Branched Actin Maintains Acetylated Microtubule Network in the Early Secretory Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Chemical Treatment and Immunofluorescence
2.3. Image Analysis of Chemically Fixed Cells
2.4. Live-Cell Imaging and Analysis
2.5. RAB1 Tracking
2.6. Statistics
3. Results
3.1. Arp2/3 Inhibition Induces an Accumulation of RAB1+ Tubules around the Centrosome
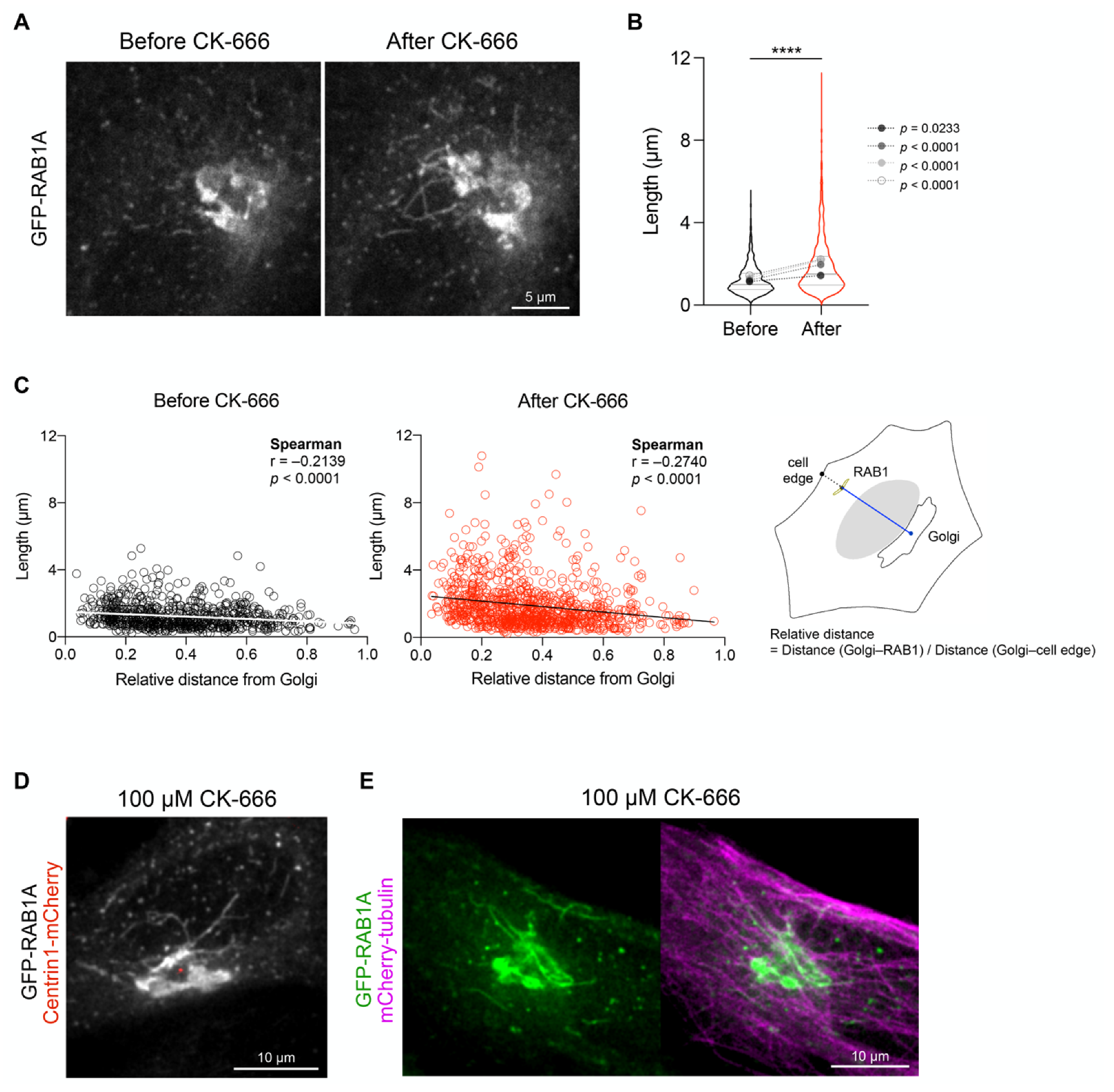
3.2. Arp2/3 Inhibition Increases the Length of GFP-RAB1A+ Tubules at the Cell Center
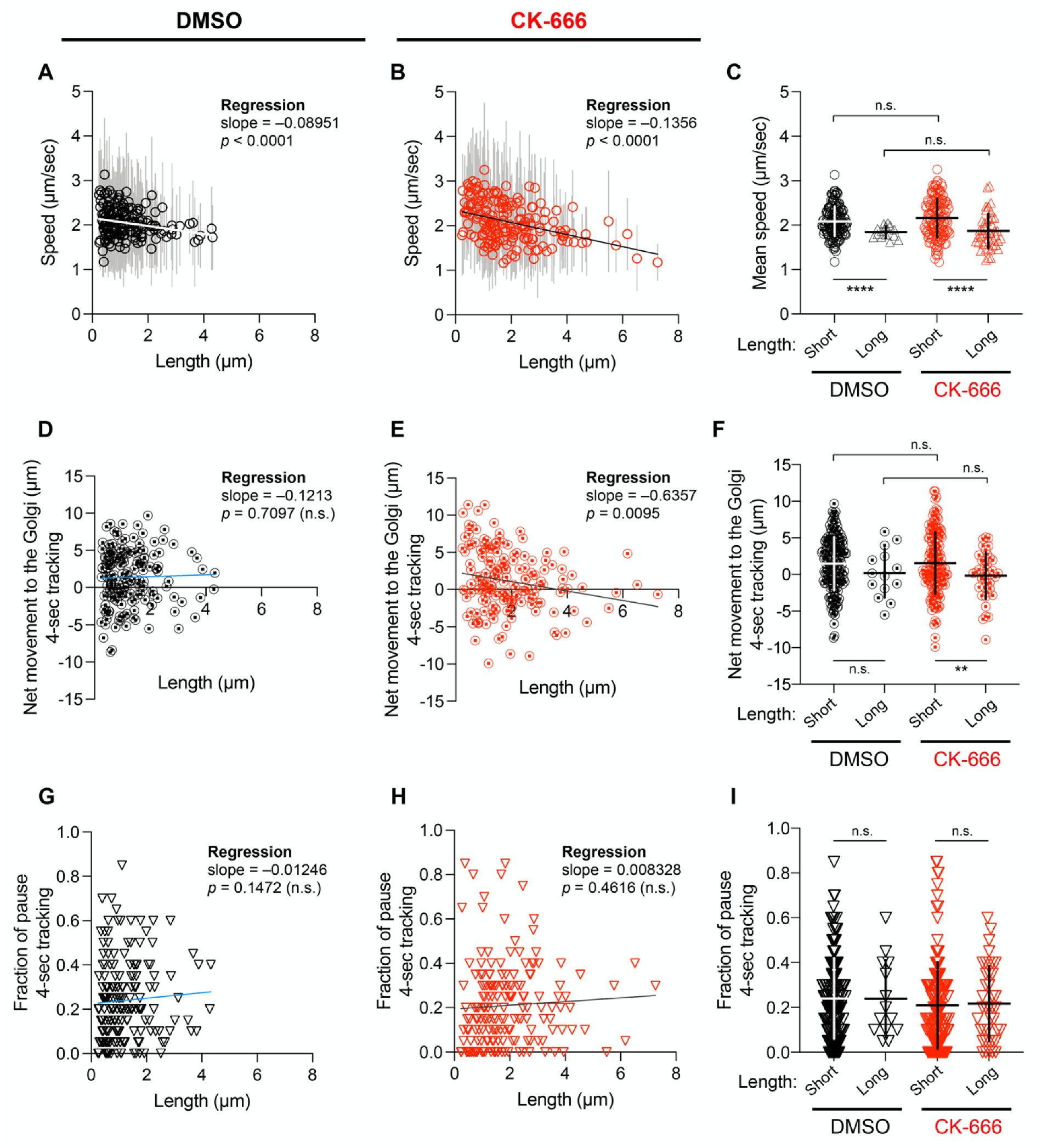
3.3. The Length of RAB1+ Tubules Correlates with Their Speed and Ability to Move towards the Golgi Apparatus
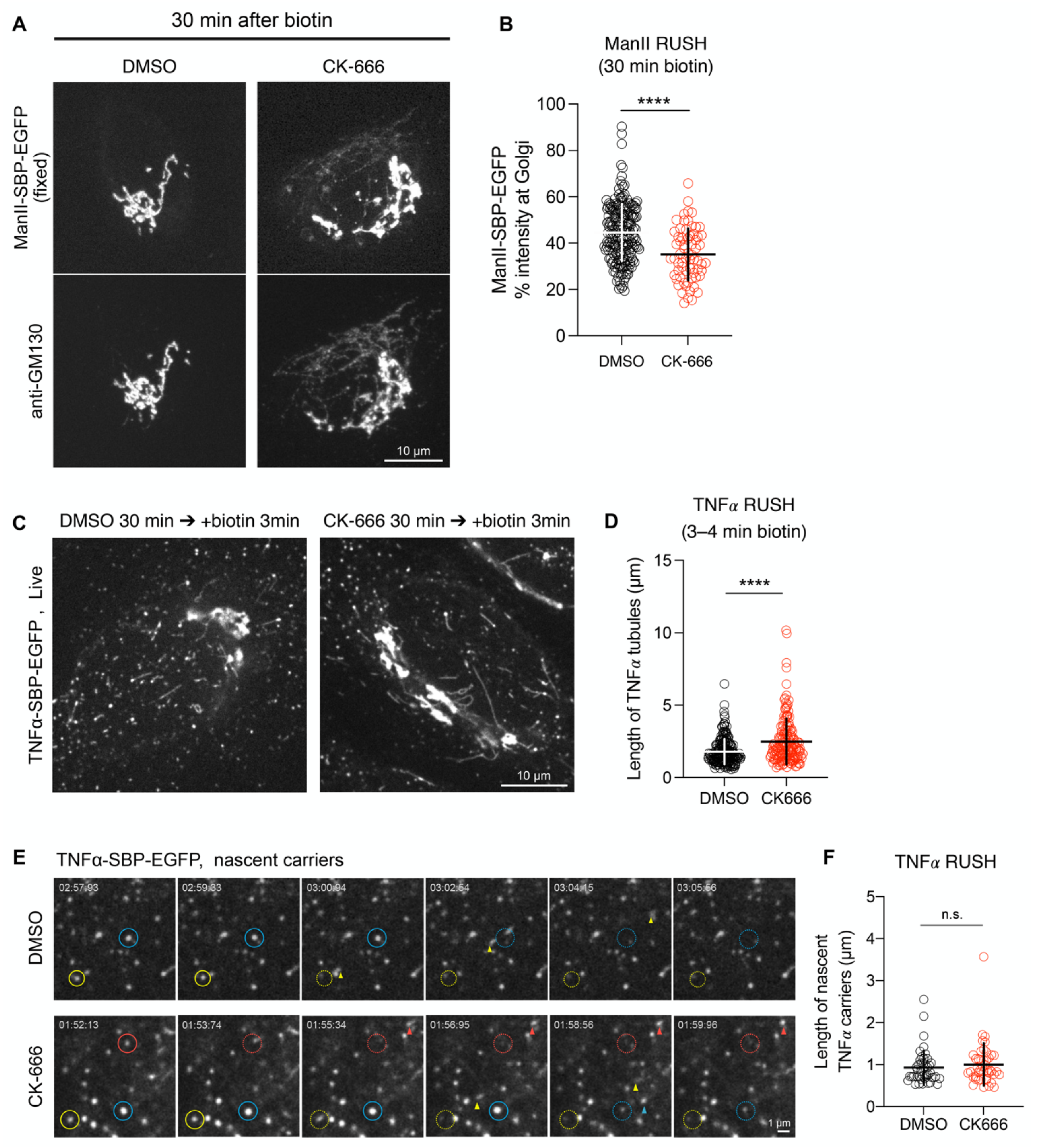
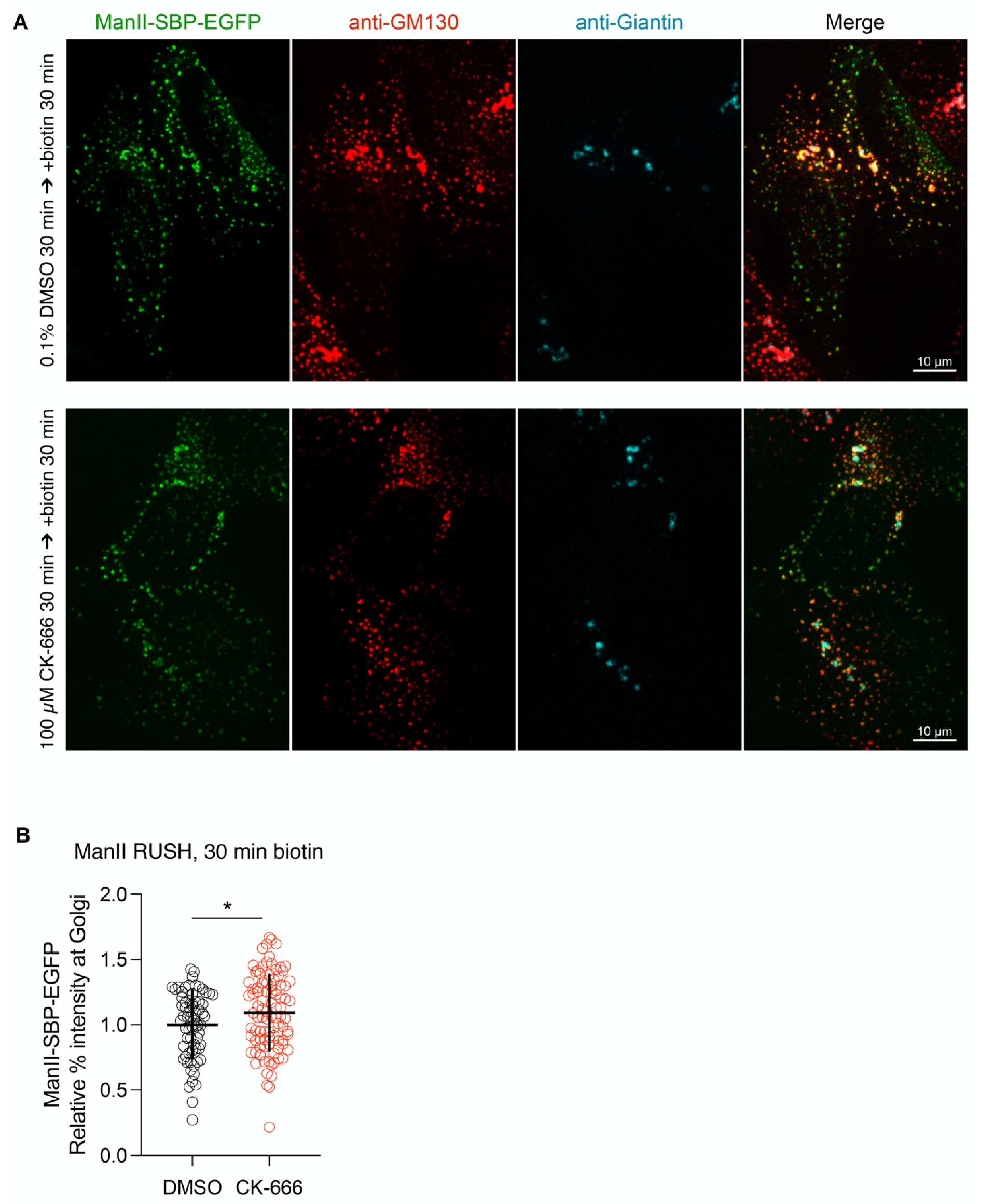
3.4. Arp2/3 Inhibition Decreases the Rate of Cargo Arrival to the Golgi Apparatus without Affecting the Early Stages of ER-to-Golgi Transport
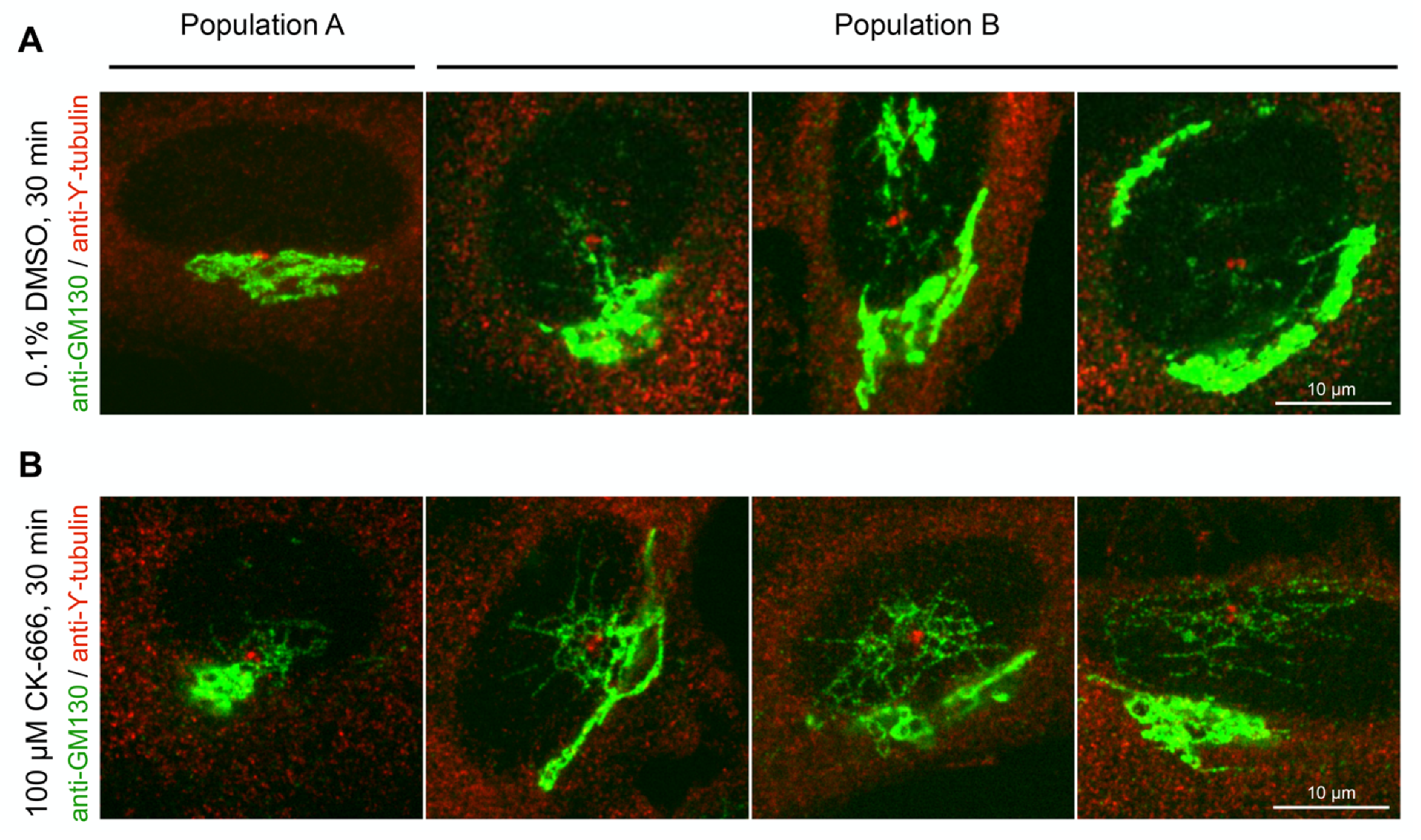
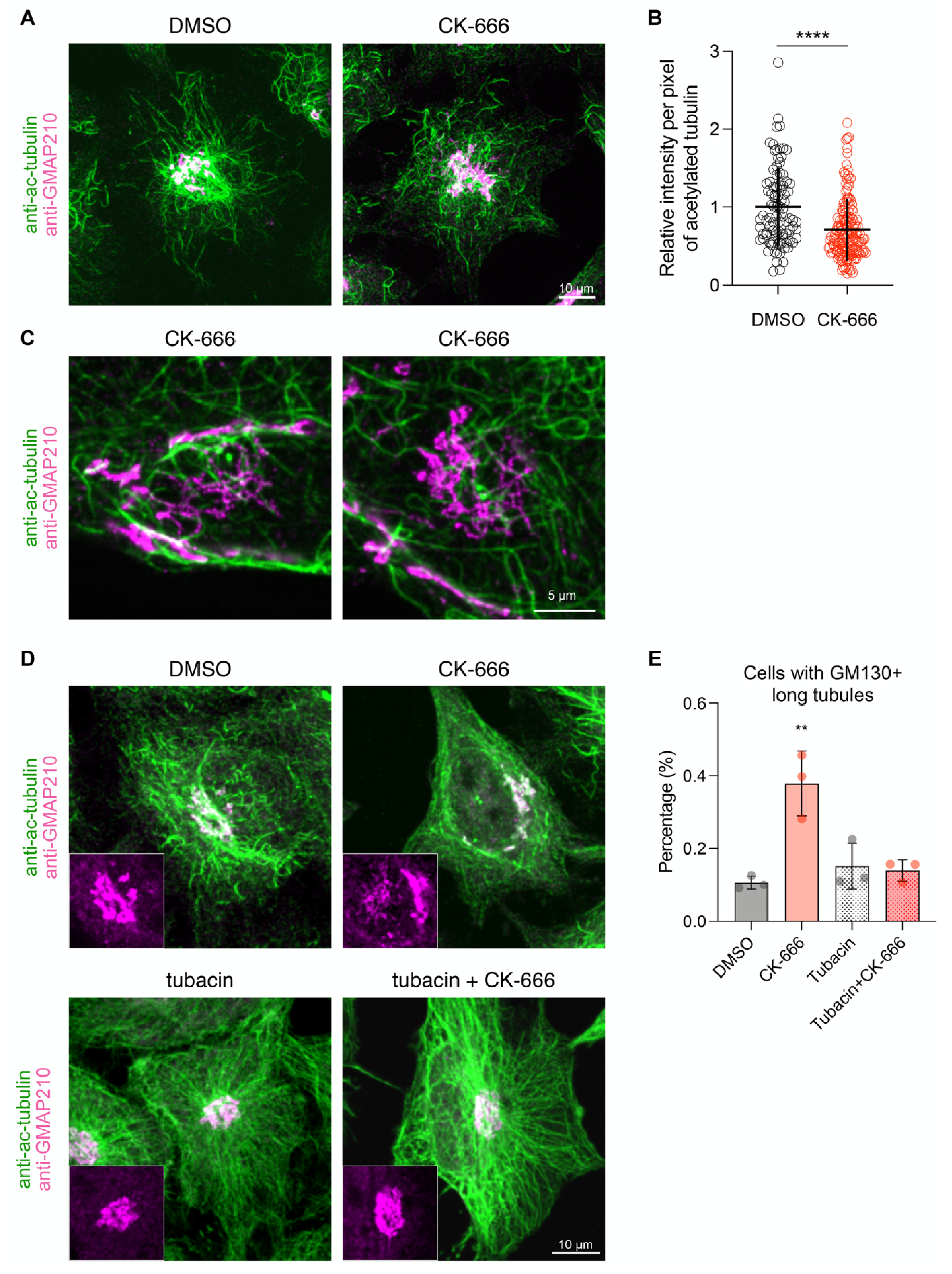
3.5. Arp2/3 Inhibition Affects Acetylated Microtubule Network around the Golgi Apparatus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirschberg, K.; Miller, C.M.; Ellenberg, J.; Presley, J.F.; Siggia, E.D.; Phair, R.D.; Lippincott-Schwartz, J. Kinetic Analysis of Secretory Protein Traffic and Characterization of Golgi to Plasma Membrane Transport Intermediates in Living Cells. J. Cell Biol. 1998, 143, 1485–1503. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, F.; Babià, T.; Ayala, I.; Kok, J.W.; Renau-Piqueras, J.; Egea, G. Actin microfilaments are essential for the cytological positioning and morphology of the Golgi complex. Eur. J. Cell Biol. 1998, 76, 9–17. [Google Scholar] [CrossRef]
- Valderrama, F.; Durán, J.M.; Babià, T.; Barth, H.; Renau-Piqueras, J.; Egea, G. Actin Microfilaments Facilitate the Retrograde Transport from the Golgi Complex to the Endoplasmic Reticulum in Mammalian Cells. Traffic 2001, 2, 717–726. [Google Scholar] [CrossRef]
- Müsch, A.; Cohen, D.; Kreitzer, G.; Rodriguez-Boulan, E. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J. 2001, 20, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Stamnes, M. Regulating the actin cytoskeleton during vesicular transport. Curr. Opin. Cell Biol. 2002, 14, 428–433. [Google Scholar] [CrossRef]
- Salvarezza, S.B.; Deborde, S.; Schreiner, R.; Campagne, F.; Kessels, M.M.; Qualmann, B.; Caceres, A.; Kreitzer, G.; Rodriguez-Boulan, E. LIM Kinase 1 and Cofilin Regulate Actin Filament Population Required for Dynamin-dependent Apical Carrier Fission from theTrans-Golgi Network. Mol. Biol. Cell 2009, 20, 438–451. [Google Scholar] [CrossRef]
- Egea, G.; Serra-Peinado, C.; Salcedo-Sicilia, L.; Gutiérrez-Martínez, E. Actin acting at the Golgi. Histochem. Cell Biol. 2013, 140, 347–360. [Google Scholar] [CrossRef]
- Gurel, P.S.; Hatch, A.L.; Higgs, H.N. Connecting the Cytoskeleton to the Endoplasmic Reticulum and Golgi. Curr. Biol. 2014, 24, R660–R672. [Google Scholar] [CrossRef]
- Guet, D.; Mandal, K.; Pinot, M.; Hoffmann, J.; Abidine, Y.; Sigaut, W.; Bardin, S.; Schauer, K.; Goud, B.; Manneville, J.-B. Mechanical Role of Actin Dynamics in the Rheology of the Golgi Complex and in Golgi-Associated Trafficking Events. Curr. Biol. 2014, 24, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Miserey-Lenkei, S.; Chalancon, G.; Bardin, S.; Formstecher, E.; Goud, B.; Echard, A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat. Cell Biol. 2010, 12, 645–654. [Google Scholar] [CrossRef]
- Carréno, S.; Engqvist-Goldstein, A.E.; Zhang, C.X.; McDonald, K.L.; Drubin, D.G. Actin dynamics coupled to clathrin-coated vesicle formation at the trans-Golgi network. J. Cell Biol. 2004, 165, 781–788. [Google Scholar] [CrossRef]
- Anitei, M.; Stange, C.; Parshina, I.; Baust, T.; Schenck, A.; Raposo, G.; Kirchhausen, T.; Hoflack, B. Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin–AP-1-coated carrier biogenesis at the TGN. Nat. Cell Biol. 2010, 12, 330–340. [Google Scholar] [CrossRef]
- Anitei, M.; Stange, C.; Czupalla, C.; Niehage, C.; Schuhmann, K.; Sala, P.; Czogalla, A.; Pursche, T.; Coskun, Ü.; Shevchenko, A.; et al. Spatiotemporal Control of Lipid Conversion, Actin-Based Mechanical Forces, and Curvature Sensors during Clathrin/AP-1-Coated Vesicle Biogenesis. Cell Rep. 2017, 20, 2087–2099. [Google Scholar] [CrossRef]
- Almeida, C.G.; Yamada, A.; Tenza, D.; Louvard, D.; Raposo, G.; Coudrier, E. Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat. Cell Biol. 2011, 13, 779–789. [Google Scholar] [CrossRef]
- Plutner, H.; Cox, A.D.; Pind, S.; Khosravi-Far, R.; Bourne, J.R.; Schwaninger, R.; Der, C.; E Balch, W. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J. Cell Biol. 1991, 115, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, E.J.; Bourne, J.R.; Khosravi-Far, R.; Der, C.; Balch, W.E. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 1992, 119, 749–761. [Google Scholar] [CrossRef]
- Campellone, K.G.; Webb, N.J.; Znameroski, E.A.; Welch, M.D. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell 2008, 134, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J.; Mathiowetz, A.J.; Hong, S.; Welch, M.D.; Campellone, K.G. Rab1 recruits WHAMM during membrane remodeling but limits actin nucleation. Mol. Biol. Cell 2016, 27, 967–978. [Google Scholar] [CrossRef]
- Kee, A.J.; Bryce, N.; Yang, L.; Polishchuk, E.; Schevzov, G.; Weigert, R.; Polishchuk, R.; Gunning, P.W.; Hardeman, E.C. ER/Golgi trafficking is facilitated by unbranched actin filaments containing Tpm4.2. Cytoskelet 2017, 74, 379–389. [Google Scholar] [CrossRef]
- Hauri, H.-P.; Schweizer, A. The endoplasmic reticulum—Golgi intermediate compartment. Curr. Opin. Cell Biol. 1992, 4, 600–608. [Google Scholar] [CrossRef]
- Saraste, J.; Kuismanen, E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin. Cell Biol. 1992, 3, 343–355. [Google Scholar] [CrossRef]
- Bannykh, S.I.; Rowe, T.; Balch, W.E. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 1996, 135, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Klumperman, J.; Schweizer, A.; Clausen, H.; Tang, B.L.; Hong, W.; Oorschot, V.; Hauri, H.P. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J. Cell Sci. 1998, 111, 3411–3425. [Google Scholar] [CrossRef]
- Stephens, D.J.; Lin-Marq, N.; Pagano, A.; Pepperkok, R.; Paccaud, J.P. COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J. Cell Sci. 2000, 113, 2177–2185. [Google Scholar] [CrossRef]
- Fan, J.-Y.; Zuber, C. Ultrastructural analysis of transitional endoplasmic reticulum and pre-Golgi intermediates: A highway for cars and trucks. Histochem. Cell Biol. 2003, 120, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Zeuschner, D.; Geerts, W.J.; Van Donselaar, E.; Humbel, B.M.; Slot, J.W.; Koster, A.; Klumperman, J. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat. Cell Biol. 2006, 8, 377–383. [Google Scholar] [CrossRef]
- Hanna, M.G.; Block, S.; Frankel, E.B.; Hou, F.; Johnson, A.; Yuan, L.; Knight, G.; Moresco, J.J.; Yates, J.R.; Ashton, R.; et al. TFG facilitates outer coat disassembly on COPII transport carriers to promote tethering and fusion with ER–Golgi intermediate compartments. Proc. Natl. Acad. Sci. USA 2017, 114, E7707–E7716. [Google Scholar] [CrossRef]
- Westrate, L.M.; Hoyer, M.J.; Nash, M.J.; Voeltz, G.K. Vesicular and uncoated Rab1-dependent cargo carriers facilitate ER to Golgi transport. J. Cell Sci. 2020, 133, jcs239814. [Google Scholar] [CrossRef] [PubMed]
- Weigel, A.V.; Chang, C.-L.; Shtengel, G.; Xu, C.S.; Hoffman, D.P.; Freeman, M.; Iyer, N.; Aaron, J.; Khuon, S.; Bogovic, J.; et al. ER-to-Golgi protein delivery through an interwoven, tubular network extending from ER. Cell 2021, 184, 2412–2429.e16. [Google Scholar] [CrossRef]
- Ben-Tekaya, H.; Miura, K.; Pepperkok, R.; Hauri, H.-P. Live imaging of bidirectional traffic from the ERGIC. J. Cell Sci. 2005, 118, 357–367. [Google Scholar] [CrossRef]
- Appenzeller-Herzog, C.; Hauri, H.-P. The ER-Golgi intermediate compartment (ERGIC): In search of its identity and function. J. Cell Sci. 2006, 119, 2173–2183. [Google Scholar] [CrossRef]
- Saraste, J.; Lahtinen, U.M.; Goud, B. Localization of the small GTP-binding protein rab1p to early compartments of the secretory pathway. J. Cell Sci. 1995, 108, 1541–1552. [Google Scholar] [CrossRef]
- Sannerud, R.; Saraste, J.; Goud, B. Retrograde traffic in the biosynthetic-secretory route: Pathways and machinery. Curr. Opin. Cell Biol. 2003, 15, 438–445. [Google Scholar] [CrossRef]
- Presley, J.F.; Cole, N.B.; Schroer, T.; Hirschberg, K.; Zaal, K.J.M.; Lippincott-Schwartz, J. ER-to-Golgi transport visualized in living cells. Nat. Cell Biol. 1997, 389, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Scales, S.; Pepperkok, R.; Kreis, T.E. Visualization of ER-to-Golgi Transport in Living Cells Reveals a Sequential Mode of Action for COPII and COPI. Cell 1997, 90, 1137–1148. [Google Scholar] [CrossRef]
- Marie, M.; Dale, H.A.; Sannerud, R.; Saraste, J. The Function of the Intermediate Compartment in Pre-Golgi Trafficking Involves its Stable Connection with the Centrosome. Mol. Biol. Cell 2009, 20, 4458–4470. [Google Scholar] [CrossRef] [PubMed]
- Saraste, J.; Goud, B. Functional Symmetry of Endomembranes. Mol. Biol. Cell 2007, 18, 1430–1436. [Google Scholar] [CrossRef]
- Saraste, J.; Marie, M. Intermediate compartment (IC): From pre-Golgi vacuoles to a semi-autonomous membrane system. Histochem. Cell Biol. 2018, 150, 407–430. [Google Scholar] [CrossRef]
- Boncompain, G.; Divoux, S.; Gareil, N.; De Forges, H.; Lescure, A.; Latreche, L.; Mercanti, V.; Jollivet, F.; Raposo, G.; Perez, F. Synchronization of secretory protein traffic in populations of cells. Nat. Methods 2012, 9, 493–498. [Google Scholar] [CrossRef]
- Sannerud, R.; Marie, M.; Nizak, C.; Dale, H.A.; Pernet-Gallay, K.; Perez, F.; Goud, B.; Saraste, J. Rab1 Defines a Novel Pathway Connecting the Pre-Golgi Intermediate Compartment with the Cell Periphery. Mol. Biol. Cell 2006, 17, 1514–1526. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; Hunt, S.D.; Stephens, D.J. Opposing microtubule motors control motility, morphology and cargo segregation during ER-to-Golgi transport. Biol Open 2014, 3, 307–313. [Google Scholar] [CrossRef]
- Fourriere, L.; Divoux, S.; Roceri, M.; Perez, F.; Boncompain, G. Microtubule-independent secretion requires functional maturation of Golgi elements. J. Cell Sci. 2016, 129, 3238–3250. [Google Scholar] [CrossRef]
- Sandoval, I.V.; Bonifacino, J.S.; Klausner, R.D.; Henkart, M.; Wehland, J. Role of microtubules in the organization and localization of the Golgi apparatus. J. Cell Biol. 1984, 99, 113s–118s. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.B.; Sciaky, N.; Marotta, A.; Song, J.; Lippincott-Schwartz, J. Golgi dispersal during microtubule disruption: Regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell 1996, 7, 631–650. [Google Scholar] [CrossRef]
- Thyberg, J.; Moskalewski, S. Relationship between the Golgi complex and microtubules enriched in detyrosinated or acetylated 𝛼-tubulin: Studies on cells recovering from nocodazole and cells in the terminal phase of cytokinesis. Z. Zellforsch. Mikrosk. Anat. 1993, 273, 457–466. [Google Scholar] [CrossRef]
- Chabin-Brion, K.; Marceiller, J.; Perez, F.; Settegrana, C.; Drechou, A.; Durand, G.; Poüs, C. The Golgi Complex Is a Microtubule-organizing Organelle. Mol. Biol. Cell 2001, 12, 2047–2060. [Google Scholar] [CrossRef]
- Rivero, S.; Cardenas, J.; Bornens, M.; Ríos, R.M. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 2009, 28, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, Y.; Huang, Y.; Zhang, C.; Li, Y.; Liu, Y.; Li, T.; Wang, W.; Liang, X.; Wu, C. Arp2/3-branched actin regulates microtubule acetylation level and affects mitochondrial distribution. J. Cell Sci. 2019, 132, jcs226506. [Google Scholar] [CrossRef]
- Delevoye, C.; Heiligenstein, X.; Ripoll, L.; Gilles-Marsens, F.; Dennis, M.; Linares, R.; Derman, L.; Gokhale, A.; Morel, E.; Faundez, V.; et al. BLOC-1 Brings Together the Actin and Microtubule Cytoskeletons to Generate Recycling Endosomes. Curr. Biol. 2016, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Saheki, Y.; Swarup, S.; Lucast, L.; Harper, J.; De Camilli, P. Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell 2016, 166, 408–423. [Google Scholar] [CrossRef]
- Dai, A.; Yu, L.; Wang, H.-W. WHAMM initiates autolysosome tubulation by promoting actin polymerization on autolysosomes. Nat. Commun. 2019, 10, 3699. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Lee, M.; Higgs, H.N. Multiple roles for actin in secretory and endocytic pathways. Curr. Biol. 2021, 31, R603–R618. [Google Scholar] [CrossRef]
- Sato, Y.; Hayashi, K.; Amano, Y.; Takahashi, M.; Yonemura, S.; Hayashi, I.; Hirose, H.; Ohno, S.; Suzuki, A. MTCL1 crosslinks and stabilizes non-centrosomal microtubules on the Golgi membrane. Nat. Commun. 2014, 5, 5266. [Google Scholar] [CrossRef]
- Sanders, A.A.W.M.; Ekaverina, I. Nucleation and Dynamics of Golgi-derived Microtubules. Front. Neurosci. 2015, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; de Heus, C.; Liu, Q.; Bouchet, B.P.; Noordstra, I.; Jiang, K.; Hua, S.; Martin, M.; Yang, C.; Grigoriev, I.; et al. Molecular Pathway of Microtubule Organization at the Golgi Apparatus. Dev. Cell 2016, 39, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Efimov, A.; Kharitonov, A.; Efimova, N.; Loncare, K.J.; Miller, P.M.; Andreyeva, N.; Gleeson, P.; Galjart, N.; Maia, A.R.; McLeod, I.X.; et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 2007, 12, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, L.; Caballero, C.; Gavilan, M.P.; Cardenas, J.; Bornens, M.; Rios, R.M. Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. J. Cell Biol. 2011, 193, 917–933. [Google Scholar] [CrossRef]
- Grigoriev, I.; Splinter, D.; Keijzer, N.; Wulf, P.S.; Demmers, J.; Ohtsuka, T.; Modesti, M.; Maly, I.V.; Grosveld, F.; Hoogenraad, C.C.; et al. Rab6 Regulates Transport and Targeting of Exocytotic Carriers. Dev. Cell 2007, 13, 305–314. [Google Scholar] [CrossRef]
- Fourriere, L.; Kasri, A.; Gareil, N.; Bardin, S.; Bousquet, H.; Pereira, D.; Perez, F.; Goud, B.; Boncompain, G.; Miserey-Lenkei, S. RAB6 and microtubules restrict protein secretion to focal adhesions. J. Cell Biol. 2019, 218, 2215–2231. [Google Scholar] [CrossRef]
- Tenorio, M.J.; Luchsinger, C.; Mardones, G.A. Protein Kinase A Activity Is Necessary for Fission and Fusion of Golgi to Endoplasmic Reticulum Retrograde Tubules. PLoS ONE 2015, 10, e0135260. [Google Scholar] [CrossRef][Green Version]
- Reed, N.A.; Cai, D.; Blasius, T.L.; Jih, G.T.; Meyhofer, E.; Gaertig, J.; Verhey, K.J. Microtubule Acetylation Promotes Kinesin-1 Binding and Transport. Curr. Biol. 2006, 16, 2166–2172. [Google Scholar] [CrossRef]
- Dompierre, J.P.; Godin, J.D.; Charrin, B.C.; Cordelières, F.P.; King, S.J.; Humbert, S.; Saudou, F. Histone Deacetylase 6 Inhibition Compensates for the Transport Deficit in Huntington’s Disease by Increasing Tubulin Acetylation. J. Neurosci. 2007, 27, 3571–3583. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Donaldson, J.G.; Schweizer, A.; Berger, E.G.; Hauri, H.-P.; Yuan, L.C.; Klausner, R.D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin a suggests an ER recycling pathway. Cell 1990, 60, 821–836. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J. Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. J. Cell Biol. 1995, 128, 293–306, Erratum in J. Cell Biol. 1995, 128, 988; Erratum in J. Cell Biol. 1995, 129, 893. [Google Scholar] [CrossRef]
- Roghi, C.; Allan, V. Dynamic association of cytoplasmic dynein heavy chain 1a with the Golgi apparatus and intermediate compartment. J. Cell Sci. 1999, 112, 4673–4685. [Google Scholar] [CrossRef]
- Portran, D.; Schaedel, L.; Xu, Z.; Théry, M.; Nachury, M.V. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 2017, 19, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Schaedel, L.; Portran, D.; Aguilar, A.; Gaillard, J.; Marinkovich, M.P.; Théry, M.; Nachury, M.V. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 2017, 356, 328–332. [Google Scholar] [CrossRef]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of α-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.; Gaillard, J.; Guérin, C.; Couté, Y.; Sillibourne, J.; Blanchoin, L.; Théry, M. The centrosome is an actin-organizing centre. Nat. Cell Biol. 2016, 18, 65–75. [Google Scholar] [CrossRef]
- Inoue, D.; Obino, D.; Pineau, J.; Farina, F.; Gaillard, J.; Guerin, C.; Blanchoin, L.; Lennon-Duménil, A.; Théry, M. Actin filaments regulate microtubule growth at the centrosome. EMBO J. 2019, 38, e99630. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimura, A.; Miserey-Lenkei, S.; Coudrier, E.; Goud, B. Branched Actin Maintains Acetylated Microtubule Network in the Early Secretory Pathway. Cells 2022, 11, 15. https://doi.org/10.3390/cells11010015
Yoshimura A, Miserey-Lenkei S, Coudrier E, Goud B. Branched Actin Maintains Acetylated Microtubule Network in the Early Secretory Pathway. Cells. 2022; 11(1):15. https://doi.org/10.3390/cells11010015
Chicago/Turabian StyleYoshimura, Azumi, Stéphanie Miserey-Lenkei, Evelyne Coudrier, and Bruno Goud. 2022. "Branched Actin Maintains Acetylated Microtubule Network in the Early Secretory Pathway" Cells 11, no. 1: 15. https://doi.org/10.3390/cells11010015
APA StyleYoshimura, A., Miserey-Lenkei, S., Coudrier, E., & Goud, B. (2022). Branched Actin Maintains Acetylated Microtubule Network in the Early Secretory Pathway. Cells, 11(1), 15. https://doi.org/10.3390/cells11010015





