S.; Arany, Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc. Natl. Acad. Sci. USA 2009, 106, 21401–21406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montell, C.; Rubin, G.M. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Holzer, P.; Izzo, A.A. The pharmacology of TRP channels. Br. J. Pharmacol. 2014, 171, 2469–2473. [Google Scholar] [CrossRef] [Green Version]
- Onohara, N.; Nishida, M.; Inoue, R.; Kobayashi, H.; Sumimoto, H.; Sato, Y.; Mori, Y.; Nagao, T.; Kurose, H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006, 25, 5305–5316. [Google Scholar] [CrossRef]
- Chen, W.; Oberwinkler, H.; Werner, F.; Gassner, B.; Nakagawa, H.; Feil, R.; Hofmann, F.; Schlossmann, J.; Dietrich, A.; Gudermann, T.; et al. Atrial natriuretic peptide-mediated inhibition of microcirculatory endothelial Ca2+ and permeability response to histamine involves cGMP-dependent protein kinase I and TRPC6 channels. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.; Hill, K.; Wang, L.; Kuebler, W.M.; Schaefer, M. Novel pharmacological TRPC inhibitors block hypoxia-induced vasoconstriction. Cell Calcium 2012, 51, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fantozzi, I.; Remillard, C.V.; Landsberg, J.W.; Kunichika, N.; Platoshyn, O.; Tigno, D.D.; Thistlethwaite, P.A.; Rubin, L.J.; Yuan, J.X. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA 2004, 101, 13861–13866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishioka, K.; Nishida, M.; Ariyoshi, M.; Jian, Z.; Saiki, S.; Hirano, M.; Nakaya, M.; Sato, Y.; Kita, S.; Iwamoto, T.; et al. Cilostazol suppresses angiotensin II-induced vasoconstriction via protein kinase A-mediated phosphorylation of the transient receptor potential canonical 6 channel. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2278–2286. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, A.J.; Krishnan, L.; Sullivan, C.J.; Williams, S.K.; Hoying, J.B. Microvascular repair: Post-angiogenesis vascular dynamics. Microcirculation 2012, 19, 676–695. [Google Scholar] [CrossRef] [Green Version]
- Numaga-Tomita, T.; Shimauchi, T.; Oda, S.; Tanaka, T.; Nishiyama, K.; Nishimura, A.; Birnbaumer, L.; Mori, Y.; Nishida, M. TRPC6 regulates phenotypic switching of vascular smooth muscle cells through plasma membrane potential-dependent coupling with PTEN. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 9785–9796. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, A.; Mederos, Y.S.M.; Gollasch, M.; Gross, V.; Storch, U.; Dubrovska, G.; Obst, M.; Yildirim, E.; Salanova, B.; Kalwa, H.; et al. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol. Cell. Biol. 2005, 25, 6980–6989. [Google Scholar] [CrossRef] [Green Version]
- Izumi, S.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. A simple and effective strategy to increase the sensitivity of fluorescence probes in living cells. J. Am. Chem. Soc. 2009, 131, 10189–10200. [Google Scholar] [CrossRef]
- Matoba, T.; Shimokawa, H.; Nakashima, M.; Hirakawa, Y.; Mukai, Y.; Hirano, K.; Kanaide, H.; Takeshita, A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Investig. 2000, 106, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- He, L.P.; Hewavitharana, T.; Soboloff, J.; Spassova, M.A.; Gill, D.L. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J. Biol. Chem. 2005, 280, 10997–11006. [Google Scholar] [CrossRef] [Green Version]
- Maier, T.; Follmann, M.; Hessler, G.; Kleemann, H.W.; Hachtel, S.; Fuchs, B.; Weissmann, N.; Linz, W.; Schmidt, T.; Lohn, M.; et al. Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br. J. Pharmacol. 2015, 172, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lozinskaya, I.; Costell, M.; Lin, Z.; Ball, J.A.; Bernard, R.; Behm, D.J.; Marino, J.P.; Schnackenberg, C.G. Characterization of small molecule TRPC3 and TRPC6 agonist and antagonists. Biophys. J. 2013, 104, 454a. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Schaefer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef] [Green Version]
- Schaper, W. Collateral circulation: Past and present. Basic Res. Cardiol. 2009, 104, 5–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitt, C.; Strauss, B.; Schwarz, E.C.; Spaeth, N.; Rast, G.; Hatzelmann, A.; Hoth, M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 2004, 279, 12427–12437. [Google Scholar] [CrossRef] [Green Version]
- Oyama, O.; Sugimoto, N.; Qi, X.; Takuwa, N.; Mizugishi, K.; Koizumi, J.; Takuwa, Y. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovasc. Res. 2008, 78, 301–307. [Google Scholar] [CrossRef]
- Lee, J.U.; Shin, J.; Song, W.; Kim, H.; Lee, S.; Jang, S.J.; Wong, S.C.; Edelberg, J.E.; Liau, G.; Hong, M.K. A novel adenoviral gutless vector encoding sphingosine kinase promotes arteriogenesis and improves perfusion in a rabbit hindlimb ischemia model. Coron. Artery Dis. 2005, 16, 451–456. [Google Scholar] [CrossRef]
- Saba, J.D.; de la Garza-Rodea, A.S. S1P lyase in skeletal muscle regeneration and satellite cell activation: Exposing the hidden lyase. Biochim. Biophys. Acta 2013, 1831, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Monet, M.; Francoeur, N.; Boulay, G. Involvement of phosphoinositide 3-kinase and PTEN protein in mechanism of activation of TRPC6 protein in vascular smooth muscle cells. J. Biol. Chem. 2012, 287, 17672–17681. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Hoffman, A.; Yang, Y.; Yan, J.; Tie, G.; Bagshahi, H.; Nowicki, P.T.; Messina, L.M. Endothelial nitric oxide synthase affects both early and late collateral arterial adaptation and blood flow recovery after induction of hind limb ischemia in mice. J. Vasc. Surg. 2010, 51, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldron, G.J.; Cole, W.C. Activation of vascular smooth muscle K+ channels by endothelium-derived relaxing factors. Clin. Exp. Pharmacol. Physiol. 1999, 26, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Hutter, C.M.; Austin, M.A.; Humphries, S.E. Familial hypercholesterolemia, peripheral arterial disease, and stroke: A HuGE minireview. Am. J. Epidemiol. 2004, 160, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Miname, M.; Makdisse, M.; Kalil Filho, R.; Santos, R.D. Association of peripheral arterial and cardiovascular diseases in familial hypercholesterolemia. Arq. Bras. Cardiol. 2014, 103, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Couffinhal, T.; Silver, M.; Kearney, M.; Sullivan, A.; Witzenbichler, B.; Magner, M.; Annex, B.; Peters, K.; Isner, J.M. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation 1999, 99, 3188–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirziu, D.; Moodie, K.L.; Zhuang, Z.W.; Singer, K.; Helisch, A.; Dunn, J.F.; Li, W.; Singh, J.; Simons, M. Delayed arteriogenesis in hypercholesterolemic mice. Circulation 2005, 112, 2501–2509. [Google Scholar] [CrossRef] [Green Version]
- Van Belle, E.; Rivard, A.; Chen, D.; Silver, M.; Bunting, S.; Ferrara, N.; Symes, J.F.; Bauters, C.; Isner, J.M. Hypercholesterolemia attenuates angiogenesis but does not preclude augmentation by angiogenic cytokines. Circulation 1997, 96, 2667–2674. [Google Scholar] [CrossRef]
- Haddad, P.; Dussault, S.; Groleau, J.; Turgeon, J.; Maingrette, F.; Rivard, A. Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: Effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis 2011, 217, 340–349. [Google Scholar] [CrossRef]
- Koo, J.H.; Kim, T.H.; Park, S.Y.; Joo, M.S.; Han, C.Y.; Choi, C.S.; Kim, S.G. Gα13 ablation reprograms myofibers to oxidative phenotype and enhances whole-body metabolism. J. Clin. Investig. 2017, 127, 3845–3860. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Koo, J.H.; Jin, X.; Kim, W.; Park, S.Y.; Park, S.; Rhee, E.P.; Choi, C.S.; Kim, S.G. Ablation of USP21 in skeletal muscle promotes oxidative fibre phenotype, inhibiting obesity and type 2 diabetes. J. Cachexia Sarcopenia Muscle 2021, 12, 1669–1689. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimauchi, T.; Numaga-Tomita, T.; Kato, Y.; Morimoto, H.; Sakata, K.; Matsukane, R.; Nishimura, A.; Nishiyama, K.; Shibuta, A.; Horiuchi, Y.; et al. A TRPC3/6 Channel Inhibitor Promotes Arteriogenesis after Hind-Limb Ischemia. Cells 2022, 11, 2041. https://doi.org/10.3390/cells11132041
Shimauchi T, Numaga-Tomita T, Kato Y, Morimoto H, Sakata K, Matsukane R, Nishimura A, Nishiyama K, Shibuta A, Horiuchi Y, et al. A TRPC3/6 Channel Inhibitor Promotes Arteriogenesis after Hind-Limb Ischemia. Cells. 2022; 11(13):2041. https://doi.org/10.3390/cells11132041
Chicago/Turabian StyleShimauchi, Tsukasa, Takuro Numaga-Tomita, Yuri Kato, Hiroyuki Morimoto, Kosuke Sakata, Ryosuke Matsukane, Akiyuki Nishimura, Kazuhiro Nishiyama, Atsushi Shibuta, Yutoku Horiuchi, and et al. 2022. "A TRPC3/6 Channel Inhibitor Promotes Arteriogenesis after Hind-Limb Ischemia" Cells 11, no. 13: 2041. https://doi.org/10.3390/cells11132041
APA StyleShimauchi, T., Numaga-Tomita, T., Kato, Y., Morimoto, H., Sakata, K., Matsukane, R., Nishimura, A., Nishiyama, K., Shibuta, A., Horiuchi, Y., Kurose, H., Kim, S. G., Urano, Y., Ohshima, T., & Nishida, M. (2022). A TRPC3/6 Channel Inhibitor Promotes Arteriogenesis after Hind-Limb Ischemia. Cells, 11(13), 2041. https://doi.org/10.3390/cells11132041






