Impact of Motile Ciliopathies on Human Development and Clinical Consequences in the Newborn
Abstract
:1. Introduction
1.1. Primary vs. Motile Ciliopathies and Human Development
1.2. Motile Cilia Structure
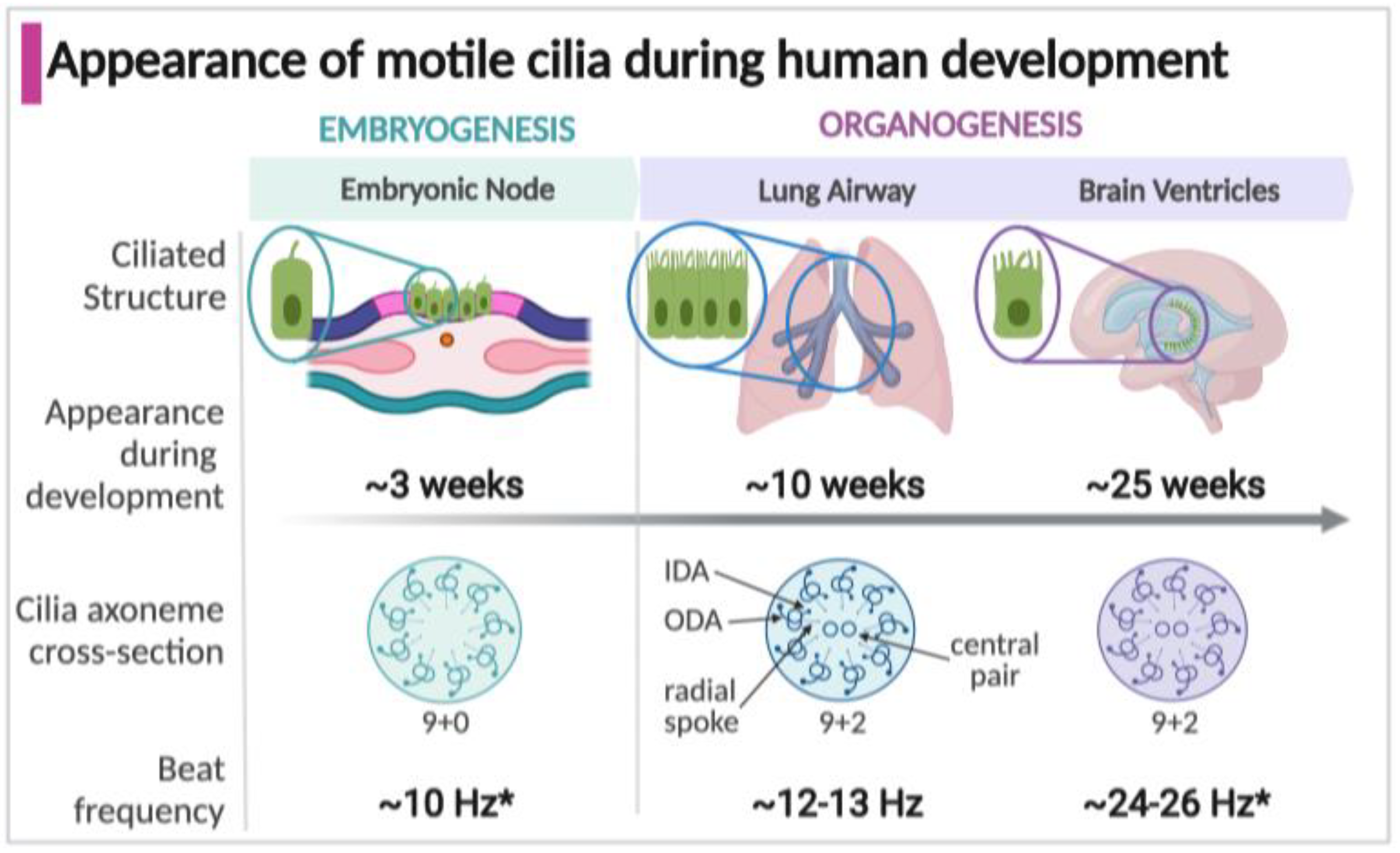
1.3. Appearance of Motile Cilia in the Human Embryo
2. Motile Cilia Disease in the Human: Primary Ciliary Dyskinesia (PCD)
3. Motile Cilia in the Embryonic Node during Human Embryogenesis
3.1. Development of Embryonic Node Motile Cilia
3.2. Left-Right Body Asymmetry
3.3. PCD Mutations Associated with Laterality Defects
3.4. Clinical Features of Laterality Defects in Neonates with PCD
3.5. Relationship of Motile Cilia and Laterality Determination with Cardiac Development
4. Motile Cilia in the Developing Human Respiratory Tract
4.1. Motile Cilia in the Lung Airway Epithelium
4.2. Development of the Motile Ciliated Airway Epithelium
4.3. Genetic Features of Airway Motile Cilia Defects in Neonates with PCD
4.4. Clinical Features of Airway Motile Cilia Defects in Neonates with PCD
4.5. Acquired Ciliary Dysfunction in the Newborn
5. Motile Cilia in the Developing Human Brain Ventricular System
5.1. Motile Cilia in the Brain Ventricular Epithelium and Cerebral Spinal Fluid Flow
5.2. Developmental Control of Motile Ciliogenesis in the Brain Ventricular Ependymal Cells
5.3. Clinical Features of Brain Ependymal Motile Cilia Dysfunction in Neonates with PCD
6. Evaluation and Management of the Newborn with Suspected PCD
6.1. Identification of the Newborn with Suspected PCD
6.2. Diagnostic Testing of the Neonate with Suspected PCD
6.3. Management of the Newborn with Suspected or Confirmed PCD
7. Conclusions and Gaps in Knowledge
- Embryonic nodal signaling is postulated but not fully defined, in particular how signaling and dysfunction in the node translates to specific cardiac laterality defects is unknown.
- In the respiratory system, it is clear that cilia are required for airway clearance, but the role of cilia in the liquid-filled lung, the effect of cilia-mediated flow on the developing lung, and exactly how motile ciliopathies cause respiratory distress at birth is not known.
- The role of ventricular motile cilia in the development of neural function, outside of the rare contribution by hydrocephalus, is unknown. Perturbations in CSF flow related to malfunctioning cilia may be insufficient to cause clinically apparent disease but may have consequences yet unknown in the developing brain.
- All motile ciliopathies are studied through the genetic disease PCD and its models, while very little is known of acquired cilia disease in neonates. The respiratory tract and brain ventricular system continue to play critical roles after development throughout adult life and an acquired ciliopathy may develop without underlying genetic disease.
Funding
Acknowledgments
Conflicts of Interest
References
- Gerdes, J.M.; Davis, E.E.; Katsanis, N. The vertebrate primary cilium in development, homeostasis, and disease. Cell 2009, 137, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Sreekumar, V.; Norris, D.P. Cilia and development. Curr. Opin. Genet. Dev. 2019, 56, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Satir, P.; Christensen, S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007, 69, 377–400. [Google Scholar] [CrossRef] [Green Version]
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Marshall, W.F. Ciliogenesis: Building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 2011, 12, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Fisher, R.L.; Bowser, S.S.; Pentecost, B.T.; Sui, H. Three-dimensional architecture of epithelial primary cilia. Proc. Natl. Acad. Sci. USA 2019, 116, 9370–9379. [Google Scholar] [CrossRef] [Green Version]
- Kurkowiak, M.; Zietkiewicz, E.; Witt, M. Recent advances in primary ciliary dyskinesia genetics. J. Med. Genet. 2015, 52, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.; Ostrowski, L.E. Motile cilia genetics and cell biology: Big results from little mice. Cell Mol. Life Sci. 2021, 78, 769–797. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Yanagisawa, H.; Kamiya, R.; Kikkawa, M. A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 2014, 346, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Stoyanova, M.; Rademacher, G.; Dutcher, S.K.; Brown, A.; Zhang, R. Structure of the Decorated Ciliary Doublet Microtubule. Cell 2019, 179, 909–922.e12. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Ohwada, N.; Takata, K. Cell biology of normal and abnormal ciliogenesis in the ciliated epithelium. Int. Rev. Cytol. 2004, 234, 101–141. [Google Scholar] [CrossRef]
- Huitorel, P. From cilia and flagella to intracellular motility and back again: A review of a few aspects of microtubule-based motility. Biol. Cell 1988, 63, 249–258. [Google Scholar] [CrossRef]
- Hirokawa, N.; Tanaka, Y.; Okada, Y.; Takeda, S. Nodal flow and the generation of left-right asymmetry. Cell 2006, 125, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Takamatsu, A.; Ishikawa, T.; Shinohara, K.; Hamada, H. Asymmetric rotational stroke in mouse node cilia during left-right determination. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2013, 87, 050701. [Google Scholar] [CrossRef] [Green Version]
- Lee, L. Riding the wave of ependymal cilia: Genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J. Neurosci. Res. 2013, 91, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Tyser, R.C.V.; Mahammadov, E.; Nakanoh, S.; Vallier, L.; Scialdone, A.; Srinivas, S. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature 2021, 600, 285–289. [Google Scholar] [CrossRef]
- Legendre, M.; Zaragosi, L.E.; Mitchison, H.M. Motile cilia and airway disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, G.J.; Driver, M.; Codd, J.; Whimster, W.F. The morphology of ciliogenesis in the developing fetal human respiratory epithelium. Pathol. Res. Pract. 1988, 183, 403–411. [Google Scholar] [CrossRef]
- Delgehyr, N.; Meunier, A.; Faucourt, M.; Bosch Grau, M.; Strehl, L.; Janke, C.; Spassky, N. Ependymal cell differentiation, from monociliated to multiciliated cells. Methods Cell Biol. 2015, 127, 19–35. [Google Scholar] [CrossRef]
- Ringers, C.; Olstad, E.W.; Jurisch-Yaksi, N. The role of motile cilia in the development and physiology of the nervous system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190156. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.J.; Howard, S.; Papadaki, L. The development of ependyma in the human fetal brain: An immunohistological and electron microscopic study. Brain Res. Dev. Brain Res. 1990, 55, 255–267. [Google Scholar] [CrossRef]
- Menard, D.; Arsenault, P. Maturation of human fetal esophagus maintained in organ culture. Anat. Rec. 1987, 217, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Spassky, N.; Meunier, A. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436. [Google Scholar] [CrossRef]
- Barberini, F.; Correr, S.; Makabe, S. Microscopical survey of the development and differentiation of the epithelium of the uterine tube and uterus in the human fetus. Ital. J. Anat. Embryol. 2005, 110, 231–237. [Google Scholar] [PubMed]
- Barberini, F.; Makabe, S.; Correr, S.; Luzi, A.; Motta, P.M. An ultrastructural study of epithelium differentiation in the human fetal fallopian tube. Acta Anat. 1994, 151, 207–219. [Google Scholar] [CrossRef]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update 2006, 12, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Saridogan, E.; Smutna, S.; Habib, A.M.; Djahanbakhch, O. The effect of ovarian steroids on epithelial ciliary beat frequency in the human Fallopian tube. Hum. Reprod. 1998, 13, 2991–2994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sironen, A.; Shoemark, A.; Patel, M.; Loebinger, M.R.; Mitchison, H.M. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell Mol. Life Sci. 2020, 77, 2029–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Saggiorato, G.; Alvarez, L.; Jikeli, J.F.; Kaupp, U.B.; Gompper, G.; Elgeti, J. Human sperm steer with second harmonics of the flagellar beat. Nat. Commun. 2017, 8, 1415. [Google Scholar] [CrossRef] [Green Version]
- Ardura-Garcia, C.; Goutaki, M.; Carr, S.B.; Crowley, S.; Halbeisen, F.S.; Nielsen, K.G.; Pennekamp, P.; Raidt, J.; Thouvenin, G.; Yiallouros, P.K.; et al. Registries and collaborative studies for primary ciliary dyskinesia in Europe. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Primers. 2020, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Stracker, T.H. Transcriptional regulation of multiciliated cell differentiation. Semin. Cell Dev. Biol. 2021, 110, 51–60. [Google Scholar] [CrossRef]
- Hjeij, R.; Lindstrand, A.; Francis, R.; Zariwala, M.A.; Liu, X.; Li, Y.; Damerla, R.; Dougherty, G.W.; Abouhamed, M.; Olbrich, H.; et al. ARMC4 mutations cause primary ciliary dyskinesia with randomization of left/right body asymmetry. Am. J. Hum. Genet. 2013, 93, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emiralioglu, N.; Taskiran, E.Z.; Kosukcu, C.; Bilgic, E.; Atilla, P.; Kaya, B.; Gunaydin, O.; Yuzbasioglu, A.; Tugcu, G.D.; Ademhan, D.; et al. Genotype and phenotype evaluation of patients with primary ciliary dyskinesia: First results from Turkey. Pediatr. Pulmonol. 2020, 55, 383–393. [Google Scholar] [CrossRef]
- Mullowney, T.; Manson, D.; Kim, R.; Stephens, D.; Shah, V.; Dell, S. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics 2014, 134, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Hjeij, R.; Onoufriadis, A.; Watson, C.M.; Slagle, C.E.; Klena, N.T.; Dougherty, G.W.; Kurkowiak, M.; Loges, N.T.; Diggle, C.P.; Morante, N.F.; et al. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am. J. Hum. Genet. 2014, 95, 257–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzaid, M.; Al-Mobaireek, K.; Almannai, M.; Mukhtar, G.; Eltahir, S.; Zafar, A.; Zada, A.P.; Alotaibi, W. Clinical and molecular characteristics of primary ciliary dyskinesia: A tertiary care centre experience. Int. J. Pediatr. Adolesc. Med. 2021, 8, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, W.; Wang, L.; Qian, L. Clinical and Genetic Spectrum of Children with Primary Ciliary Dyskinesia in China. J. Pediatr. 2020, 225, 157–165.e5. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Leigh, M.W.; Carson, J.L.; Davis, S.D.; Dell, S.D.; Ferkol, T.W.; Olivier, K.N.; Sagel, S.D.; Rosenfeld, M.; Burns, K.A.; et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax 2012, 67, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Zariwala, M.A.; Leigh, M.W.; Ceppa, F.; Kennedy, M.P.; Noone, P.G.; Carson, J.L.; Hazucha, M.J.; Lori, A.; Horvath, J.; Olbrich, H.; et al. Mutations of DNAI1 in primary ciliary dyskinesia: Evidence of founder effect in a common mutation. Am. J. Respir. Crit. Care Med. 2006, 174, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Omran, H.; Kobayashi, D.; Olbrich, H.; Tsukahara, T.; Loges, N.T.; Hagiwara, H.; Zhang, Q.; Leblond, G.; O’Toole, E.; Hara, C.; et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature 2008, 456, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Zhang, Y.; Yang, J.; Wang, Y.; Tan, H.; Wang, H.; Lei, T.; Li, X.; Zhang, X.; Xiong, W.; et al. Novel compound heterozygous DNAAF2 mutations cause primary ciliary dyskinesia in a Han Chinese family. J. Assist. Reprod. Genet. 2020, 37, 2159–2170. [Google Scholar] [CrossRef]
- Tarkar, A.; Loges, N.T.; Slagle, C.E.; Francis, R.; Dougherty, G.W.; Tamayo, J.V.; Shook, B.; Cantino, M.; Schwartz, D.; Jahnke, C.; et al. DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat. Genet. 2013, 45, 995–1003. [Google Scholar] [CrossRef]
- Knowles, M.R.; Ostrowski, L.E.; Loges, N.T.; Hurd, T.; Leigh, M.W.; Huang, L.; Wolf, W.E.; Carson, J.L.; Hazucha, M.J.; Yin, W.; et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am. J. Hum. Genet. 2013, 93, 711–720. [Google Scholar] [CrossRef] [Green Version]
- Zariwala, M.A.; Gee, H.Y.; Kurkowiak, M.; Al-Mutairi, D.A.; Leigh, M.W.; Hurd, T.W.; Hjeij, R.; Dell, S.D.; Chaki, M.; Dougherty, G.W.; et al. ZMYND10 is mutated in primary ciliary dyskinesia and interacts with LRRC6. Am. J. Hum. Genet. 2013, 93, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Antony, D.; Becker-Heck, A.; Zariwala, M.A.; Schmidts, M.; Onoufriadis, A.; Forouhan, M.; Wilson, R.; Taylor-Cox, T.; Dewar, A.; Jackson, C.; et al. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum. Mutat. 2013, 34, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Olbrich, H.; Schmidts, M.; Werner, C.; Onoufriadis, A.; Loges, N.T.; Raidt, J.; Banki, N.F.; Shoemark, A.; Burgoyne, T.; Al Turki, S.; et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am. J. Hum. Genet. 2012, 91, 672–684. [Google Scholar] [CrossRef] [Green Version]
- Knowles, M.R.; Ostrowski, L.E.; Leigh, M.W.; Sears, P.R.; Davis, S.D.; Wolf, W.E.; Hazucha, M.J.; Carson, J.L.; Olivier, K.N.; Sagel, S.D.; et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am. J. Respir. Crit. Care Med. 2014, 189, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Kott, E.; Legendre, M.; Copin, B.; Papon, J.F.; Dastot-Le Moal, F.; Montantin, G.; Duquesnoy, P.; Piterboth, W.; Amram, D.; Bassinet, L.; et al. Loss-of-function mutations in RSPH1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am. J. Hum. Genet. 2013, 93, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljassim, F.; Elmelhat, A.M. A term newborn with lung collapse since birth. Dubai Med. J. 2019, 2, 158–162. [Google Scholar] [CrossRef]
- Wallmeier, J.; Frank, D.; Shoemark, A.; Nothe-Menchen, T.; Cindric, S.; Olbrich, H.; Loges, N.T.; Aprea, I.; Dougherty, G.W.; Pennekamp, P.; et al. De Novo Mutations in FOXJ1 Result in a Motile Ciliopathy with Hydrocephalus and Randomization of Left/Right Body Asymmetry. Am. J. Hum. Genet. 2019, 105, 1030–1039. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Kaspy, K.; Daniels, M.L.A.; Stonebraker, J.R.; Nguyen, V.H.; Joyal, L.; Knowles, M.R.; Zariwala, M.A. Autosomal dominant variants in FOXJ1 causing primary ciliary dyskinesia in two patients with obstructive hydrocephalus. Mol. Genet. Genomic. Med. 2021, 9, e1726. [Google Scholar] [CrossRef]
- Boon, M.; Wallmeier, J.; Ma, L.; Loges, N.T.; Jaspers, M.; Olbrich, H.; Dougherty, G.W.; Raidt, J.; Werner, C.; Amirav, I.; et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Commun. 2014, 5, 4418. [Google Scholar] [CrossRef]
- Levin, M.; Johnson, R.L.; Stern, C.D.; Kuehn, M.; Tabin, C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 1995, 82, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, S.; Tanaka, Y.; Okada, Y.; Takeda, S.; Harada, A.; Kanai, Y.; Kido, M.; Hirokawa, N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998, 95, 829–837. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.; Somlo, S.; Makova, S.; Tian, X.; Brueckner, M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 2003, 114, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Blum, M.; Andre, P.; Muders, K.; Schweickert, A.; Fischer, A.; Bitzer, E.; Bogusch, S.; Beyer, T.; van Straaten, H.W.; Viebahn, C. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation 2007, 75, 133–146. [Google Scholar] [CrossRef]
- Little, R.B.; Norris, D.P. Right, left and cilia: How asymmetry is established. Semin. Cell Dev. Biol. 2021, 110, 11–18. [Google Scholar] [CrossRef]
- Okada, Y.; Takeda, S.; Tanaka, Y.; Belmonte, J.I.; Hirokawa, N. Mechanism of nodal flow: A conserved symmetry breaking event in left-right axis determination. Cell 2005, 121, 633–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshiba, S.; Shiratori, H.; Kuo, I.Y.; Kawasumi, A.; Shinohara, K.; Nonaka, S.; Asai, Y.; Sasaki, G.; Belo, J.A.; Sasaki, H.; et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 2012, 338, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feistel, K.; Blum, M. Three types of cilia including a novel 9+4 axoneme on the notochordal plate of the rabbit embryo. Dev. Dyn. 2006, 235, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Odate, T.; Takeda, S.; Narita, K.; Kawahara, T. 9 + 0 and 9 + 2 cilia are randomly dispersed in the mouse node. J. Electron Microsc. 2016, 65, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Tavares, B.; Jacinto, R.; Sampaio, P.; Pestana, S.; Pinto, A.; Vaz, A.; Roxo-Rosa, M.; Gardner, R.; Lopes, T.; Schilling, B.; et al. Notch/Her12 signalling modulates, motile/immotile cilia ratio downstream of Foxj1a in zebrafish left-right organizer. Elife 2017, 6, e25165. [Google Scholar] [CrossRef]
- Kramer-Zucker, A.G.; Olale, F.; Haycraft, C.J.; Yoder, B.K.; Schier, A.F.; Drummond, I.A. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development 2005, 132, 1907–1921. [Google Scholar] [CrossRef] [Green Version]
- Caspary, T.; Larkins, C.E.; Anderson, K.V. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell 2007, 12, 767–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brody, S.L.; Yan, X.H.; Wuerffel, M.K.; Song, S.K.; Shapiro, S.D. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am. J. Respir. Cell Mol. Biol. 2000, 23, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasser, R.F.; Cork, R.J. The Virtual Human Embryo Project. Digitally Reproduced Embryonic Morphology. 2011. Available online: https://www.ehd.org/virtual-human-embryo,RRID:SCR_006921 (accessed on 1 November 2021).
- Shiratori, H.; Hamada, H. The left-right axis in the mouse: From origin to morphology. Development 2006, 133, 2095–2104. [Google Scholar] [CrossRef] [Green Version]
- Hamada, H.; Meno, C.; Watanabe, D.; Saijoh, Y. Establishment of vertebrate left-right asymmetry. Nat. Rev. Genet. 2002, 3, 103–113. [Google Scholar] [CrossRef]
- Beckers, A.; Alten, L.; Viebahn, C.; Andre, P.; Gossler, A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc. Natl. Acad. Sci. USA 2007, 104, 15765–15770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minegishi, K.; Hashimoto, M.; Ajima, R.; Takaoka, K.; Shinohara, K.; Ikawa, Y.; Nishimura, H.; McMahon, A.P.; Willert, K.; Okada, Y.; et al. A Wnt5 Activity Asymmetry and Intercellular Signaling via PCP Proteins Polarize Node Cells for Left-Right Symmetry Breaking. Dev. Cell 2017, 40, 439–452.e4. [Google Scholar] [CrossRef] [Green Version]
- Cartwright, J.H.; Piro, O.; Tuval, I. Fluid-dynamical basis of the embryonic development of left-right asymmetry in vertebrates. Proc. Natl. Acad. Sci. USA 2004, 101, 7234–7239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonaka, S.; Yoshiba, S.; Watanabe, D.; Ikeuchi, S.; Goto, T.; Marshall, W.F.; Hamada, H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005, 3, e268. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.E.; Krikov, S.; Riehle-Colarusso, T.; Frias, J.L.; Belmont, J.; Anderka, M.; Geva, T.; Getz, K.D.; Botto, L.D.; National Birth Defects Prevention Study. Laterality defects in the national birth defects prevention study (1998–2007): Birth prevalence and descriptive epidemiology. Am. J. Med. Genet. A 2014, 164A, 2581–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, M.J.; Shapiro, A.J.; Kennedy, M.P. Congenital Heart Disease and Primary Ciliary Dyskinesia. Paediatr. Respir. Rev. 2016, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Davis, S.D.; Ferkol, T.; Dell, S.D.; Rosenfeld, M.; Olivier, K.N.; Sagel, S.D.; Milla, C.; Zariwala, M.A.; Wolf, W.; et al. Laterality defects other than situs inversus totalis in primary ciliary dyskinesia: Insights into situs ambiguus and heterotaxy. Chest 2014, 146, 1176–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Liu, H.; Chen, Y.; Yan, X.; Zhu, X. Rsph9 is critical for ciliary radial spoke assembly and central pair microtubule stability. Biol. Cell. 2019, 111, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Best, S.; Shoemark, A.; Rubbo, B.; Patel, M.P.; Fassad, M.R.; Dixon, M.; Rogers, A.V.; Hirst, R.A.; Rutman, A.; Ollosson, S.; et al. Risk factors for situs defects and congenital heart disease in primary ciliary dyskinesia. Thorax 2019, 74, 203–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Xia, H.; Deng, S. Genetic basis of human left-right asymmetry disorders. Expert Rev. Mol. Med. 2015, 16, e19. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.E.; Ticho, B.S.; Houde, K.; Westgate, M.N.; Holmes, L.B. Heterotaxy: Associated conditions and hospital-based prevalence in newborns. Genet. Med. 2000, 2, 157–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, S.S. Non-cardiac issues in patients with heterotaxy syndrome. Ann. Pediatr. Cardiol. 2014, 7, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Anderson, R.H.; Cohen, M.I.; Atz, A.M.; Fogel, M.; Gruber, P.J.; Lopez, L.; Rome, J.J.; Weinberg, P.M. Controversies, genetics, diagnostic assessment, and outcomes relating to the heterotaxy syndrome. Cardiol. Young 2007, 17 (Suppl. 2), 29–43. [Google Scholar] [CrossRef]
- Goutaki, M.; Halbeisen, F.S.; Barbato, A.; Crowley, S.; Harris, A.; Hirst, R.A.; Karadag, B.; Martinu, V.; Morgan, L.; O’Callaghan, C.; et al. Late Diagnosis of Infants with PCD and Neonatal Respiratory Distress. J. Clin. Med. 2020, 9, 2871. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.P.; Omran, H.; Leigh, M.W.; Dell, S.; Morgan, L.; Molina, P.L.; Robinson, B.V.; Minnix, S.L.; Olbrich, H.; Severin, T.; et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 2007, 115, 2814–2821. [Google Scholar] [CrossRef] [Green Version]
- Francis, R.J.; Christopher, A.; Devine, W.A.; Ostrowski, L.; Lo, C. Congenital heart disease and the specification of left-right asymmetry. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2102–H2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Klena, N.T.; Gabriel, G.C.; Liu, X.; Kim, A.J.; Lemke, K.; Chen, Y.; Chatterjee, B.; Devine, W.; Damerla, R.R.; et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature 2015, 521, 520–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanjundappa, R.; Kong, D.; Shim, K.; Stearns, T.; Brody, S.L.; Loncarek, J.; Mahjoub, M.R. Regulation of cilia abundance in multiciliated cells. Elife 2019, 8, e44039. [Google Scholar] [CrossRef]
- Chilvers, M.A.; Rutman, A.; O’Callaghan, C. Functional analysis of cilia and ciliated epithelial ultrastructure in healthy children and young adults. Thorax 2003, 58, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, C.; Smith, K.; Wilkinson, M.; Morgan, D.; Priftis, K. Ciliary beat frequency in newborn infants. Arch. Dis. Child. 1991, 66, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, W.V.; Lu, J. Regulation of early lung morphogenesis: Questions, facts and controversies. Development 2006, 133, 1611–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warburton, D. Overview of Lung Development in the Newborn Human. Neonatology 2017, 111, 398–401. [Google Scholar] [CrossRef] [Green Version]
- Morrisey, E.E.; Cardoso, W.V.; Lane, R.H.; Rabinovitch, M.; Abman, S.H.; Ai, X.; Albertine, K.H.; Bland, R.D.; Chapman, H.A.; Checkley, W.; et al. Molecular determinants of lung development. Ann. Am. Thorac. Soc. 2013, 10, S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Pan, J.; Driscoll, J.A.; Wisner, J.W.; Huang, T.; Gunsten, S.P.; You, Y.; Brody, S.L. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2010, 43, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Perl, A.K.; Wert, S.E.; Nagy, A.; Lobe, C.G.; Whitsett, J.A. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. USA 2002, 99, 10482–10487. [Google Scholar] [CrossRef] [Green Version]
- Burri, P.H. Fetal and postnatal development of the lung. Annu. Rev. Physiol. 1984, 46, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupnikov, M.R.; Yang, Y.; Mori, M.; Lu, J.; Cardoso, W.V. Jagged and Delta-like ligands control distinct events during airway progenitor cell differentiation. Elife 2019, 8, e50487. [Google Scholar] [CrossRef]
- Tsao, P.N.; Vasconcelos, M.; Izvolsky, K.I.; Qian, J.; Lu, J.; Cardoso, W.V. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 2009, 136, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Quigley, I.; Omran, H.; Kintner, C. Multicilin drives centriole biogenesis via E2f proteins. Genes Dev. 2014, 28, 1461–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, E.R.; Wallingford, J.B. Multiciliated cells. Curr. Biol. 2014, 24, R973–R982. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhu, L.; Zhu, Y.; Cao, J.; Li, S.; Huang, Q.; Xu, T.; Huang, X.; Yan, X.; Zhu, X. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat. Cell Biol. 2013, 15, 1434–1444. [Google Scholar] [CrossRef]
- Ruiz Garcia, S.; Deprez, M.; Lebrigand, K.; Cavard, A.; Paquet, A.; Arguel, M.J.; Magnone, V.; Truchi, M.; Caballero, I.; Leroy, S.; et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development 2019, 146, dev177428. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Nguyen, Q.P.H.; Nanjundappa, R.; Delgehyr, N.; Megherbi, A.; Doherty, R.; Thompson, J.; Jackson, C.; Albulescu, A.; Heng, Y.M.; et al. Super-Resolution Microscopy and FIB-SEM Imaging Reveal Parental Centriole-Derived, Hybrid Cilium in Mammalian Multiciliated Cells. Dev. Cell 2020, 55, 224–236.e6. [Google Scholar] [CrossRef]
- You, Y.; Huang, T.; Richer, E.J.; Schmidt, J.E.; Zabner, J.; Borok, Z.; Brody, S.L. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L650–L657. [Google Scholar] [CrossRef] [Green Version]
- Marshall, C.B.; Mays, D.J.; Beeler, J.S.; Rosenbluth, J.M.; Boyd, K.L.; Santos Guasch, G.L.; Shaver, T.M.; Tang, L.J.; Liu, Q.; Shyr, Y.; et al. p73 Is Required for Multiciliogenesis and Regulates the Foxj1-Associated Gene Network. Cell Rep. 2016, 14, 2289–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huizar, R.L.; Lee, C.; Boulgakov, A.A.; Horani, A.; Tu, F.; Marcotte, E.M.; Brody, S.L.; Wallingford, J.B. A liquid-like organelle at the root of motile ciliopathy. Elife 2018, 7, e38497. [Google Scholar] [CrossRef]
- Hosie, P.H.; Fitzgerald, D.A.; Jaffe, A.; Birman, C.S.; Rutland, J.; Morgan, L.C. Presentation of primary ciliary dyskinesia in children: 30 years’ experience. J. Paediatr. Child Health 2015, 51, 722–726. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Davis, S.D.; Polineni, D.; Manion, M.; Rosenfeld, M.; Dell, S.D.; Chilvers, M.A.; Ferkol, T.W.; Zariwala, M.A.; Sagel, S.D.; et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 197, e24–e39. [Google Scholar] [CrossRef]
- Reuter, S.; Moser, C.; Baack, M. Respiratory distress in the newborn. Pediatr. Rev. 2014, 35, 417–428, quiz 429. [Google Scholar] [CrossRef]
- Davis, S.D.; Rosenfeld, M.; Lee, H.S.; Ferkol, T.W.; Sagel, S.D.; Dell, S.D.; Milla, C.; Pittman, J.E.; Shapiro, A.J.; Sullivan, K.M.; et al. Primary Ciliary Dyskinesia: Longitudinal Study of Lung Disease by Ultrastructure Defect and Genotype. Am. J. Respir. Crit. Care Med. 2019, 199, 190–198. [Google Scholar] [CrossRef]
- Leigh, M.W.; Ferkol, T.W.; Davis, S.D.; Lee, H.S.; Rosenfeld, M.; Dell, S.D.; Sagel, S.D.; Milla, C.; Olivier, K.N.; Sullivan, K.M.; et al. Clinical Features and Associated Likelihood of Primary Ciliary Dyskinesia in Children and Adolescents. Ann. Am. Thorac. Soc. 2016, 13, 1305–1313. [Google Scholar] [CrossRef]
- Machogu, E.; Gaston, B. Respiratory Distress in the Newborn with Primary Ciliary Dyskinesia. Children 2021, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Kuek, L.E.; Lee, R.J. First contact: The role of respiratory cilia in host-pathogen interactions in the airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L603–L619. [Google Scholar] [CrossRef]
- Kumar, A.; Bhat, B.V. Epidemiology of respiratory distress of newborns. Indian J. Pediatr. 1996, 63, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.J.; Rennie, J.M.; Milton, P.J. Neonatal respiratory morbidity and mode of delivery at term: Influence of timing of elective caesarean section. Br. J. Obstet. Gynaecol. 1995, 102, 101–106. [Google Scholar] [CrossRef]
- Khasawneh, W.; Sindiani, A.; Rawabdeh, S.A.; Aleshawi, A.; Kanaan, D. Indications and Clinical Profile of Neonatal Admissions: A Cross-Sectional Descriptive Analysis from a Single Academic Center in Jordan. J. Multidiscip. Healthc. 2020, 13, 997–1006. [Google Scholar] [CrossRef]
- Hossain, T.; Kappelman, M.D.; Perez-Atayde, A.R.; Young, G.J.; Huttner, K.M.; Christou, H. Primary ciliary dyskinesia as a cause of neonatal respiratory distress: Implications for the neonatologist. J. Perinatol. 2003, 23, 684–687. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Zariwala, M.A.; Ferkol, T.; Davis, S.D.; Sagel, S.D.; Dell, S.D.; Rosenfeld, M.; Olivier, K.N.; Milla, C.; Daniel, S.J.; et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr. Pulmonol. 2016, 51, 115–132. [Google Scholar] [CrossRef]
- Hillman, N.H.; Kallapur, S.G.; Pillow, J.J.; Moss, T.J.; Polglase, G.R.; Nitsos, I.; Jobe, A.H. Airway injury from initiating ventilation in preterm sheep. Pediatr. Res. 2010, 67, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Looi, K.; Evans, D.J.; Garratt, L.W.; Ang, S.; Hillas, J.K.; Kicic, A.; Simpson, S.J. Preterm birth: Born too soon for the developing airway epithelium? Paediatr. Respir. Rev. 2019, 31, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.M.; O’Brodovich, H. Airway epithelial damage in premature infants with respiratory failure. Am. Rev. Respir. Dis. 1988, 137, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Look, D.C.; Walter, M.J.; Williamson, M.R.; Pang, L.; You, Y.; Sreshta, J.N.; Johnson, J.E.; Zander, D.S.; Brody, S.L. Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function. Am. J. Pathol. 2001, 159, 2055–2069. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.M.; Kulkarni, H.; Radhakrishnan, P.; Rutman, A.; Bankart, M.J.; Williams, G.; Hirst, R.A.; Easton, A.J.; Andrew, P.W.; O’Callaghan, C. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur. Respir. J. 2014, 43, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.; Pitt, T.; Taylor, G.; Watson, D.; MacDermot, J.; Sykes, D.; Roberts, D.; Cole, P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J. Clin. Investig. 1987, 79, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Takeda, S. Cilia in the choroid plexus: Their roles in hydrocephalus and beyond. Front. Cell Neurosci. 2015, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro-Cervello, C.; Cebrian-Silla, A.; Soriano-Navarro, M.; Garcia-Tarraga, P.; Matias-Guiu, J.; Gomez-Pinedo, U.; Molina Aguilar, P.; Alvarez-Buylla, A.; Luquin, M.R.; Garcia-Verdugo, J.M. The adult macaque spinal cord central canal zone contains proliferative cells and closely resembles the human. J. Comp. Neurol. 2014, 522, 1800–1817. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Sikand, K.; Chilvers, M.A. Analysis of ependymal ciliary beat pattern and beat frequency using high speed imaging: Comparison with the photomultiplier and photodiode methods. Cilia 2012, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, C.; Sikand, K.; Rutman, A. Respiratory and brain ependymal ciliary function. Pediatr. Res. 1999, 46, 704–707. [Google Scholar] [CrossRef]
- Bystron, I.; Blakemore, C.; Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 2008, 9, 110–122. [Google Scholar] [CrossRef]
- Spassky, N.; Merkle, F.T.; Flames, N.; Tramontin, A.D.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 2005, 25, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Meunier, A.; Sawamoto, K.; Spassky, N. Patterning and Cell Type Specification in the Developing CNS and PNS: Comprehensive Developmental Neuroscience; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 1. [Google Scholar]
- Nonami, Y.; Narita, K.; Nakamura, H.; Inoue, T.; Takeda, S. Developmental changes in ciliary motility on choroid plexus epithelial cells during the perinatal period. Cytoskeleton (Hoboken) 2013, 70, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, Z.; Kusne, Y.; Duran-Moreno, M.; Cabrales, E.; Gil-Perotin, S.; Ortiz, C.; Chen, B.; Garcia-Verdugo, J.M.; Sanai, N.; Alvarez-Buylla, A. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat. Commun. 2017, 8, 13759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzadeh, Z.; Merkle, F.T.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyrousi, C.; Arbi, M.; Pilz, G.A.; Pefani, D.E.; Lalioti, M.E.; Ninkovic, J.; Gotz, M.; Lygerou, Z.; Taraviras, S. Mcidas and GemC1 are key regulators for the generation of multiciliated ependymal cells in the adult neurogenic niche. Development 2015, 142, 3661–3674. [Google Scholar] [CrossRef] [Green Version]
- Jacquet, B.V.; Muthusamy, N.; Sommerville, L.J.; Xiao, G.; Liang, H.; Zhang, Y.; Holtzman, M.J.; Ghashghaei, H.T. Specification of a Foxj1-dependent lineage in the forebrain is required for embryonic-to-postnatal transition of neurogenesis in the olfactory bulb. J. Neurosci. 2011, 31, 9368–9382. [Google Scholar] [CrossRef] [Green Version]
- Jacquet, B.V.; Salinas-Mondragon, R.; Liang, H.; Therit, B.; Buie, J.D.; Dykstra, M.; Campbell, K.; Ostrowski, L.E.; Brody, S.L.; Ghashghaei, H.T. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development 2009, 136, 4021–4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Alvarez, G.; Daclin, M.; Shihavuddin, A.; Lansade, P.; Fortoul, A.; Faucourt, M.; Clavreul, S.; Lalioti, M.E.; Taraviras, S.; Hippenmeyer, S.; et al. Adult Neural Stem Cells and Multiciliated Ependymal Cells Share a Common Lineage Regulated by the Geminin Family Members. Neuron 2019, 102, 159–172.e7. [Google Scholar] [CrossRef] [Green Version]
- Guirao, B.; Meunier, A.; Mortaud, S.; Aguilar, A.; Corsi, J.M.; Strehl, L.; Hirota, Y.; Desoeuvre, A.; Boutin, C.; Han, Y.G.; et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 2010, 12, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Banizs, B.; Pike, M.M.; Millican, C.L.; Ferguson, W.B.; Komlosi, P.; Sheetz, J.; Bell, P.D.; Schwiebert, E.M.; Yoder, B.K. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 2005, 132, 5329–5339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlucchi, M.; de Santi, M.M.; Bertoni, E.; Spinelli, E.; Timpano, S.; Padoan, R. Ciliary aplasia associated with hydrocephalus: An extremely rare occurrence. Eur. Arch. Otorhinolaryngol. 2012, 269, 2295–2299. [Google Scholar] [CrossRef]
- Ibanez-Tallon, I.; Pagenstecher, A.; Fliegauf, M.; Olbrich, H.; Kispert, A.; Ketelsen, U.P.; North, A.; Heintz, N.; Omran, H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 2004, 13, 2133–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tully, H.M.; Dobyns, W.B. Infantile hydrocephalus: A review of epidemiology, classification and causes. Eur. J. Med. Genet. 2014, 57, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, S.; Gupta, N.; Wrensch, M.; Zhao, S.; Wu, Y.W. Prevalence of congenital hydrocephalus in California, 1991–2000. Pediatr. Neurol. 2011, 45, 67–71. [Google Scholar] [CrossRef]
- Kahle, K.T.; Kulkarni, A.V.; Limbrick, D.D., Jr.; Warf, B.C. Hydrocephalus in children. Lancet 2016, 387, 788–799. [Google Scholar] [CrossRef]
- Amirav, I.; Wallmeier, J.; Loges, N.T.; Menchen, T.; Pennekamp, P.; Mussaffi, H.; Abitbul, R.; Avital, A.; Bentur, L.; Dougherty, G.W.; et al. Systematic Analysis of CCNO Variants in a Defined Population: Implications for Clinical Phenotype and Differential Diagnosis. Hum. Mutat. 2016, 37, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Wallmeier, J.; Bracht, D.; Alsaif, H.S.; Dougherty, G.W.; Olbrich, H.; Cindric, S.; Dzietko, M.; Heyer, C.; Teig, N.; Thiels, C.; et al. Mutations in TP73 cause impaired mucociliary clearance and lissencephaly. Am. J. Hum. Genet. 2021, 108, 1318–1329. [Google Scholar] [CrossRef]
- Kosaki, K.; Ikeda, K.; Miyakoshi, K.; Ueno, M.; Kosaki, R.; Takahashi, D.; Tanaka, M.; Torikata, C.; Yoshimura, Y.; Takahashi, T. Absent inner dynein arms in a fetus with familial hydrocephalus-situs abnormality. Am. J. Med. Genet. A 2004, 129A, 308–311. [Google Scholar] [CrossRef]
- Zengin Akkus, P.; Gharibzadeh Hizal, M.; Ilter Bahadur, E.; Ozmert, E.N.; Eryilmaz Polat, S.; Ozdemir, G.; Karahan, S.; Yalcin, E.; Dogru Ersoz, D.; Kiper, N.; et al. Developmental and behavioral problems in preschool-aged primary ciliary dyskinesia patients. Eur. J. Pediatr. 2019, 178, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.G.; Horani, A.; Shapiro, A.J. Progress in Diagnosing Primary Ciliary Dyskinesia: The North American Perspective. Diagnostics 2021, 11, 1278. [Google Scholar] [CrossRef]
- Behan, L.; Dimitrov, B.D.; Kuehni, C.E.; Hogg, C.; Carroll, M.; Evans, H.J.; Goutaki, M.; Harris, A.; Packham, S.; Walker, W.T.; et al. PICADAR: A diagnostic predictive tool for primary ciliary dyskinesia. Eur. Respir. J. 2016, 47, 1103–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurland, G.; Deterding, R.R.; Hagood, J.S.; Young, L.R.; Brody, A.S.; Castile, R.G.; Dell, S.; Fan, L.L.; Hamvas, A.; Hilman, B.C.; et al. An official American Thoracic Society clinical practice guideline: Classification, evaluation, and management of childhood interstitial lung disease in infancy. Am. J. Respir. Crit. Care Med. 2013, 188, 376–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, C.R.; Scherer, S.W.; Zariwala, M.A.; Lau, L.; Paton, T.A.; Stockley, T.; Jobling, R.K.; Ray, P.N.; Knowles, M.R.; Consortium, F.C.; et al. Whole-Exome Sequencing and Targeted Copy Number Analysis in Primary Ciliary Dyskinesia. G3 (Bethesda) 2015, 5, 1775–1781. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.A.; Gove, K.; Walker, W.; Lucas, J.S. Nasal nitric oxide screening for primary ciliary dyskinesia: Systematic review and meta-analysis. Eur. Respir. J. 2014, 44, 1589–1599. [Google Scholar] [CrossRef]
- Leigh, M.W.; Hazucha, M.J.; Chawla, K.K.; Baker, B.R.; Shapiro, A.J.; Brown, D.E.; Lavange, L.M.; Horton, B.J.; Qaqish, B.; Carson, J.L.; et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann. Am. Thorac. Soc. 2013, 10, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Buechel, F.; Usemann, J.; Aline, A.; Salfeld, P.; Moeller, A.; Jung, A. Feasibility of nasal NO screening in healthy newborns. Pediatr. Pulmonol. 2021, 57, 231–238. [Google Scholar] [CrossRef]
- Paff, T.; Omran, H.; Nielsen, K.G.; Haarman, E.G. Current and Future Treatments in Primary Ciliary Dyskinesia. Int. J. Mol. Sci. 2021, 22, 9834. [Google Scholar] [CrossRef]
- El-Abiad, N.M.; Clifton, S.; Nasr, S.Z. Long-term use of nebulized human recombinant DNase1 in two siblings with primary ciliary dyskinesia. Respir. Med. 2007, 101, 2224–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbato, A.; Frischer, T.; Kuehni, C.E.; Snijders, D.; Azevedo, I.; Baktai, G.; Bartoloni, L.; Eber, E.; Escribano, A.; Haarman, E.; et al. Primary ciliary dyskinesia: A consensus statement on diagnostic and treatment approaches in children. Eur. Respir. J. 2009, 34, 1264–1276. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.Y.; Park, Y.; Jeong, J.H.; Kim, C.K.; Min, Y.G.; Chi, J.G. The effect of regular salbutamol on lung function and bronchial responsiveness in patients with primary ciliary dyskinesia. Chest 2000, 117, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Bassler, D.; Shinwell, E.S.; Hallman, M.; Jarreau, P.H.; Plavka, R.; Carnielli, V.; Meisner, C.; Engel, C.; Koch, A.; Kreutzer, K.; et al. Long-Term Effects of Inhaled Budesonide for Bronchopulmonary Dysplasia. N. Engl. J. Med. 2018, 378, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.E.; Thomas, S.; Heather, S.; Bush, A. Airway response of children with primary ciliary dyskinesia to exercise and beta2-agonist challenge. Eur. Respir. J. 1998, 11, 1389–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joskova, M.; Mokry, J.; Franova, S. Respiratory Cilia as a Therapeutic Target of Phosphodiesterase Inhibitors. Front. Pharmacol. 2020, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Wright, Z.; Larrew, T.W.; Eskandari, R. Pediatric Hydrocephalus: Current State of Diagnosis and Treatment. Pediatr. Rev. 2016, 37, 478–490. [Google Scholar] [CrossRef]
- Paes, B.; Carbonell-Estrany, X. Respiratory syncytial virus prophylaxis for children with chronic lung disease: Have we got the criteria right? Expert Rev. Anti. Infect. Ther. 2019, 17, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Kobbernagel, H.E.; Buchvald, F.F.; Haarman, E.G.; Casaulta, C.; Collins, S.A.; Hogg, C.; Kuehni, C.E.; Lucas, J.S.; Moser, C.E.; Quittner, A.L.; et al. Efficacy and safety of azithromycin maintenance therapy in primary ciliary dyskinesia (BESTCILIA): A multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir. Med. 2020, 8, 493–505. [Google Scholar] [CrossRef]
- Kreicher, K.L.; Schopper, H.K.; Naik, A.N.; Hatch, J.L.; Meyer, T.A. Hearing loss in children with primary ciliary dyskinesia. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Behan, L.; Rubbo, B.; Lucas, J.S.; Dunn Galvin, A. The patient’s experience of primary ciliary dyskinesia: A systematic review. Qual. Life Res. 2017, 26, 2265–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Motile Cilia-Containing Structures during Development | Developmental Function | Clinical Complication of Motile Ciliopathies in the Newborn |
|---|---|---|
| Embryonic node Nodal pit cells | Laterality determination Cardiac formation Visceral organ position | Situs anomalies Congenital heart disease |
| Respiratory tract Airway epithelial cells | Directed airway fluid movement | Respiratory distress of the newborn Lung atelectasis |
| Brain ventricles Ependymal cells | Directed cerebral spinal fluid movement | Ventricular enlargement Hydrocephalus |
| Functional Class and Gene | Percent of PCD Caused by the Pathogenic Variant | Neonatal Respiratory Distress | Laterality Defect | Hydrocephalus | Ref |
|---|---|---|---|---|---|
| Outer dynein arm | |||||
| ARMC4 (ODAD2) | <3% | Yes | Yes | NR | [35,36] |
| CCDC103 | <4% | Yes | Yes | NR | [36,37] |
| CCDC151 (ODAD3) | <3% | Yes | Yes | Yes | [38] |
| DNAH1 | <1% | Yes | Yes | NR | [36] |
| DNAH5 | 15–29% | Yes | Yes | NR | [36,37,39,40] |
| DNAH11 | 6–9% | Yes | Yes | NR | [37,40,41] |
| DNAI1 | 2–10% | Yes | Yes | NR | [36,39,42] |
| TTC25 | <1% | Yes | Yes | NR | [36] |
| Dynein axonemal assembly factor | |||||
| DNAAF2 (KTU) | <1% | Yes | Yes | NR | [37,43,44] |
| DNAAF3 | <1% | Yes | Yes | NR | [39,40] |
| DNAAF4 (DYX1C1) | <1% | Yes | Yes | NR | [39,45] |
| LRRC6 (DNAAAF11) | <1% | Yes | Yes | NR | [37] |
| SPAG1 | <4% | Yes | Yes | NR | [37,46] |
| ZMYND10 | <2–4% | Yes | Yes | NR | [47] |
| Ruler protein | |||||
| CCDC39 | 4–9% | Yes | Yes | NR | [36,37,48] |
| CCDC40 | 3–4% | Yes | Yes | NR | [36,37,48] |
| Central Pair | |||||
| HYDIN | <1% | Yes | NR | NR | [36,40,49] |
| Radial Spoke | |||||
| RSPH1 | <1% | Yes (rare) | NR | NR | [50,51] |
| RSPH4A | <1% | Yes | NR | NR | [36] |
| Multiciliogenesis | |||||
| CCNO | <1% | Yes | NR | Yes | [36,37,52] |
| FOXJ1 | <1% | Yes | Yes | Yes | [53,54] |
| MCIDAS | <1% | Yes | NR | Yes | [55] |
| Criteria for Neonatal Evaluation of PCD |
|
| Diagnostic Testing for PCD in the Neonatal Period |
|
| System | Workup |
|---|---|
| Respiratory | Chest X-ray |
| Cardiac | Echocardiogram |
| Gastrointestinal | Abdominal ultrasound (with attention to visceral organ laterality, splenic abnormalities) |
| Neurologic | Occipital frontal head circumference (OFC) Head ultrasound |
| Genetic | Genetic testing for cystic fibrosis, immunodeficiency, surfactant deficiency Comprehensive PCD genetic panel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyland, R.M.; Brody, S.L. Impact of Motile Ciliopathies on Human Development and Clinical Consequences in the Newborn. Cells 2022, 11, 125. https://doi.org/10.3390/cells11010125
Hyland RM, Brody SL. Impact of Motile Ciliopathies on Human Development and Clinical Consequences in the Newborn. Cells. 2022; 11(1):125. https://doi.org/10.3390/cells11010125
Chicago/Turabian StyleHyland, Rachael M., and Steven L. Brody. 2022. "Impact of Motile Ciliopathies on Human Development and Clinical Consequences in the Newborn" Cells 11, no. 1: 125. https://doi.org/10.3390/cells11010125
APA StyleHyland, R. M., & Brody, S. L. (2022). Impact of Motile Ciliopathies on Human Development and Clinical Consequences in the Newborn. Cells, 11(1), 125. https://doi.org/10.3390/cells11010125






