Abstract
Breast cancer, as the most prevalent cancer in women, is responsible for more than 15% of new cancer cases and about 6.9% of all cancer-related death in the US. A major cause of therapeutic failure in breast cancer is the development of resistance to chemotherapy, especially for triple-negative breast cancer (TNBC). Therefore, how to overcome chemoresistance is the major challenge to improve the life expectancy of breast cancer patients. Our studies demonstrate that TNBC cells surviving the chronic treatment of chemotherapeutic drugs show significantly higher expression of the dual serine/threonine and tyrosine protein kinase (DSTYK) than non-treated parental cells. In our in vitro cellular models, DSTYK knockout via the CRISPR/Cas9-mediated technique results in apoptotic cell death of chemoresistant cells upon drug treatment. Moreover, DSTYK knockout promotes chemotherapeutic drug-induced tumor cell death in an orthotopic mouse model. These findings suggest that DSTYK exerts an important and previously unknown role in promoting chemoresistance. Our studies provide fundamental insight into the role of DSTYK in chemoresistance in TNBC cells and lay the foundation for the development of new strategies targeting DSTYK for improving TNBC therapy.
1. Introduction
Chemoresistance is the main reason for the failure of anti-breast cancer chemotherapy [1,2]. Triple-negative breast cancer (TNBC), defined as estrogen receptor (ER) negative, progesterone receptor (PR) negative, and human epidermal growth factor receptor 2 (HER2) negative, is the most lethal subtype of breast cancer due to its highly-aggressive characteristics, heterogeneity, and the availability of few treatment methods [3,4]. Currently, chemotherapy remains a standard neoadjuvant treatment of TNBC [5,6]. Unfortunately, chemoresistance is the most common treatment failure in TNBC patients [7].
Numerous mechanisms have been reported to explain the chemoresistance in TNBC cells including autophagy induction, unpredicted drug efflux, drug target shift, drug loss-of-function, cell death inhibition, epigenetic modifications, epithelial-to-mesenchymal transition (EMT), increased DNA damage repair, and cancer stem cells (CSCs) [8,9,10,11,12,13,14,15,16,17,18]. Recent studies to characterize TNBC tumors relying on the profiling of their transcriptome, proteome, epigenome, genome, and immuno-microenvironment significantly improved our understanding of the molecular heterogeneity of TNBC [17]. However, the discovery of effective and efficient treatment strategies is still an unmet clinical requirement. Therefore, it is urgent to find a strategy to overcome chemoresistance and save TNBC patients’ lives.
DSTYK, as a dual serine/threonine and tyrosine protein kinase, is expressed in multiple human tissues, including the brain, heart, kidney, lung, colon, and muscle [18]. Knockdown of DSTYK in zebrafish resulted in developmental defects in the spine, and further mechanistic studies suggested that DSTYK has involved in the fibroblast growth factor (FGF) signaling pathway by increasing the phosphorylation of extracellular-signal-regulated kinase (ERK) signaling in zebrafish [19]. In addition, DSTYK mutation was shown to upregulate the expression of two metastasis-related molecules, MMP2 and MMP9, in HPC3 cells by activating ERK1/2 signaling [19]. Furthermore, our group found that DSTYK promotes metastasis and chemoresistance via EMT in colorectal cancer [20]. To date, there is no available inhibitor targeting DSTYK for chemotherapy.
One popular chemotherapeutic drug combination, doxorubicin (DOX) plus docetaxel (DXL), for TNBC treatment has shown significant therapeutic efficacy in breast cancer therapy and has been frequently employed in clinical trials [21,22,23,24]. In this study, we employed the DOX and DXL combination (DOX/DXL) to select chemoresistant cells from two triple-negative breast cancer cell lines, SUM102PT and MDA-MB-468 cells. The cells surviving after DOX/DXL combination treatment show significant characteristics of CSCs and chemoresistance. Previous reports found that DSTYK promotes chemoresistance in colorectal cancer cells [20] and inhibits skin cell apoptosis [25]. Here, we report that DSTYK knockout (DSTYKKO) significantly attenuates the chemoresistance in TNBC cells, making chemoresistant cells sensitive to chemotherapeutic drug treatment in both in vitro and in vivo models. Therefore, we identified DSTYK as a potential chemotherapeutic target in the treatment of TNBC.
2. Materials and Methods
2.1. Xenografts Experiments in Mice
All of the animal experimental procedures are consistent with our mice protocols that were approved by the Institutional Animal Care and Use Committee at East Tennessee State University (ETSU). NOD.CB17-Prkdcscid/J mice (SCID) FVB mice, 4–6 weeks old, were purchased from the Jackson Laboratory and are maintained at the ETSU animal facility under the specified pathogen-free conditions. In all of the experiments, female mice were randomly selected. In the xenograft mouse model, approximately 2 × 105 cells were injected into the flanks of 7–8-week-old SCID mice. When the tumor volume reached around 50 mm3, mice received therapy by intraperitoneal (i.p.) injection of the chemotherapeutic agent (DOX/DXL). DOX/DXL was administered to mice 2 times/week until some tumors reach a volume of ~1000 mm3. The tumors were sized every 3 days using calipers and the volume was calculated using a standard formula: Width2 × length × 0.5. Then, mice were euthanized by carbon dioxide and subjected to necropsy and tumor collection. Final tumors were weighed.
2.2. Cell Culture and Reagents
SUM102PT cells (BioIVT, NY, USA) were cultured in Ham’s F12 media with 5% FBS, 0.1% BSA, 5 µg/mL insulin, 1 µg/mL of hydrocortisone, 5 mM ethanolamine, 10 mM HEPES, 5 µg/mL apo-transferrin, 10 nM of triiodothyronine, 50 nM sodium selenite, and 10 ng/mL EGF. MDA-MB-468 cells (ATCC) were cultured in a DMEM medium containing 10% FBS and 1% antibiotic and antimycotic cocktail (Millipore, MO, USA). The cells were maintained at 37 °C in 95% air and 5% CO2 for all of the experiments.
2.3. Annexin V-FITC/7-AAD Double-Staining Assay
Cells were seeded in 12-well plates and incubated overnight for attachment, then the media was replaced with new media for the drug treatment. After the treatment, all of the floating and adherent cells were collected by trypsinization and centrifugation, followed by washing two times in phosphate-buffered saline (1 × PBS) before staining with Annexin V-FITC and 7-AAD, according to the manufacturer’s protocol (Cell Signaling Technology). Briefly, cells were resuspended in 500 µL 1× binding buffer containing 5 µL Annexin V-fluorescein isothiocyanate (FITC) and 10 µL 7-AAD in the dark for 15 min. For 7-ADD compensation, cells were incubated in a 70 °C water bath for 30 min. Then, we mixed live and dead cells, and thereafter, stained them with 7-ADD for compensation [26]. We used unstained cells as a negative control. For Annexin V compensation, we treated MDA-MB-468 cells with 0.5 and 5 µM doxorubicin for 24 h. Then, cells were stained with Annexin V [27]. The stained cells were analyzed with a flow cytometer, and the data were analyzed using FlowJo cell software (FlowJo, OR, USA) to measure apoptotic cells, live cells, and dead cells. Early apoptosis was designated as annexin V positive/7-AAD negative and late apoptosis was designated as annexin V positive/7-AAD positive. Necrosis was defined as annexin V negative/7-AAD positive.
2.4. MTT Assay
The MTT assay was performed as previously described [28]. Briefly, cells were seeded in a 96-well dish with 200 μL media and incubated in a 37 °C and 5% CO2 incubator. After 24 h, various concentrations of DOX/DXL or control vehicle were added. Following the treatment for 48 h, cell viability was assessed by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide; Sigma-Aldrich, St. Louis, MO, USA) assay, according to the manufacturer’s protocol. Viable fraction is expressed as the percentage of vehicle-treated control cells.
2.5. Western Blotting
Western blot (WB) analyses were performed as previously described [28]. Total proteins were separated by electrophoresis of a specified amount of whole cell lysate through 8–10% SDS-polyacrylamide gels, followed by transferring proteins to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with a 5% non-fat milk solution and incubated with primary antibodies for caspase-3, ERK, and p-ERK (Cell Signaling Biotechnology, Danvers, MA, USA), DSTYK (Santa Cruz Biotechnology, Dallas, TX, USA), GAPDH and Hsp90 (Santa Cruz Biotechnology). Secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA) conjugated to horseradish peroxidase and ECL (Bio-rad, Hercules, CA, USA) were used to detect signals, which were visualized by chemiluminescence. At least three independent experiments were performed for each WB assay.
2.6. Real Time-Quantitative PCR (RT-qPCR)
Total RNA was extracted using the RNeasy®Mini Kit (Qiagen, Germantown, MD, USA) and cDNA was synthesized from 1 μg of total RNA using High-Capacity cDNA Reverse Transcription Kits (Bio-Rad) plus SYB® Gene Expression Assay Mix, sterile water, and Fast Universal PCR Master Mix (Applied Biosystems, Waltham, MA, USA) to measure the expression of specific genes. Gene expression levels were assessed by real-time RT-qPCR using the Taqman® fast advanced master mix (Thermo Scientific, Waltham, MA, USA) and the CFX96 RT-PCR Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA). The PCR primer sequences used are: DSTYK forward primer: GAAGAGAAGTACCTCCAGC; DSTYK reverse primer: CAAGAAATCATTCACCAAGT. β-actin forward primer: CACCATTGGCAATGAGCGGTTC; β-actin reserve primer: AGGTCTTTGCGGATGTCCACGT. Gene expression was calculated and presented as fold changes.
2.7. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End-Labeling (TUNEL) Assay
The TUNEL assay was performed as described previously [20], following the supplier’s protocol, ABP Biosciences (catalog number: A050). Briefly, cells were cultured on coverslips in 24-well plates and fixed in 4% paraformaldehyde in PBS. The TdT reaction was performed for 1 h at 37 °C in a humidified chamber. Finally, the cells were stained with 4, 6-diamidino-2-phenylindole (DAPI) for 5 min. The fluorescence signal was examined and photos were taken with a Leica fluorescence microscope.
2.8. DSTYK Knockout (DSTYKKO) via Crispr/Cas9
Cells were transfected with DSTYK Crispr/Cas9 Knockout (KO) Plasmid (Santa Cruz, sc-404521), and HDR plasmids (Santa Cruz, sc-404521-HDR) using lipofectamine 3000 (Thermo Fisher Scientific), according to the manufacturer’s instructions. 48 h after transfection, cells were cultured in puromycin-containing media for the selection and cloning of DSTYK knockout (DSTYKKO) cells. Successful DSTYKKO cells were confirmed by WB analysis.
2.9. Immunohistochemistry
Tumors derived from parent cells and resistant cells were collected following our previous immunohistochemistry protocol [20]. Paraffin-embedded tissue sections (5 µm thick) were incubated with an anti-DSTYK antibody overnight at 4 °C. The signals were detected using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA), following the manufacturer’s protocol. Hematoxylin was used for counterstaining. The signal intensity was scored using the following scale: 0 (negative), 1 (weak), 2 (moderate), 3 (strong), 4 (middle strong), 5 (very strong). The staining was considered to be positive if the sum of distribution and intensity scores was greater than 1.
2.10. Statistical Analysis
Data was analyzed using Prism 7 software and is expressed as the mean ± S.D. Comparisons among multiple groups were made using a one-way ANOVA at a 95% confidence level (Turkey’s honest significance test). Comparisons between two groups were made using a parametric paired t-test for normally distributed data or non-parametric Wilcoxon paired t-test for non-normal distributions. Mice and sample groups were n > 3, unless otherwise indicated. Data was analyzed using ANOVA or the unpaired student t-test, and differences were considered significant at p-values of <0.05 (*), <0.01 (**), or <0.001 (***). Survival curves were generated using GraphPad Prism software.
3. Results
3.1. The Expression of DSTYK Is Related to the Survival Probability of TNBC Patients
It has been reported that DSTYK plays a predominant role in suppressing caspase-dependent apoptosis in some dermal cell types exposed to ultraviolet (UV) light [25], and we previously observed that DSTYK promotes metastasis and chemoresistance in colorectal cancer cells [20]. However, the role of DSTYK in the chemoresistance of TNBC cells has yet to be reported. Statistical analysis of the patient dataset indicates that a high DSTYK level is correlated with a high probability of death in breast cancer patients (Figure 1A, The Human Protein Atlas), TNBC patients (Figure 1B, Kaplan-Meier Plotter), and TNBC patients treated with chemotherapy only (Figure 1C, Kaplan-Meier Plotter). Furthermore, the image derived from NDEx v2.5.2 based on all of the available online databases provides more evidence for us to study DSTYK-related signaling pathways. DSTYK has been predicted to be involved in regulating apoptosis and cell death [25]. It may also participate in determining the TNBC cell fate by interacting with CDK13 [29], IKBKE [30], and/or TRIM25 [31], etc., (Supplemental Figure S1). These statistical data suggest that DSTYK may play a key role in chemoresistance in TNBC.
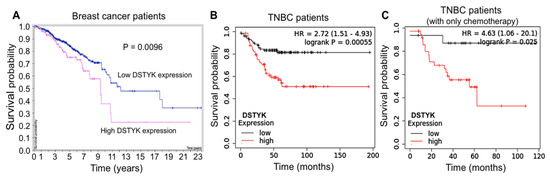
Figure 1.
Statistical analysis of DSTYK-related survival probability in breast cancer patients. (A) Survival curves of breast cancer patients with differential DSTYK expression published in The Human Protein Atlas (HPA). Patients include all four cancer stages and those stages are not available (n/a). The survival probabilities were generated and are publicly available at website (www.proteinatlas.org, accessed on 14 December 2021). (B) Survival curves of TNBC patients and (C) TNBC patients only treated with chemotherapy from the Kaplan-Meier Plotter dataset statistical analysis (https://kmplot.com/analysis/index.php?p=service&cancer=breast, accessed on 14 December 2021).
3.2. Establishment of Chemoresistant Cell Lines
SUM102PT and MDA-MB-468 cells are two typical TNBC cell lines. The SUM102PT cell line was derived from a patient who had ductal carcinoma in situ (DCIS) with microinvasion and is considered as the only breast cancer cell line derived from this early stage TNBC [32]. MDA-MB-468 cells were extracted from a pleural effusion of the mammary gland and breast tissues, and it is commonly used to study TNBC metastasis, migration, and proliferation. These two cell lines were treated with DOX/DXL combination starting with a low concentration (Figure 2A). Dead cells were washed out, and then the surviving cells were collected and cultured in the complete culture medium absence of any drug. Thereafter, these cells were treated again with DOX/DXL at a higher concentration. The same selection was repeated for 5–6 months. Eventually, we acquired resistant SUM102PTR and MDA-MB-468R cells that survived in the presence of 10 µM DOX and 100 nM DXL (Figure 2A). The higher drug concentrations did not significantly induce cell death. We confirmed that the combination of DOX and DXL failed to induce significant cells death among the drug-resistant cell population using the MTT cell survival assay (Figure 2B,C) and the TUNEL assay that stains apoptotic cells (Figure 2D). Moreover, flow cytometry analyses were performed to detect cell surface apoptotic marker annexin V and the loss of plasma membrane integrity (uptake of 7-ADD) [28]. Our results showed that after the DOX/DXL treatment, there were no significant increases in annexin-V and 7-AAD signals in chemoresistant cells, whereas the majority of parental cells showed strong annexin V and 7AAD signals indicative of significant cell death (Figure 2E,F). These results indicate that SUM102PTR and MDA-MB-468R cell lines are resistant to the chemotherapeutic drug treatment and are optimum cell models for studying chemoresistance in TNBC cells.
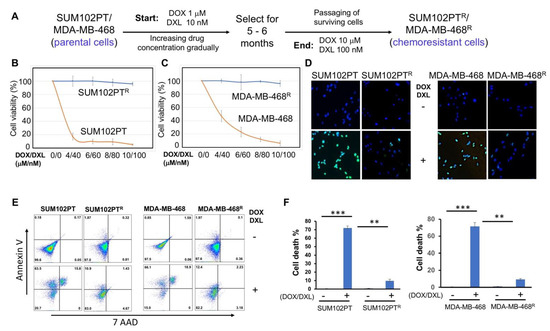
Figure 2.
Schematic diagram of the procedure for generating chemoresistant TNBC cells. (A) SUM102PT and MDA-MB-468 cells were exposed to DOX/DXL. The concentration of drugs was gradually increased (DOX/DXL:1 µM/10 nM, 2 µM/20 nM, 4 µM/40 nM, 6 µM/60 nM, 8 µM/80 nM, 10 µM/100 nM) until stable proliferation at 10 µM/100 nM was established. (B) MTT assays to compare chemoresistance between SUM102PT and SUM102PTR cells, and (C) between MDA-MB-468 and MDA-MB-468R cells. The data presented represent the mean values ± SD (n = 3). (D) TUNEL apoptosis assays comparing the chemoresistance between SUM102PT and SUM102PTR, and between MDA-MB-468 and MDA-MB-468R cells. (E) Representative images from flow cytometry analyses to compare the levels of cell death by staining with cell surface apoptotic marker annexin V and the necrotic marker 7-ADD between parental cells and resistant SUM102PT cells treated with or without DOX/DXL (2 µM/20 nM) or MDA-MB-468 cells treated with or without DOX/DXL (4 µM/40 nM). (F) The quantification of the results from (E). The data represent mean values ± SD; ** p < 0.01; *** p < 0.001.
3.3. DSTYK Expression Is Upregulated in Chemoresistant Cells
Furthermore, since previous findings that DSTYK is involved in cell apoptosis and chemoresistance in colorectal cancer cells [20], we planned to determine whether DSTYK also plays a role in the chemoresistance in SUM102PTR and MDA-MB-468R cells. First, we performed RT-PCR and qPCR analyses and the results indicate that the mRNA expression of DSTYK is considerably higher in chemoresistant cells than in parental cells for both SUM102PT and MDA-MB-468 cell lines (Figure 3A,B).
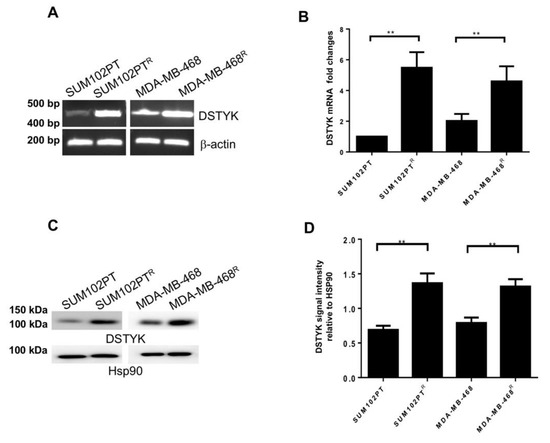
Figure 3.
DSTYK expression correlates with chemoresistance. (A) The mRNA level of DSTYK between parental and chemoresistant cells by reverse-transcription PCR (RT-PCR) and (B) qPCR. (C,D) WB and quantification to detect the expressions of DSTYK in SUM102PT and SUM102PTR cells, and MDA-MB-468 and MDA-MB-468R cells, respectively. Hsp90 was used as the loading control. Experiments were repeated three times; ** p < 0.01; one-way ANOVA.
To compare the protein levels of DSTYK between SUM102PT and SUM102PTR cells, and between MDA-MB-468 and MDA-MB-468R cells, we performed immunoblotting assays. Our results demonstrate that DSTYK protein levels in chemoresistant cells are significantly higher than in parental cells (Figure 3C,D). Together, these results indicate that DSTYK is significantly upregulated in chemoresistant cells and suggest that DSTYK may play a role in chemoresistance in SUM102PTR and MDA-MB-468R cells.
3.4. DSTYKKO Attenuates Chemoresistance in Chemoresistant Cells
To test whether DSTYK is essential for chemoresistance, we not only employed the Crispr/Cas9 technique to stably knock out DSTYK (DSTYKKO) expression in both SUM102PTR and MDA-MB-468R cells, but also restored DSTYK expression in KO (DSTYKKO-RE) cells. Then, we performed MTT assays to compare the chemotherapeutic response between control, DSTYKKO, and DSTYKKO-RE chemoresistant cells (Figure 4A). The results reveal that DSTYKKO chemoresistant cells showed dramatic cell death after the drug treatment, but the cell death was rescued in DSTYKKO-RE chemoresistant cells. Furthermore, WB analyses indicate that the apoptosis marker cleaved-caspase 3 is significantly induced by the DOX/DXL combination treatment in the DSTYKKO chemoresistant cells, whereas in control chemoresistant cells, cleaved-caspase 3 is barely induced. We did not observe any significant difference in the basal level of cleaved-caspase 3 between control chemoresistant and DSTYKKO chemoresistant cells, which suggests that DSTYKKO per se does not lower the viability of cells (Figure 4B–F). Clonogenic assays confirmed that much more apoptotic events occur in DSTYKKO cells upon DOX/DXL treatment than in control cells (Figure 4G). Similarly, we knocked out DSTYK expression via Crispr/Cas9 in parental SUM102PT and MDA-MB-468 cells, followed by MTT assays to determine whether DSTYKKO will cause more chemosensitivity. The results clearly demonstrate that more cell death is induced by DOX/DXL treatment in parental DSTYKKO cells than in parental control cells in both SUM102PT and MDA-MB-468 cells (Figure 4H,I). In addition, DSTYKKO does not lower the growth rate of cells before the drug treatment (Supplementary Figure S3), which further supports that DSTYK provides protection against chemotherapeutic drug-induced cell death. Moreover, there is no significant differences for cell death between DSTYK KO-RE and control parental cells (Figure 4H,I). Furthermore, we measured the phosphorylated ERK in control, DSTYKKO, and DSTYKRE-KO cells. We found that DSTYK upregulates ERK phosphorylation, as shown in Supplemental Figure S2. All of these data indicate that DSTYK plays a key role in chemoresistance through inhibiting chemotherapeutic drug-induced cell death.
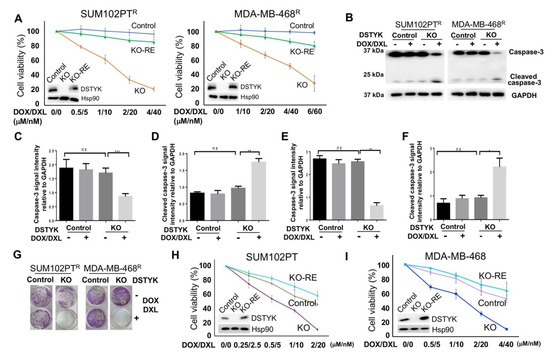
Figure 4.
DSTYK is essential to the chemoresistance in TNBC cells. (A) MTT assays to compare the chemoresistance treated with different concentrations of DOX/DXL. The data presented represents the mean values ± SD (n = 3). The levels of cellular DSTYK protein are indicated by the included WB. Hsp90 was used as the loading control. (B) WBs to detect the apoptosis marker cleaved-caspase 3 before and after DOX/DXL treatment (0.5/5 μM/nM for SUM102PTR cells; 1/10 μM/nM for MDA-MB-468R cells). GAPDH was used as the loading control. (C–F) Quantification analyses of (B). (G) Clonogenic assays to confirm that chemoresistant cells regain chemosensitivity after DSTYKKO. DOX/DXL 4 μM/40 nm for SUM102PTR cells and 6 μM/60 nm for MDA-MB-468R cells. (H,I) MTT assays to compare the chemoresistance among parental control cells, parental DSTYKKO cells, and parental DSTYKKO-RE cells. Experiments were repeated three times; ns, not significant; * p < 0.05; **: p < 0.01; *** p < 0.001, one-way ANOVA.
3.5. DSTYK Plays a Key Role in Chemoresistance through Inhibiting Apoptosis
Flow cytometry analysis was performed to detect cell surface apoptotic marker annexin V and 7-ADD [28]. Our results show that after DOX/DXL treatment, there were few annexin V-positive control chemoresistant cells, whereas the majority of DSTYKKO chemoresistant cells showed strong annexin V signals indicative of apoptosis (Figure 5A, B). To confirm, DSTYK plays a key role in chemoresistance by inhibiting apoptosis, we also performed flow cytometry analysis in DSTYKKO-RE chemoresistant cells, our results showed that after DOX/DXL treatment, there were few annexin V-positive DSTYKKO-RE chemoresistant cells that confirms restoring DSTYK expression rescued DSTYKKO-induced apoptosis after DOX/DXL treatment in chemoresistant cells (Supplemental Figure S4). Furthermore, in the DSTYKKO chemoresistant cells, cells proliferations are not downregulated by DSTYKKO per se (Figure 5C,D). These results indicate that DSTYKKO sensitizes these cells to chemotherapeutic drug treatment, suggesting that DSTYK plays a key role in chemoresistance by inhibiting the apoptosis signaling pathway in TNBC.
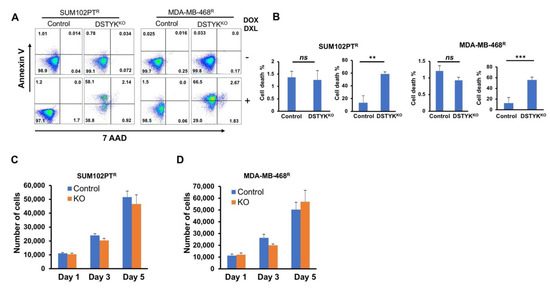
Figure 5.
DSTYKKO sensitizes chemoresistant cells to chemotherapy drug treatment. (A) Representative images from flow cytometry analyses to compare the levels of cell surface apoptotic marker annexin V and the necrotic marker 7-ADD between control and DSTYKKO groups in SUM102PTR cells or MDA-MB-468R cells with or without DOX/DXL. DOX/DXL 2 μM/20 nm for SUM102PTR cells and 4 μM/40 nm for MDA-MB-468R cells. (B) The data in (A) are quantified. For each cell line, the left panel is the result for non-drug treated and the right panel is the result for drug-treated. The data represents mean values ± SD; ns, not significant; ** p < 0.01; *** p < 0.001. (C,D) DSTYKKO resistant cell growth for 5 days and total numbers of cells were counted on days 1, 3, and 5 (n = 3 independent experiments) in SUM102PTR cells and MDA-MB-468R cells.
3.6. Knockout of DSTYK Facilitates Tumor Regression during Drug Treatment in an In Vivo Orthotopic Mouse Model
We employed an orthotopic mouse model to provide in vivo evidence that DSTYKKO can attenuate chemoresistance. Approximately 2 × 105 SUM102PTR control or DSTYKKO cells were subcutaneously implanted into the 4th mammary fat pads of 7-week-old immunodeficient NOD/SCID FVB mice. In each mouse, the control cells were implanted into the right 4th mammary fat pad and the DSTYKKO cells were implanted into the left 4th mammary fat pad. After 3–4 weeks, seven mice carrying two tumors with similar sizes on each side were selected. A combination of DOX (4–5 mg/kg)/DXL (1–2 mg/kg) was administrated intraperitoneally (i.p.) into the mice 2 times/week for 3–4 weeks. After DOX/DXL treatment, the tumors developed from DSTYKKO cells regressed significantly when compared to those from control resistant cells (Figure 6A–C). In confirmation of these data, similar results were observed in the same experiments performed with MDA-MB-468R cells (Figure 6D–F). Moreover, we also checked the tumor growth curve in control and DSTYKKO parental cells with DOX/DXL treatment. We found that control tumors are more resistant to DOX/DXL treatment when compared with DSTYKKO tumors (Supplemental Figure S5). IHC staining showed that DSTYK expression is significantly higher in chemoresistant tumors when compared with parental tumors, as shown in Supplemental Figure S6. These results indicate that DSTYK can attenuate the chemosensitivity of tumor cells by inhibiting chemotherapeutic drug-induced tumor cell death. Taken together, all of the above results support that DSTYK plays a pivotal role in the chemoresistance of TNBC cells, and targeting DSTYK in TNBC chemotherapy may significantly improve the treatment efficacy.
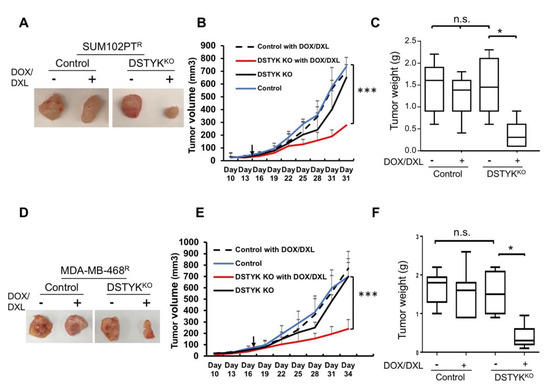
Figure 6.
DSTYK promotes chemoresistance in vivo. (A) Representative images of one set of tumors derived from control and DSTYKKO SUM102PTR cells. (B) The graph represents the tumor growth volume observed for the different groups (as labeled) derived from SUM102PTR cells. The data represents mean values ± SD. (C) Quantification of tumors after excision and being weighed at the end point; n = 7; n.s., not significant; * p < 0.05; *** p < 0.001, one-way ANOVA. (D) Representative images of one set of tumors derived from control and DSTYKKO MDA-MB-468R cells. (E) The graph represents the tumor growth volume observed for the different groups derived from MDA-MB-468R cells (as labeled) until reaching the endpoint. The data represents mean values ± SD. (F) Quantification of tumors after excision and weighing at the end point; n = 7; n.s., not significant; * p < 0.05; *** p < 0.001, one-way ANOVA.
4. Discussion
TNBC is a very deadly type of breast cancer and, presently, there is no ideal target available for effective therapy [33]. Chemotherapy remains the primary choice for most TNBC patients [9,34]. Successful chemotherapeutic treatments can eliminate cancer by inducing cancer cells to undergo apoptotic cell death [35], and, ideally, apoptosis is induced by chemotherapeutic agents in all TNBC cells [36,37,38]. Unfortunately, most TNBC patients will eventually develop resistance to chemotherapy, evading drug-induced cell death (deregulated apoptosis) and allowing the tumors in most TNBC patients to relapse and advance into metastasis, which is the reason for more than 90% of TNBC patients death [39]. Therefore, targeting deregulated apoptosis is an attractive approach to TNBC therapy [40]. As such, it is crucial to find a target for overcoming TNBC chemoresistance.
To identify the potential target that is responsible for TNBC chemoresistance, we employed two typical TNBC cell lines, SUM102PT and MDA-MB-468 cells. We further mimicked the clinical treatment process and eventually selected their chemoresistant cell clones, SUM102PTR and MDA-MB-468R cells. Based on our previous research in colorectal cancer cells [20], we found that DSTYK is significantly upregulated in chemoresistant TNBC cells when compared to their corresponding parental cells. Moreover, we found that DSTYK knockout (DSTYKKO) significantly attenuates the chemoresistance and renders chemoresistant cells regain sensitivity to chemotherapeutic drug treatment.
Although DSTYK is expressed in various tissues [41], its biological function is still elusive. It has been reported that DSTYK plays a predominant role in regulating both caspase-dependent and -independent apoptosis [42], and the underlying mechanisms are still under investigation. DSTYK was reported to promote ERK activation, and ERK inhibition has been proposed to improve cancer therapy. However, ERK inhibition did not significantly improve the TNBC treatment [43]. We speculate that shifting from ERK inhibitors to DSTYK inhibitors may hold greater promise in improving the TNBC treatment, since DSTYK may regulate the functions of many downstream factors in addition to ERK. We observed that DSTYK promotes metastasis and chemoresistance in colorectal cancer cells [20]. Here, we further found that DSTYK also plays a pivotal role in inhibiting chemotherapeutic agent-induced cell death in TNBC cells with both cellular and orthotopic models. Our studies indicate that DSTYK is a novel target to attenuate chemoresistance due to its inhibitory effect on apoptosis signaling in TNBC cells. DSTYK inactivation will affect the functions of many targets that may also participate in regulating the systematic apoptotic or survival processes. Therefore, inhibiting DSTYK activity will have a greater effect on TNBC treatment than inhibiting ERK activity, which will significantly impact on TNBC treatment regimen. Moreover, the large-scale statistical analyses of patient data support that a high DSTYK level is correlated with a high probability of death in breast cancer patients, TNBC patients, and TNBC patients treated with chemotherapy only.
It has been reported that the B-RafV600E inhibitor dabrafenib selectively inhibits the activity of receptor-interacting protein kinase 3 over receptor-interacting protein kinase 1, receptor-interacting protein kinase 2, and DSTYK. However, the inhibitory effect of dabrafenib on DSTYK was not clearly described [44]. To date, no specific inhibitors directly targeting DSTYK have been reported due to its novel role in the chemoresistance of breast cancer. We cannot exclude other kinase inhibitors, such as CDKs inhibitor purvalanol B, staurosporine, K-252a, and SB218078 (CHK1 inhibitor), which are published in “guidetopharmacology.org”, may affect DSTYK kinase activity, such as to execute indirectly more or less an inhibitory effect on DSTYK, but their biological functions are complicated since they target different kinases. Therefore, it is urgent to design a novel inhibitor specifically targeting DSTYK. To identify the mechanism of DSTYK in TNBC, we also predicted DSTYK signaling pathways in cancers using NDEx v2.5.2, the Network Data Exchange (NDEx) online data commons (www.ndexbio.org, accessed on 14 December 2021). This provides the direction for us to study DSTYK in clinical research. Since the next generation sequencing (NGS) is frequently employed in medical research, it may be necessary to perform more detailed expression profiling of primary TNBC specimens and their corresponding chemoresistant recurred specimens to identify more potential druggable targets including DSTYK, in order to achieve the success in improving the TNBC treatment.
5. Conclusions
Overall, our findings will significantly advance the medical strategy to the current dilemma of TNBC chemotherapy [45,46]. Our discovery will present DSTYK as a promising druggable target to facilitate the elimination of chemoresistant TNBC cells within TNBC.
Supplementary Materials
The following figures are available online at https://www.mdpi.com/article/10.3390/cells11010097/s1, Figure S1: The predicted DSTYK-involved signaling pathway; Figure S2: DSTYK restoration rescues the chemoresistant phenotype; Figure S3: Cell growth rate comparison between control parental cells and DSTYKKO parental cells; Figure S4: DSTYKKO-RE rescues cell death of chemoresistant cells during chemotherapeutic drug treatment; Figure S5: The in vivo tumor growth and drug sensitivity of TNBC are dependent on DSTYK expression; Figure S6: DSTYK plays a key role in chemoresistant TNBC.
Author Contributions
S.C.O., S.R. and J.W. performed experiments. J.Z. and Y.J. designed, wrote and revised the manuscript with comments and suggestions of Z.Q.Y. and P.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by ETSU startup funding.
Institutional Review Board Statement
All of the animal experimental procedures are consistent with our animal protocols that were approved by the Institutional Animal Care and Use Committee at East Tennessee State University (ETSU).
Informed Consent Statement
The informed consent in this study was applied from all subjects involved.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, 108800. [Google Scholar] [CrossRef]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A.B. Multi-Targeted Agents in Cancer Cell Chemosensitization: What We Learnt from Curcumin Thus Far. Recent Pat. Anticancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef]
- da Silva, J.L.; Cardoso Nunes, N.C.; Izetti, P.; de Mesquita, G.G.; de Melo, A.C. Triple negative breast cancer: A thorough review of biomarkers. Crit. Rev. Oncol. Hematol. 2020, 145, 102855. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Omarini, C.; Guaitoli, G.; Pipitone, S.; Moscetti, L.; Cortesi, L.; Cascinu, S.; Piacentini, F. Neoadjuvant treatments in triple-negative breast cancer patients: Where we are now and where we are going. Cancer Manag. Res. 2018, 10, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Narayan, V.; Vaughn, D. Pharmacokinetic and toxicity considerations in the use of neoadjuvant chemotherapy for bladder cancer. Expert Opin. Drug Metab. Toxicol. 2015, 11, 731–742. [Google Scholar] [CrossRef]
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer (Dove Med. Press) 2016, 8, 93–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedeljkovic, M.; Damjanovic, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Strietz, J.; Bleilevens, A.; Stickeler, E.; Maurer, J. Chemotherapeutic Stress Influences Epithelial-Mesenchymal Transition and Stemness in Cancer Stem Cells of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2020, 21, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [Green Version]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Maugeri-Sacca, M.; Vigneri, P.; De Maria, R. Cancer stem cells and chemosensitivity. Clin. Cancer Res. 2011, 17, 4942–4947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Shimono, Y.; Zabala, M.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, A.; Yan, Y.; Gerson, S.L. Concise Reviews: Cancer Stem Cell Targeted Therapies: Toward Clinical Success. Stem Cells Transl. Med. 2019, 8, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Nunes, T.; Hamdan, D.; Leboeuf, C.; El Bouchtaoui, M.; Gapihan, G.; Nguyen, T.T.; Meles, S.; Angeli, E.; Ratajczak, P.; Lu, H.; et al. Targeting Cancer Stem Cells to Overcome Chemoresistance. Int. J. Mol. Sci. 2018, 19, 4036. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.L.; Kuo, Y.C.; Ho, Y.S.; Huang, Y.H. Triple-Negative Breast Cancer: Current Understanding and Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers 2019, 11, 1334. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Yang, Y.; Shang, L.; Jun, F.; Liu, Q. A DSTYK mutation activates ERK1/2 signaling to promote intraspinal dissemination in a case of solitary fibrous tumor/hemangiopericytoma. Lab. Investig. 2019, 99, 1501–1514. [Google Scholar] [CrossRef]
- Zhang, J.; Miller, Z.; Musich, P.R.; Thomas, A.E.; Yao, Z.Q.; Xie, Q.; Howe, P.H.; Jiang, Y. DSTYK Promotes Metastasis and Chemoresistance via EMT in Colorectal Cancer. Front. Pharmacol. 2020, 11, 1250. [Google Scholar] [CrossRef]
- Baltali, E.; Ozisik, Y.; Guler, N.; Firat, D.; Altundag, K. Combination of docetaxel and doxorubicin as first-line chemotherapy in metastatic breast cancer. Tumori 2001, 87, 18–19. [Google Scholar] [CrossRef]
- Evans, T.R.; Yellowlees, A.; Foster, E.; Earl, H.; Cameron, D.A.; Hutcheon, A.W.; Coleman, R.E.; Perren, T.; Gallagher, C.J.; Quigley, M.; et al. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: An anglo-celtic cooperative oncology group study. J. Clin. Oncol. 2005, 23, 2988–2995. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Raab, G.; Caputo, A.; Schutte, M.; Hilfrich, J.; Blohmer, J.U.; Gerber, B.; Costa, S.D.; Merkle, E.; Eidtmann, H.; et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: The GEPARDUO study of the German Breast Group. J. Clin. Oncol. 2005, 23, 2676–2685. [Google Scholar] [CrossRef]
- Wali, V.B.; Langdon, C.G.; Held, M.A.; Platt, J.T.; Patwardhan, G.A.; Safonov, A.; Aktas, B.; Pusztai, L.; Stern, D.F.; Hatzis, C. Systematic Drug Screening Identifies Tractable Targeted Combination Therapies in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.W.; Hsu, C.K.; Michael, M.; Nanda, A.; Liu, L.; McMillan, J.R.; Pourreyron, C.; Takeichi, T.; Tolar, J.; Reid, E.; et al. Large Intragenic Deletion in DSTYK Underlies Autosomal-Recessive Complicated Spastic Paraparesis, SPG23. Am. J. Hum. Genet. 2017, 100, 364–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 50, e2597. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, D.; Shen, P.; Yu, Y.; Yan, Y.; You, W. Hsa_circ_0092276 promotes doxorubicin resistance in breast cancer cells by regulating autophagy via miR-348/ATG7 axis. Transl. Oncol. 2021, 14, 101045. [Google Scholar] [CrossRef]
- Jiang, Y.; Woosley, A.N.; Sivalingam, N.; Natarajan, S.; Howe, P.H. Cathepsin-B-mediated cleavage of Disabled-2 regulates TGF-beta-induced autophagy. Nat. Cell Biol. 2016, 18, 851–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quereda, V.; Bayle, S.; Vena, F.; Frydman, S.M.; Monastyrskyi, A.; Roush, W.R.; Duckett, D.R. Therapeutic Targeting of CDK12/CDK13 in Triple-Negative Breast Cancer. Cancer Cell 2019, 36, 545–558.e547. [Google Scholar] [CrossRef] [PubMed]
- Barbie, T.U.; Alexe, G.; Aref, A.R.; Li, S.; Zhu, Z.; Zhang, X.; Imamura, Y.; Thai, T.C.; Huang, Y.; Bowden, M.; et al. Targeting an IKBKE cytokine network impairs triple-negative breast cancer growth. J. Clin. Investig. 2014, 124, 5411–5423. [Google Scholar] [CrossRef]
- Walsh, L.A.; Alvarez, M.J.; Sabio, E.Y.; Reyngold, M.; Makarov, V.; Mukherjee, S.; Lee, K.W.; Desrichard, A.; Turcan, S.; Dalin, M.G.; et al. An Integrated Systems Biology Approach Identifies TRIM25 as a Key Determinant of Breast Cancer Metastasis. Cell Rep. 2017, 20, 1623–1640. [Google Scholar] [CrossRef] [Green Version]
- Sartor, C.I.; Dziubinski, M.L.; Yu, C.L.; Jove, R.; Ethier, S.P. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997, 57, 978–987. [Google Scholar] [PubMed]
- Jiao, X.; Wang, M.; Zhang, Z.; Li, Z.; Ni, D.; Ashton, A.W.; Tang, H.Y.; Speicher, D.W.; Pestell, R.G. Leronlimab, a humanized monoclonal antibody to CCR5, blocks breast cancer cellular metastasis and enhances cell death induced by DNA damaging chemotherapy. Breast Cancer Res. 2021, 23, 11. [Google Scholar] [CrossRef]
- Hu, M.H.; Wu, T.Y.; Huang, Q.; Jin, G. New substituted quinoxalines inhibit triple-negative breast cancer by specifically downregulating the c-MYC transcription. Nucleic Acids Res. 2019, 47, 10529–10542. [Google Scholar] [CrossRef]
- Montinaro, A.; Areso Zubiaur, I.; Saggau, J.; Kretz, A.L.; Ferreira, R.M.M.; Hassan, O.; Kitzig, E.; Muller, I.; El-Bahrawy, M.A.; von Karstedt, S.; et al. Potent pro-apoptotic combination therapy is highly effective in a broad range of cancers. Cell Death Differ. 2021. [Google Scholar] [CrossRef]
- Dewangan, J.; Srivastava, S.; Mishra, S.; Pandey, P.K.; Divakar, A.; Rath, S.K. Chetomin induces apoptosis in human triple-negative breast cancer cells by promoting calcium overload and mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2018, 495, 1915–1921. [Google Scholar] [CrossRef]
- Shen, L.W.; Jiang, X.X.; Li, Z.Q.; Li, J.; Wang, M.; Jia, G.F.; Ding, X.; Lei, L.; Gong, Q.H.; Gao, N. Cepharanthine sensitizes human triple negative breast cancer cells to chemotherapeutic agent epirubicin via inducing cofilin oxidation-mediated mitochondrial fission and apoptosis. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, W.; Miyazaki, K.; Asano, Y.; Kubota, S.; Tanaka, N. Kruppel-Like Factor 4 and Its Activator APTO-253 Induce NOXA-Mediated, p53-Independent Apoptosis in Triple-Negative Breast Cancer Cells. Genes 2021, 12, 539. [Google Scholar] [CrossRef]
- Wu, Q.; Siddharth, S.; Sharma, D. Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs. Cancers 2021, 13, 3697. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, Y.; Zhang, R.; Wang, Z.; Xu, M.; Zhang, D.; Huang, J.; Luo, F.; Li, F.; Ni, Z.; et al. Dstyk mutation leads to congenital scoliosis-like vertebral malformations in zebrafish via dysregulated mTORC1/TFEB pathway. Nat. Commun. 2020, 11, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zha, J.; Zhou, Q.; Xu, L.G.; Chen, D.; Li, L.; Zhai, Z.; Shu, H.B. RIP5 is a RIP-homologous inducer of cell death. Biochem. Biophys. Res. Commun. 2004, 319, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Torres-Adorno, A.M.; Lee, J.; Kogawa, T.; Ordentlich, P.; Tripathy, D.; Lim, B.; Ueno, N.T. Histone Deacetylase Inhibitor Enhances the Efficacy of MEK Inhibitor through NOXA-Mediated MCL1 Degradation in Triple-Negative and Inflammatory Breast Cancer. Clin. Cancer Res. 2017, 23, 4780–4792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.X.; Feng, J.M.; Wang, Y.; Li, X.H.; Chen, X.X.; Su, Y.; Shen, Y.Y.; Chen, Y.; Xiong, B.; Yang, C.H.; et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis. 2014, 5, e1278. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, J.; Kraft, A.S. Bax-induced apoptotic cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 529–531. [Google Scholar] [CrossRef] [Green Version]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 2011, 1813, 521–531. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).