Channels and Transporters of the Pulmonary Lamellar Body in Health and Disease
Abstract
:1. Introduction
2. Ion Channels and Transporters on Lamellar Bodies
3. Physiological Role for Ion Channels and Transporters on Lamellar Bodies
4. Pathophysiology Linked to Ion Channels and Transporters on Lamellar Bodies
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Schmitz, G.; Muller, G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 1991, 32, 1539–1570. [Google Scholar] [CrossRef]
- Wertz, P. Epidermal Lamellar Granules. Skin Pharmacol. Physiol. 2018, 31, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Matoltsy, A.G.; Parakkal, P.F. Membrane-coating granules of keratinizing epithelia. J. Cell Biol. 1965, 24, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Campiche, M. Les inclusions lamellaires des cellules alvéolaires dans le poumon du Raton. J. Ultrastruct. Res. 1960, 3, 302–312. [Google Scholar] [CrossRef]
- Dietl, P.; Haller, T. Exocytosis of lung surfactant: From the secretory vesicle to the air-liquid interface. Annu. Rev. Physiol. 2005, 67, 595–621. [Google Scholar] [CrossRef]
- Raposo, G.; Marks, M.S.; Cutler, D.F. Lysosome-related organelles: Driving post-Golgi compartments into specialisation. Curr. Opin. Cell Biol. 2007, 19, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Weaver, T.E.; Na, C.L.; Stahlman, M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin. Cell Dev. Biol. 2002, 13, 263–270. [Google Scholar] [CrossRef]
- Castillo-Sánchez, J.C.; Cruz, A.; Pérez-Gil, J. Structural hallmarks of lung surfactant: Lipid-protein interactions, membrane structure and future challenges. Arch. Biochem. Biophys. 2021, 703, 108850. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Wimmer, B.H.; Winter, S.L.; Kolovou, A.; Laketa, V.; Chlanda, P. Post-correlation on-lamella cryo-CLEM reveals the membrane architecture of lamellar bodies. Commun. Biol. 2021, 4, 137. [Google Scholar] [CrossRef]
- Olmeda, B.; Martínez-Calle, M.; Pérez-Gil, J. Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann. Anat. 2017, 209, 78–92. [Google Scholar] [CrossRef]
- Mason, R.J.; Voelker, D.R. Regulatory mechanisms of surfactant secretion. Biochim. Biophys. Acta 1998, 1408, 226–240. [Google Scholar] [CrossRef] [Green Version]
- Frick, M.; Eschertzhuber, S.; Haller, T.; Mair, N.; Dietl, P. Secretion in alveolar type II cells at the interface of constitutive and regulated exocytosis. Am. J. Respir. Cell Mol. Biol. 2001, 25, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Chander, A.; Fisher, A.B. Regulation of lung surfactant secretion. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1990, 258, L241–L253. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Wert, S.E.; Weaver, T.E. Diseases of pulmonary surfactant homeostasis. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 371–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicary, G.W.; Vergne, Y.; Santiago-Cornier, A.; Young, L.R.; Roman, J. Pulmonary fibrosis in Hermansky-Pudlak syndrome. Ann. Am. Thorac. Soc. 2016, 13, 1839–1846. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Díaz, P.; Nakajima, E.; Sorkhdini, P.; Hernandez-Gutierrez, A.; Eberle, A.; Yang, D.; Zhou, Y. Hermansky-Pudlak Syndrome and Lung Disease: Pathogenesis and Therapeutics. Front. Pharmacol. 2021, 12, 644671. [Google Scholar] [CrossRef]
- Beers, M.F.; Mulugeta, S. The biology of the ABCA3 lipid transporter in lung health and disease. Cell Tissue Res. 2017, 367, 481–493. [Google Scholar] [CrossRef]
- Daniels, C.B.; Orgeig, S. Pulmonary surfactant: The key to the evolution of air breathing. News Physiol. Sci. 2003, 18, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rodriguez, E.; Pérez-Gil, J. Structure-function relationships in pulmonary surfactant membranes: From biophysics to therapy. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1568–1585. [Google Scholar] [CrossRef] [Green Version]
- Neergaard, K. Neue Auffassungen über einen Grundbegriff der Atemmechanik. Z. Gesamte Exp. Med. 1929, 66, 373–394. [Google Scholar] [CrossRef]
- Pattle, R.E. Surface Lining of Lung Alveoli. Physiol. Rev. 1965, 45, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.A. Lung surfactant: A personal perspective. Annu. Rev. Physiol. 1997, 59, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.; Avery, M.E. Time of appearance of lung surfactant in the foetal mouse. Nature 1962, 193, 688–689. [Google Scholar] [CrossRef]
- Avery, M.E.; Mead, J. Surface Properties in Relation to Atelectasis and Hyaline Membrane Disease. AMA. J. Dis. Child. 1959, 97, 517–523. [Google Scholar] [CrossRef]
- Avery, M.E.; Taeusch, H.; Floros, J. Surfactant replacement. N. Engl. J. Med. 1986, 315, 825–826. [Google Scholar] [CrossRef]
- Ryan, U.S.; Ryan, J.W.; Smith, D.S. Alveolar type II cells: Studies on the mode of release of lamellar bodies. Tissue Cell 1975, 7, 587–599. [Google Scholar] [CrossRef]
- Balis, J.U.; Conen, P.E. The role of alveolar inclusion bodies in the developing lung. Lab. Investig. 1964, 13, 1215–1229. [Google Scholar] [PubMed]
- Kikkawa, Y.; Smith, F. Cellular and biochemical aspects of pulmonary surfactant in health and disease. Lab. Investig. 1983, 49, 122–139. [Google Scholar]
- Veldhuizen, R.; Nag, K.; Orgeig, S.; Possmayer, F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta-Mol. Basis Dis. 1998, 1408, 90–108. [Google Scholar] [CrossRef]
- Askin, F.B.; Kuhn, C. The cellular origin of pulmonary surfactant. Lab. Investig. 1971, 25, 260–268. [Google Scholar]
- Veldhuizen, E.J.A.; Haagsman, H.P. Role of pulmonary surfactant components in surface film formation and dynamics. Biochim. Biophys. Acta-Biomembr. 2000, 1467, 255–270. [Google Scholar] [CrossRef] [Green Version]
- Schürch, S.; Green, F.H.Y.; Bachofen, H. Formation and structure of surface films: Captive bubble surfactometry. Biochim. Biophys. Act-Mol. Basis Dis. 1998, 1408, 180–202. [Google Scholar] [CrossRef] [Green Version]
- Mason, R.J.; Greene, K.; Voelker, D.R. Surfactant protein A and surfactant protein D in health and disease. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1998, 275, L1–L13. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Beirag, N.; Murugaiah, V.; Chou, Y.C.; Kuo, W.S.; Kao, H.F.; Madan, T.; Kishore, U.; Wang, J.Y. Human Surfactant Protein D Binds Spike Protein and Acts as an Entry Inhibitor of SARS-CoV-2 Pseudotyped Viral Particles. Front. Immunol. 2021, 12, 641360. [Google Scholar] [CrossRef]
- Watson, A.; Madsen, J.; Clark, H.W. SP-A and SP-D: Dual Functioning Immune Molecules with Antiviral and Immunomodulatory Properties. Front. Immunol. 2021, 11, 622598. [Google Scholar] [CrossRef]
- Osanai, K.; Mason, R.J.; Voelker, D.R. Trafficking of newly synthesized surfactant protein A in isolated rat alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.B.; Dodia, C.; Ruckert, P.; Tao, J.Q.; Bates, S.R. Pathway to lamellar bodies for surfactant protein A. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 299, L51–L58. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Ikegami, M.; Moon, C.; Naren, A.P.; Shannon, J.M. Lysophosphatidylcholine acyltransferase 1 (LPCAT1) specifically interacts with phospholipid transfer protein StarD10 to facilitate surfactant phospholipid trafficking in alveolar type II Cells. J. Biol. Chem. 2015, 290, 18559–18574. [Google Scholar] [CrossRef] [Green Version]
- Yamano, G.; Funahashi, H.; Kawanami, O.; Zhao, L.X.; Ban, N.; Uchida, Y.; Morohoshi, T.; Ogawa, J.; Shioda, S.; Inagaki, N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001, 508, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Mulugeta, S.; Gray, J.M.; Notarfrancesco, K.L.; Gonzales, L.W.; Koval, M.; Feinstein, S.I.; Ballard, P.L.; Fisher, A.B.; Shuman, H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J. Biol. Chem. 2002, 277, 22147–22155. [Google Scholar] [CrossRef] [Green Version]
- Miklavc, P.; Mair, N.; Wittekindt, O.H.; Haller, T.; Dietl, P.; Felder, E.; Timmler, M.; Frick, M. Fusion-activated Ca 2+ entry via vesicular P2X 4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 14503–14508. [Google Scholar] [CrossRef] [Green Version]
- Kook, S.; Wang, P.; Young, L.R.; Schwake, M.; Saftig, P.; Weng, X.; Meng, Y.; Neculai, D.; Marks, M.S.; Gonzales, L.; et al. Impaired lysosomal integral membrane protein 2-dependent peroxiredoxin 6 delivery to lamellar bodies accounts for altered alveolar phospholipid content in adaptor protein-3-deficient pearl mice. J. Biol. Chem. 2016, 291, 8414–8427. [Google Scholar] [CrossRef] [Green Version]
- Roszell, B.R.; Tao, J.Q.; Yu, K.J.; Huang, S.; Bates, S.R. Characterization of the Niemann-Pick C pathway in alveolar type II cells and lamellar bodies of the lung. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L919–L932. [Google Scholar] [CrossRef] [Green Version]
- Kook, S.; Wang, P.; Meng, S.; Jetter, C.S.; Sucre, J.M.S.; Benjamin, J.T.; Gokey, J.J.; Hanby, H.A.; Jaume, A.; Goetzl, L.; et al. AP-3-dependent targeting of flippase ATP8A1 to lamellar bodies suppresses activation of YAP in alveolar epithelial type 2 cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2025208118. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, S.J.; Spitzer, A.R.; Chander, A. Ionic regulation of proton chemical (pH) and electrical gradients in lung lamellar bodies. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1997, 273, L427–L436. [Google Scholar] [CrossRef] [PubMed]
- Chintagari, N.R.; Mishra, A.; Su, L.; Wang, Y.; Ayalew, S.; Hartson, S.D.; Liu, L. Vacuolar ATPase regulates surfactant secretion in rat alveolar type II cells by modulating lamellar body calcium. PLoS ONE 2010, 5, e9228. [Google Scholar] [CrossRef] [Green Version]
- Sun-Wada, G.H.; Murata, Y.; Namba, M.; Yamamoto, A.; Wada, Y.; Futai, M. Mouse Proton Pump ATPase C Subunit Isoforms (C2-a and C2-b) Specifically Expressed in Kidney and Lung. J. Biol. Chem. 2003, 278, 44843–44851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fois, G.; Winkelmann, V.E.; Bareis, L.; Staudenmaier, L.; Hecht, E.; Ziller, C.; Ehinger, K.; Schymeinsky, J.; Kranz, C.; Frick, M. ATP is stored in lamellar bodies to activate vesicular P2X4 in an autocrine fashion upon exocytosis. J. Gen. Physiol. 2018, 150, 277–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadsworth, S.J.; Chander, A. H+- and K+-dependence of Ca2+ uptake in lung lamellar bodies. J. Membr. Biol. 2000, 174, 41–51. [Google Scholar] [CrossRef]
- Chander, A.; Johnson, R.G.; Reicherter, J.; Fisher, B. Lung lamellar bodies maintain an acidic internal pH. J. Biol. Chem. 1986, 261, 6126–6131. [Google Scholar] [CrossRef]
- Ridsdale, R.; Lewis, D.F.; Weaver, T.E.; Akinbi, H.T. Proteomic analysis of lamellar bodies isolated from amniotic fluid: Implications for function. Am. J. Perinatol. 2012, 29, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chintagari, N.R.; Narayanaperumal, J.; Ayalew, S.; Hartson, S.; Liu, L. Proteomic analysis of lamellar bodies isolated from rat lungs. BMC Cell Biol. 2008, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridsdale, R.; Na, C.L.; Xu, Y.; Greis, K.D.; Weaver, T. Comparative proteomic analysis of lung lamellar bodies and lysosome-related organelles. PLoS ONE 2011, 6, e16482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller-Bals, S.; Bates, S.R.; Notarfrancesco, K.; Tao, J.Q.; Fisher, A.B.; Shuman, H. Surface-expressed lamellar body membrane is recycled to lamellar bodies. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L631–L640. [Google Scholar] [CrossRef]
- Fois, G.; Föhr, K.J.; Kling, C.; Fauler, M.; Wittekindt, O.H.; Dietl, P.; Frick, M. P2X4 receptor re-sensitization depends on a protonation/deprotonation cycle mediated by receptor internalization and recycling. J. Physiol. 2018, 596, 4893–4907. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Valencia, J.C.; Huang, H.; Chi, A.; Shabanowitz, J.; Hearing, V.J.; Appella, E.; Wu, C. Comparative bioinformatics analyses and profiling of lysosome-related organelle proteomes. Int. J. Mass Spectrom. 2007, 259, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Goerke, J. Pulmonary surfactant: Functions and molecular composition. Biochim. Biophys. Acta 1998, 1408, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Kos, V.; Ford, R.C. The ATP-binding cassette family: A structural perspective. Cell. Mol. Life Sci. 2009, 66, 3111–3126. [Google Scholar] [CrossRef]
- Tusnády, G.E.; Sarkadi, B.; Simon, I.; Váradi, A. Membrane topology of human ABC proteins. FEBS Lett. 2006, 580, 1017–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, N.; Matsumura, Y.; Sakai, H.; Takanezawa, Y.; Sasaki, M.; Arai, H.; Inagaki, N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J. Biol. Chem. 2007, 282, 9628–9634. [Google Scholar] [CrossRef] [Green Version]
- Cerrada, A.; Haller, T.; Cruz, A.; Pérez-Gil, J. Pneumocytes Assemble Lung Surfactant as Highly Packed/Dehydrated States with Optimal Surface Activity. Biophys. J. 2015, 109, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravasio, A.; Olmeda, B.; Bertocchi, C.; Haller, T.; Pérez-Gil, J. Lamellar bodies form solid three-dimensional films at the respiratory air-liquid interface. J. Biol. Chem. 2010, 285, 28174–28182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, T.; Dietl, P.; Stockner, H.; Frick, M.; Mair, N.; Tinhofer, I.; Ritsch, A.; Enhorning, G.; Putz, G. Tracing surfactant transformation from cellular release to insertion into an air-liquid interface. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 286, L1009–L1015. [Google Scholar] [CrossRef]
- Cheong, N.; Zhang, H.; Madesh, M.; Zhao, M.; Yu, K.; Dodia, C.; Fisher, A.B.; Savani, R.C.; Shuman, H. ABCA3 is critical for lamellar body biogenesis in vivo. J. Biol. Chem. 2007, 282, 23811–23817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, M.L.; Xavier, R.; Haley, K.J.; Welti, R.; Goss, J.L.; Brown, C.E.; Zhuang, D.Z.; Bell, S.A.; Lu, N.; Mckee, M.; et al. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J. Lipid Res. 2007, 48, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Sakai, H.; Sasaki, M.; Ban, N.; Inagaki, N. ABCA3-mediated choline-phospholipids uptake into intracellular vesicles in A549 cells. FEBS Lett. 2007, 581, 3139–3144. [Google Scholar] [CrossRef] [Green Version]
- Cheong, N.; Madesh, M.; Gonzales, L.W.; Zhao, M.; Yu, K.; Ballard, P.L.; Shuman, H. Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J. Biol. Chem. 2006, 281, 9791–9800. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, A.I.; Maxfield, F.R. Niemann-Pick type C disease: Molecular mechanisms and potential therapeutic approaches. J. Neurochem. 2011, 116, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Saha, P.; Lib, J.; Blobel, G.; Pfeffer, S.R. Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2. Proc. Natl. Acad. Sci. USA 2016, 113, 10079–10084. [Google Scholar] [CrossRef] [Green Version]
- Heybrock, S.; Kanerva, K.; Meng, Y.; Ing, C.; Liang, A.; Xiong, Z.J.; Weng, X.; Ah Kim, Y.; Collins, R.; Trimble, W.; et al. Lysosomal integral membrane protein-2 (LIMP-2/SCARB2) is involved in lysosomal cholesterol export. Nat. Commun. 2019, 10, 3521. [Google Scholar] [CrossRef]
- Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef] [Green Version]
- Manevich, Y.; Shuvaeva, T.; Dodia, C.; Kazi, A.; Feinstein, S.I.; Fisher, A.B. Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A2 activities. Arch. Biochem. Biophys. 2009, 485, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooney, S.A.; Young, S.L.; Mendelson, C.R. Molecular and cellular processing of lung surfactant. FASEB J. 1994, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Feinstein, S.I.; Ho, Y.S. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J. Lipid Res. 2005, 46, 1248–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.B.; Dodia, C.; Yu, K.; Manevich, Y.; Feinstein, S.I. Lung phospholipid metabolism in transgenic mice overexpressing peroxiredoxin 6. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2006, 1761, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.S.; Cheng, T.W.; Ysselstein, D.; Heybrock, S.; Hoth, L.R.; Chrunyk, B.A.; Am Ende, C.W.; Krainc, D.; Schwake, M.; Saftig, P.; et al. Lysosomal integral membrane protein-2 as a phospholipid receptor revealed by biophysical and cellular studies. Nat. Commun. 2017, 8, 1908. [Google Scholar] [CrossRef] [Green Version]
- Roszell, B.R.; Tao, J.Q.; Yu, K.J.; Gao, L.; Huang, S.; Ning, Y.; Feinstein, S.I.; Vite, C.H.; Bates, S.R. Pulmonary Abnormalities in Animal Models Due to Niemann-Pick Type C1 (NPC1) or C2 (NPC2) Disease. PLoS ONE 2013, 8, e67084. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-Terminal Domain of NPC1 Reveals Distinct Subdomains for Binding and Transfer of Cholesterol. Cell 2009, 137, 1213–1224. [Google Scholar] [CrossRef] [Green Version]
- Rituper, B.; Guček, A.; Lisjak, M.; Gorska, U.; Šakanović, A.; Bobnar, S.T.; Lasič, E.; Božić, M.; Abbineni, P.S.; Jorgačevski, J.; et al. Vesicle cholesterol controls exocytotic fusion pore. Cell Calcium 2021, 101, 102503. [Google Scholar] [CrossRef]
- Palmgren, M.; Østerberg, J.T.; Nintemann, S.J.; Poulsen, L.R.; López-Marqués, R.L. Evolution and a revised nomenclature of P4 ATPases, a eukaryotic family of lipid flippases. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 1135–1151. [Google Scholar] [CrossRef]
- Best, J.T.; Xu, P.; Graham, T.R. Phospholipid flippases in membrane remodeling and transport carrier biogenesis. Curr. Opin. Cell Biol. 2019, 59, 8–15. [Google Scholar] [CrossRef]
- Soupene, E. ATP8A1 activity and phosphatidylserine transbilayer movement. J. Receptor. Ligand Channel Res. 2008, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fairn, G.D.; Schieber, N.L.; Ariotti, N.; Murphy, S.; Kuerschner, L.; Webb, R.I.; Grinstein, S.; Parton, R.G. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J. Cell Biol. 2011, 194, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Ansari, I.U.H.; Longacre, M.J.; Paulusma, C.C.; Stoker, S.W.; Kendrick, M.A.; MacDonald, M.J. Characterization of P4 ATPase phospholipid translocases (flippases) in human and rat pancreatic beta cells: Their gene silencing inhibits insulin secretion. J. Biol. Chem. 2015, 290, 23110–23123. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, M.J.; Ade, L.; Ntambi, J.M.; Ansari, I.U.H.; Stoker, S.W. Characterization of phospholipids in insulin secretory granules and mitochondria in pancreatic beta cells and their changes with glucose stimulation. J. Biol. Chem. 2015, 290, 11075–11092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckenhoff, R.C.; Somlyo, A.P. Rat lung type II cell and lamellar body: Elemental composition in situ. Am. J. Physiol.-Cell Physiol. 1988, 254, C614-20. [Google Scholar] [CrossRef]
- Eckenhoff, R.G.; Rannels, S.R.; Fisher, A.B. Secretory granule calcium loss after isolation of rat alveolar type II cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1991, 260, L129–L135. [Google Scholar] [CrossRef]
- Malacrida, L.; Astrada, S.; Briva, A.; Bollati-Fogolín, M.; Gratton, E.; Bagatolli, L.A. Spectral phasor analysis of LAURDAN fluorescence in live A549 lung cells to study the hydration and time evolution of intracellular lamellar body-like structures. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2625–2635. [Google Scholar] [CrossRef]
- Beers, M.F. Inhibition of cellular processing of surfactant protein C by drugs affecting intracellular pH gradients. J. Biol. Chem. 1996, 271, 14361–14370. [Google Scholar] [CrossRef] [Green Version]
- Serrano, A.G.; Cabré, E.J.; Pérez-Gil, J. Identification of a segment in the precursor of pulmonary surfactant protein SP-B, potentially involved in pH-dependent membrane assembly of the protein. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Bañares-Hidalgo, A.; Pérez-Gil, J.; Estrada, P. Acidic pH triggers conformational changes at the NH2-terminal propeptide of the precursor of pulmonary surfactant protein B to form a coiled coil structure. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1738–1751. [Google Scholar] [CrossRef] [Green Version]
- Chander, A.; Sen, N.; Wu, A.M.; Higgins, S.; Wadsworth, S.; Spitzer, A.R. Methylamine decreases trafficking and packaging of newly synthesized phosphatidylcholine in lamellar bodies in alveolar type II cells. Biochem. J. 1996, 318, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.B.; Dodia, C. Lysosomal-type PLA2 and turnover of alveolar DPPC. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 280, L748-54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.B.; Dodia, C. Role of acidic Ca2+-independent phospholipase A2 in synthesis of lung dipalmitoyl phosphatidylcholine. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1997, 272, L238-43. [Google Scholar] [CrossRef]
- Rice, W.R.; Dorn, C.C.; Singleton, F.M. P2-purinoceptor regulation of surfactant phosphatidylcholine secretion. Relative roles of calcium and protein kinase C. Biochem. J. 1990, 266, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Haller, T.; Auktor, K.; Frick, M.; Mair, N.; Dietl, A.P. Threshold calcium levels for lamellar body exocytosis in type II pneumocytes. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L893–L900. [Google Scholar] [CrossRef]
- Fois, G.; Hobi, N.; Felder, E.; Ziegler, A.; Miklavc, P.; Walther, P.; Radermacher, P.; Haller, T.; Dietl, P. A new role for an old drug: Ambroxol triggers lysosomal exocytosis via pH-dependent Ca2+ release from acidic Ca2+ stores. Cell Calcium 2015, 58, 628–637. [Google Scholar] [CrossRef]
- Cao, Q.; Zhong, X.Z.; Zou, Y.; Murrell-Lagnado, R.; Zhu, M.X.; Dong, X.P. Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion. J. Cell Biol. 2015, 209, 879–894. [Google Scholar] [CrossRef] [Green Version]
- Cang, C.; Bekele, B.; Ren, D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 2014, 10, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Li, G.; Zhang, X.; Xu, H.; Abraham, S.N. A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell 2015, 161, 1306–1319. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Zou, Y.; Zhong, X.Z.; Cao, Q.; Zhao, K.; Zhu, M.X.; Murrell-Lagnado, R.; Dong, X.P. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J. Biol. Chem. 2014, 289, 17658–17667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rovere, R.M.L.; Roest, G.; Bultynck, G.; Parys, J.B. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 2016, 60, 74–87. [Google Scholar] [CrossRef]
- Neuland, K.; Sharma, N.; Frick, M. Synaptotagmin-7 links fusion-activated Ca2+ entry and fusion pore dilation. J. Cell Sci. 2014, 127, 5218–5227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklavc, P.; Albrecht, S.; Wittekindt, O.H.; Schullian, P.; Haller, T.; Dietl, P. Existence of exocytotic hemifusion intermediates with a lifetime of up to seconds in type II pneumocytes. Biochem. J. 2009, 424, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Dietl, P.; Haller, T.; Frick, M. Spatio-temporal aspects, pathways and actions of Ca2+ in surfactant secreting pulmonary alveolar type II pneumocytes. Cell Calcium 2012, 52, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, T.; Dietl, P.; Pfaller, K.; Frick, M.; Mair, N.; Paulmichl, M.; Hess, M.W.; Fürst, J.; Maly, K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 2001, 155, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.E.; Korbmacher, J.P.; Hecht, E.; Hobi, N.; Wittekindt, O.H.; Dietl, P.; Kranz, C.; Frick, M. Fusion-activated cation entry (FACE) via P2X4 couples surfactant secretion and alveolar fluid transport. FASEB J. 2013, 27, 1772–1783. [Google Scholar] [CrossRef]
- Miklavc, P.; Thompson, K.E.; Frick, M. A new role for P2X4 receptors as modulators of lung surfactant secretion. Front. Cell. Neurosci. 2013, 7, 171. [Google Scholar] [CrossRef] [Green Version]
- Murrell-Lagnado, R.D.; Frick, M. P2X4 and lysosome fusion. Curr. Opin. Pharmacol. 2019, 47, 126–132. [Google Scholar] [CrossRef]
- Verkman, A.S. Water permeability measurement in living cells and complex tissues. J. Membr. Biol. 2000, 173, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Vitkova, V.; Genova, J.; Bivas, I. Permeability and the hidden area of lipid bilayers. Eur. Biophys. J. 2004, 33, 706–714. [Google Scholar] [CrossRef]
- Olbrich, K.; Rawicz, W.; Needham, D.; Evans, E. Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 2000, 79, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Borgnia, M.; Nielsen, S.; Engel, A.; Agre, P. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 1999, 68, 425–458. [Google Scholar] [CrossRef] [PubMed]
- Haller, T.; Dietl, P.; Deetjen, P.; Völkl, H. The lysosomal compartment as intracellular calcium store in MDCK cells: A possible involvement in InsP3-mediated Ca2+ release. Cell Calcium 1996, 19, 157–165. [Google Scholar] [CrossRef]
- Wolters, P.J.; Blackwell, T.S.; Eickelberg, O.; Loyd, J.E.; Kaminski, N.; Jenkins, G.; Maher, T.M.; Molina-Molina, M.; Noble, P.W.; Raghu, G.; et al. Time for a change: Is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir. Med. 2018, 6, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Noble, P.W.; Barkauskas, C.E.; Jiang, D. Pulmonary fibrosis: Patterns and perpetrators. J. Clin. Investig. 2012, 122, 2756–2762. [Google Scholar] [CrossRef] [Green Version]
- Bagnato, G.; Harari, S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur. Respir. Rev. 2015, 24, 102–114. [Google Scholar] [CrossRef]
- Tscherny, V.V.; Markin, V.S.; Decoursey, T.E. The Voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 1995, 105, 861–896. [Google Scholar] [CrossRef] [Green Version]
- Bullard, J.E.; Wert, S.E.; Nogee, L.M. ABCA3 Deficiency: Neonatal Respiratory Failure and Interstitial Lung Disease. Semin. Perinatol. 2006, 30, 327–334. [Google Scholar] [CrossRef]
- Garmany, T.H.; Moxley, M.A.; White, F.V.; Dean, M.; Hull, W.M.; Whitsett, J.A.; Nogee, L.M.; Hamvas, A. Surfactant composition and function in patients with ABCA3 mutations. Pediatr. Res. 2006, 59, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Shulenin, S.; Nogee, L.M.; Annilo, T.; Wert, S.E.; Whitsett, J.A.; Dean, M. ABCA3 Gene Mutations in Newborns with Fatal Surfactant Deficiency. N. Engl. J. Med. 2004, 350, 1296–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, J.P.; Pinheiro, L.; Costa, M.; Silva, A.; Gonçalves, A.; Pereira, A. Novel ABCA3 mutations as a cause of respiratory distress in a term newborn. Gene 2014, 534, 417–420. [Google Scholar] [CrossRef]
- Bullard, J.E.; Wert, S.E.; Whitsett, J.A.; Dean, M.; Nogee, L.M. ABCA3 mutations associated with pediatric interstitial lung disease. Am. J. Respir. Crit. Care Med. 2005, 172, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Crossno, P.F.; Polosukhin, V.V.; Blackwell, T.S.; Johnson, J.E.; Markin, C.; Moore, P.E.; Worrell, J.A.; Stahlman, M.T.; Phillips, J.A.; Loyd, J.E.; et al. Identification of early interstitial lung disease in an individual with genetic variations in ABCA3 and SFTPC. Chest 2010, 137, 969–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epaud, R.; Delestrain, C.; Louha, M.; Simon, S.; Fanen, P.; Tazi, A. Combined pulmonary fibrosis and emphysema syndrome associated with ABCA3 mutations. Eur. Respir. J. 2014, 43, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Ota, C.; Kimura, M.; Kure, S. ABCA3 mutations led to pulmonary fibrosis and emphysema with pulmonary hypertension in an 8-year-old girl. Pediatr. Pulmonol. 2016, 51, E21–E23. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, R.; Kaltenborn, E.; Frixel, S.; Wittmann, T.; Liebisch, G.; Schmitz, G.; Griese, M. ABCA3 protects alveolar epithelial cells against free cholesterol induced cell death. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.L.; Bi-Karchin, J.; Le, L.; Marks, M.S. The road to lysosome-related organelles: Insights from Hermansky-Pudlak syndrome and other rare diseases. Traffic 2019, 20, 404–435. [Google Scholar] [CrossRef] [Green Version]
- Huizing, M.; Helip-Wooley, A.; Westbroek, W.; Gunay-Aygun, M.; Gahl, W.A. Disorders of lysosome-related organelle biogenesis: Clinical and molecular genetics. Annu. Rev. Genomics Hum. Genet. 2008, 9, 359–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, F.M.; D’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Prim. 2018, 4, 27. [Google Scholar] [CrossRef]
- Faverio, P.; Stainer, A.; De Giacomi, F.; Gasperini, S.; Motta, S.; Canonico, F.; Pieruzzi, F.; Monzani, A.; Pesci, A.; Biondi, A. Molecular pathways and respiratory involvement in lysosomal storage diseases. Int. J. Mol. Sci. 2019, 20, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staretz-Chacham, O.; Aviram, M.; Morag, I.; Goldbart, A.; Hershkovitz, E. Pulmonary involvement in Niemann-Pick C type 1. Eur. J. Pediatr. 2018, 177, 1609–1615. [Google Scholar] [CrossRef]
- Ballout, R.A.; Sviridov, D.; Bukrinsky, M.I.; Remaley, A.T. The lysosome: A potential juncture between SARS-CoV-2 infectivity and Niemann-Pick disease type C, with therapeutic implications. FASEB J. 2020, 34, 7253–7264. [Google Scholar] [CrossRef] [PubMed]
- Dell’Angelica, E.C. Lysosome-related organelles. FASEB J. 2000, 14, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Luzio, J.P.; Hackmann, Y.; Dieckmann, N.M.G.; Griffiths, G.M. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb. Perspect. Biol. 2014, 6, a016840. [Google Scholar] [CrossRef] [Green Version]
- Guttentag, S.H.; Akhtar, A.; Tao, J.Q.; Atochina, E.; Rusiniak, M.E.; Swank, R.T.; Bates, S.R. Defective surfactant secretion in a mouse model of Hermansky-Pudlak syndrome. Am. J. Respir. Cell Mol. Biol. 2005, 33, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Atochina-Vasserman, E.N.; Bates, S.R.; Zhang, P.; Abramova, H.; Zhang, Z.; Gonzales, L.; Tao, J.Q.; Gochuico, B.R.; Gahl, W.; Guo, C.J.; et al. Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome. Am. J. Respir. Crit. Care Med. 2011, 184, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Young, L.R.; Gulleman, P.M.; Bridges, J.P.; Weaver, T.E.; Deutsch, G.H.; Blackwell, T.S.; McCormack, F.X. The alveolar epithelium determines susceptibility to lung fibrosis in Hrmansky-Pdlak syndrome. Am. J. Respir. Crit. Care Med. 2012, 186, 1014–1024. [Google Scholar] [CrossRef] [Green Version]
- Young, L.R.; Pasula, R.; Gulleman, P.M.; Deutsch, G.H.; McCormack, F.X. Susceptibility of hermansky-pudlak mice to bleomycin-induced type II cell apoptosis and fibrosis. Am. J. Respir. Cell Mol. Biol. 2007, 37, 67–74. [Google Scholar] [CrossRef]
- Peng, R.; Wu, L.A.; Wang, Q.; Qi, J.; Gao, G.F. Cell entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Delorey, T.M.; Ziegler, C.G.K.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446. [Google Scholar] [CrossRef]
- Qian, Z.; Travanty, E.A.; Oko, L.; Edeen, K.; Berglund, A.; Wang, J.; Ito, Y.; Holmes, K.V.; Mason, R.J. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am. J. Respir. Cell Mol. Biol. 2013, 48, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by the Transmembrane Protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A Transmembrane Serine Protease Is Linked to the Severe Acute Respiratory Syndrome Coronavirus Receptor and Activates Virus Entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Glowacka, I.; Bertram, S.; Muller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [Green Version]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Schuler, B.A.; Habermann, A.C.; Plosa, E.J.; Taylor, C.J.; Jetter, C.; Negretti, N.M.; Kapp, M.E.; Benjamin, J.T.; Gulleman, P.; Nichols, D.S.; et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J. Clin. Investig. 2021, 131, e140766. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.C.; Bosch, B.J.; Li, F.; Li, W.; Kyoung, H.L.; Ghiran, S.; Vasilieva, N.; Dermody, T.S.; Harrison, S.C.; Dormitzer, P.R.; et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006, 281, 3189–3203. [Google Scholar] [CrossRef] [Green Version]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, S.; Wu, Y.; Hou, W.; Yuan, L.; Shen, C.; Wang, J.; Ye, J.; Zheng, Q.; Ma, J.; et al. Virus-Free and Live-Cell Visualizing SARS-CoV-2 Cell Entry for Studies of Neutralizing Antibodies and Compound Inhibitors. Small Methods 2021, 5, 2001031. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef] [PubMed]
- Blaess, M.; Kaiser, L.; Sauer, M.; Csuk, R.; Deigner, H.P. COVID-19/SARS-CoV-2 infection: Lysosomes and lysosomotropism implicate new treatment strategies and personal risks. Int. J. Mol. Sci. 2020, 21, 4953. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Qin, P.; Huang, Y.W. Lysosomal ion channels involved in cellular entry and uncoating of enveloped viruses: Implications for therapeutic strategies against SARS-CoV-2. Cell Calcium 2021, 94, 102360. [Google Scholar] [CrossRef]
- Haller, T.; Ortmayr, J.; Friedrich, F.; Völkl, H.; Dietl, P. Dynamics of surfactant release in alveolar type II cells. Proc. Natl. Acad. Sci. USA 1998, 95, 1579–1584. [Google Scholar] [CrossRef] [Green Version]
- Mair, N.; Haller, T.; Dietl, P.; Shaul, P.W. Exocytosis in alveolar type II cells revealed by cell capacitance and fluorescence measurements. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 276, L376–L382. [Google Scholar] [CrossRef]
- Yayoi, Y.; Ohsawa, Y.; Koike, M.; Zhang, G.; Kominami, E.; Uchiyama, Y. Specific localization of lysosomal aminopeptidases in type II alveolar epithelial cells of the rat lung. Arch. Histol. Cytol. 2001, 64, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, G.E.R.; Gilmore, L.B. Hydrolases of pulmonary lysosomes and lamellar bodies. J. Biol. Chem. 1982, 257, 9211–9220. [Google Scholar] [CrossRef]
- Huang, J.; Hume, A.J.; Abo, K.M.; Werder, R.B.; Villacorta-Martin, C.; Alysandratos, K.D.; Beermann, M.L.; Simone-Roach, C.; Lindstrom-Vautrin, J.; Olejnik, J.; et al. SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell 2020, 27, 962–973. [Google Scholar] [CrossRef]
- Nardacci, R.; Colavita, F.; Castilletti, C.; Lapa, D.; Matusali, G.; Meschi, S.; Del Nonno, F.; Colombo, D.; Capobianchi, M.R.; Zumla, A.; et al. Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis. Cell Death Dis. 2021, 12, 263. [Google Scholar] [CrossRef]
- Ou, T.; Mou, H.; Zhang, L.; Ojha, A.; Choe, H.; Farzan, M. Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLoS Pathog. 2021, 17, e1009212. [Google Scholar] [CrossRef]
- Gorshkov, K.; Chen, C.Z.; Bostwick, R.; Rasmussen, L.; Tran, B.N.; Cheng, Y.S.; Xu, M.; Pradhan, M.; Henderson, M.; Zhu, W.; et al. The SARS-CoV-2 Cytopathic Effect Is Blocked by Lysosome Alkalizing Small Molecules. ACS Infect. Dis. 2021, 7, 1389–1408. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Gripp, B.; Hoffmann, M.; Pöhlmann, S.; Hoertel, N.; Edwards, M.J.; Kamler, M.; Kornhuber, J.; Becker, K.A.; Gulbins, E. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J. Biol. Chem. 2021, 296, 100701. [Google Scholar] [CrossRef]
- Huppert, L.A.; Matthay, M.A.; Ware, L.B. Pathogenesis of Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef]
- Swenson, K.E.; Swenson, E.R. Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury. Crit. Care Clin. 2021, 37, 749–776. [Google Scholar] [CrossRef]
- Riteau, N.; Gasse, P.; Fauconnier, L.; Gombault, A.; Couegnat, M.; Fick, L.; Kanellopoulos, J.; Quesniaux, V.F.J.; Marchand-Adam, S.; Crestani, B.; et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 774–783. [Google Scholar] [CrossRef]
- Kanellopoulos, J.M.; Almeida-da-Silva, C.L.C.; Rüütel Boudinot, S.; Ojcius, D.M. Structural and Functional Features of the P2X4 Receptor: An Immunological Perspective. Front. Immunol. 2021, 12, 645834. [Google Scholar] [CrossRef]
- Wirsching, E.; Fauler, M.; Fois, G.; Frick, M. P2 purinergic signaling in the distal lung in health and disease. Int. J. Mol. Sci. 2020, 21, 4973. [Google Scholar] [CrossRef]
- Diem, K.; Fauler, M.; Fois, G.; Hellmann, A.; Winokurow, N.; Schumacher, S.; Kranz, C.; Frick, M. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J. 2020, 34, 12785–12804. [Google Scholar] [CrossRef]
- Wauters, E.; Van Mol, P.; Garg, A.D.; Jansen, S.; Van Herck, Y.; Vanderbeke, L.; Bassez, A.; Boeckx, B.; Malengier-Devlies, B.; Timmerman, A.; et al. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res. 2021, 31, 272–290. [Google Scholar] [CrossRef]
- Ji, J.; Sun, L.; Luo, Z.; Zhang, Y.; Xianzheng, W.; Liao, Y.; Tong, X.; Shan, J. Potential Therapeutic Applications of Pulmonary Surfactant Lipids in the Host Defence Against Respiratory Viral Infections. Front. Immunol. 2021, 12, 730022. [Google Scholar] [CrossRef]
- Kaczmarek-Hájek, K.; Lörinczi, É.; Hausmann, R.; Nicke, A. Molecular and functional properties of P2X receptors-recent progress and persisting challenges. Purinergic Signal. 2012, 8, 375–417. [Google Scholar] [CrossRef] [Green Version]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Bryant, A.; Lawrie, T.A.; Dowswell, T.; Fordham, E.J.; Mitchell, S.; Hill, S.R.; Tham, T.C. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am. J. Ther. 2021, 28, e434–e460. [Google Scholar] [CrossRef]
- Haller, T.; Cerrada, A.; Pfaller, K.; Braubach, P.; Felder, E. Polarized light microscopy reveals physiological and drug-induced changes in surfactant membrane assembly in alveolar type II pneumocytes. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 1152–1161. [Google Scholar] [CrossRef]
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 5–11. [Google Scholar] [CrossRef]
- Patel, S.D.; Bono, T.R.; Rowe, S.M.; Solomon, G.M. CFTR targeted therapies: Recent advances in cystic fibrosis and possibilities in other diseases of the airways. Eur. Respir. Rev. 2020, 29, 190068. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 1783–1784. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Dřevínek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR Potentiator in Patients with Cystic Fibrosis and the G551D Mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef] [Green Version]
- Dukovski, D.; Villella, A.; Bastos, C.; King, R.; Finley, D.; Kelly, J.W.; Morimoto, R.I.; Hartl, F.U.; Munoz, B.; Lee, P.S.; et al. Amplifiers co-translationally enhance CFTR biosynthesis via PCBP1-mediated regulation of CFTR mRNA. J. Cyst. Fibros. 2020, 19, 733–741. [Google Scholar] [CrossRef] [Green Version]
- High, K.D.; Roncarolo, M.G. Gene Therapy. N. Engl. J. Med. 2019, 381, 455–464. [Google Scholar] [CrossRef]
- Moriyama, Y.; Nomura, M. Clodronate: A Vesicular ATP Release Blocker. Trends Pharmacol. Sci. 2018, 39, 13–23. [Google Scholar] [CrossRef]
- Magalhaes, J.; Gegg, M.E.; Migdalska-Richards, A.; Schapira, A.H. Effects of ambroxol on the autophagy-lysosome pathway and mitochondria in primary cortical neurons. Sci. Rep. 2018, 8, 1385. [Google Scholar] [CrossRef]
- Sheth, J.; Joseph, J.J.; Shah, K.; Muranjan, M.; Mistri, M.; Sheth, F. Pulmonary manifestations in Niemann-Pick type C disease with mutations in NPC2 gene: Case report and review of literature. BMC Med. Genet. 2017, 18, 5. [Google Scholar] [CrossRef] [Green Version]
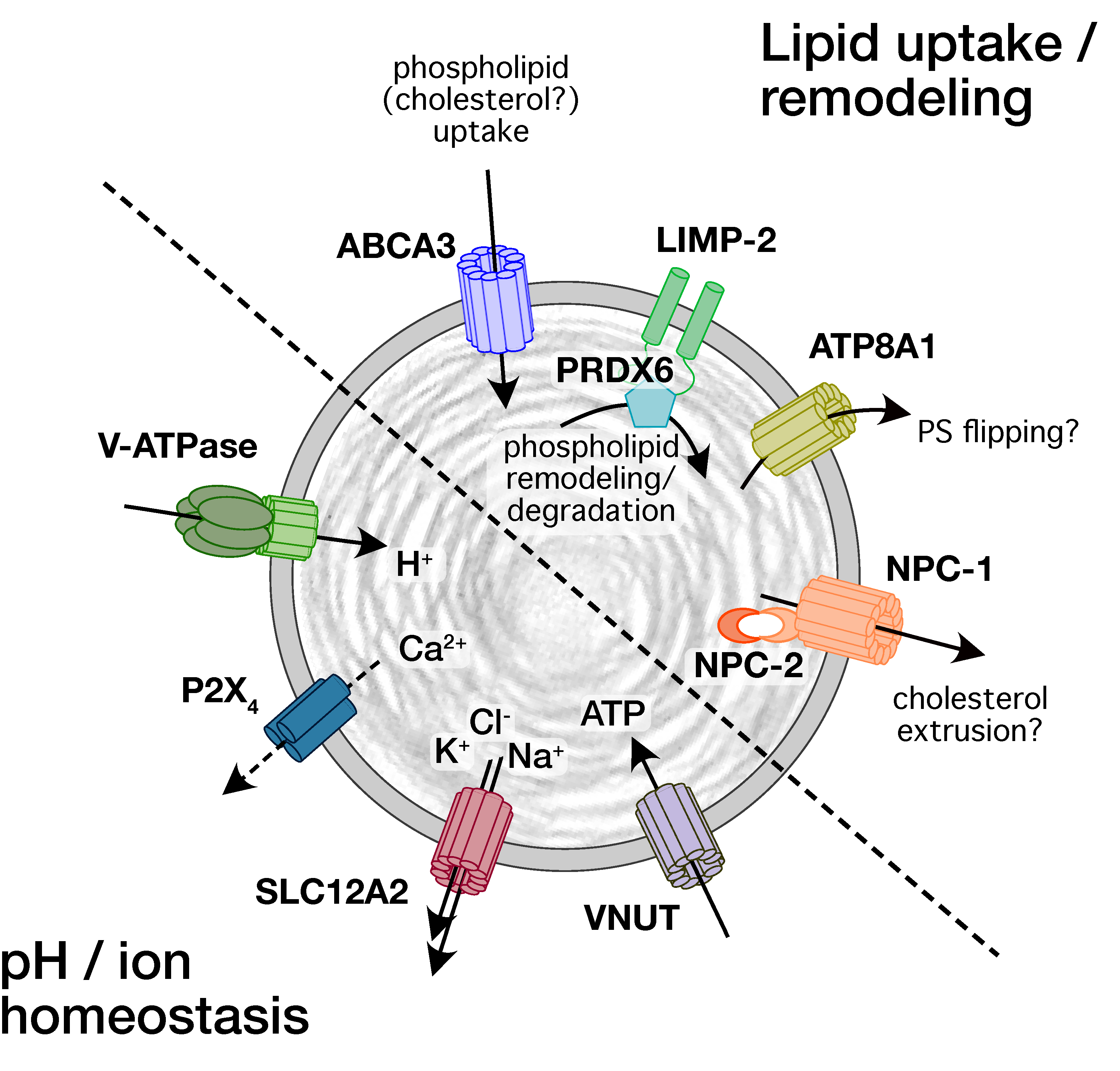
| Ion Channel Transporter | Detection | Physiological Function | Role in Lung Disease |
|---|---|---|---|
| ABCA3 | Immuno-EM, IF [39] | LB biogenesis, lipid uptake [17,60,64] | Surfactant-related lung disorders [120,121] respiratory distress in newborns [122,123] interstitial lung diseases (ILDs), fibrosis [124,125,126] |
| ATP8A1 | WB, IF [44] | Suggested: LB priming for exocytosis [44] | Possible involvement in fibrosis [44] |
| LIMP-2/SCARB2 | WB, IF [42] | Possibly role in luminal localization of PRDX6 for regulation of LB phospholipid content [42] | n.d. (possibly fibrosis [42]) |
| NPC1 | WB, IF [43] | n.d. (possible role in regulating LB cholesterol content) | ILD, fibrosis [132,190] |
| NPC2 | WB, IF [43] | n.d. | Fibrosis [190] |
| V-ATPase subunits | WB [45,46,47] IF [46,47] | Acidification of LB lumen [45,50] | n.d. |
| P2X4 | WB, IF [41] | Ca2+ release/entry (FACE) facilitates surfactant secretion and activation, alveolar fluid resorption [41,107] | n.d. |
| SLC12A2 | WB [45] | Na+, K+, 2Cl- efflux [45] | n.d. |
| VNUT | WB, IF [48] | ATP uptake [48] | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietl, P.; Frick, M. Channels and Transporters of the Pulmonary Lamellar Body in Health and Disease. Cells 2022, 11, 45. https://doi.org/10.3390/cells11010045
Dietl P, Frick M. Channels and Transporters of the Pulmonary Lamellar Body in Health and Disease. Cells. 2022; 11(1):45. https://doi.org/10.3390/cells11010045
Chicago/Turabian StyleDietl, Paul, and Manfred Frick. 2022. "Channels and Transporters of the Pulmonary Lamellar Body in Health and Disease" Cells 11, no. 1: 45. https://doi.org/10.3390/cells11010045
APA StyleDietl, P., & Frick, M. (2022). Channels and Transporters of the Pulmonary Lamellar Body in Health and Disease. Cells, 11(1), 45. https://doi.org/10.3390/cells11010045






