Tuber Yield Formation and Sugar Composition of Yacon Genotypes Grown in Central Europe
Abstract
1. Introduction
2. Materials and Methods
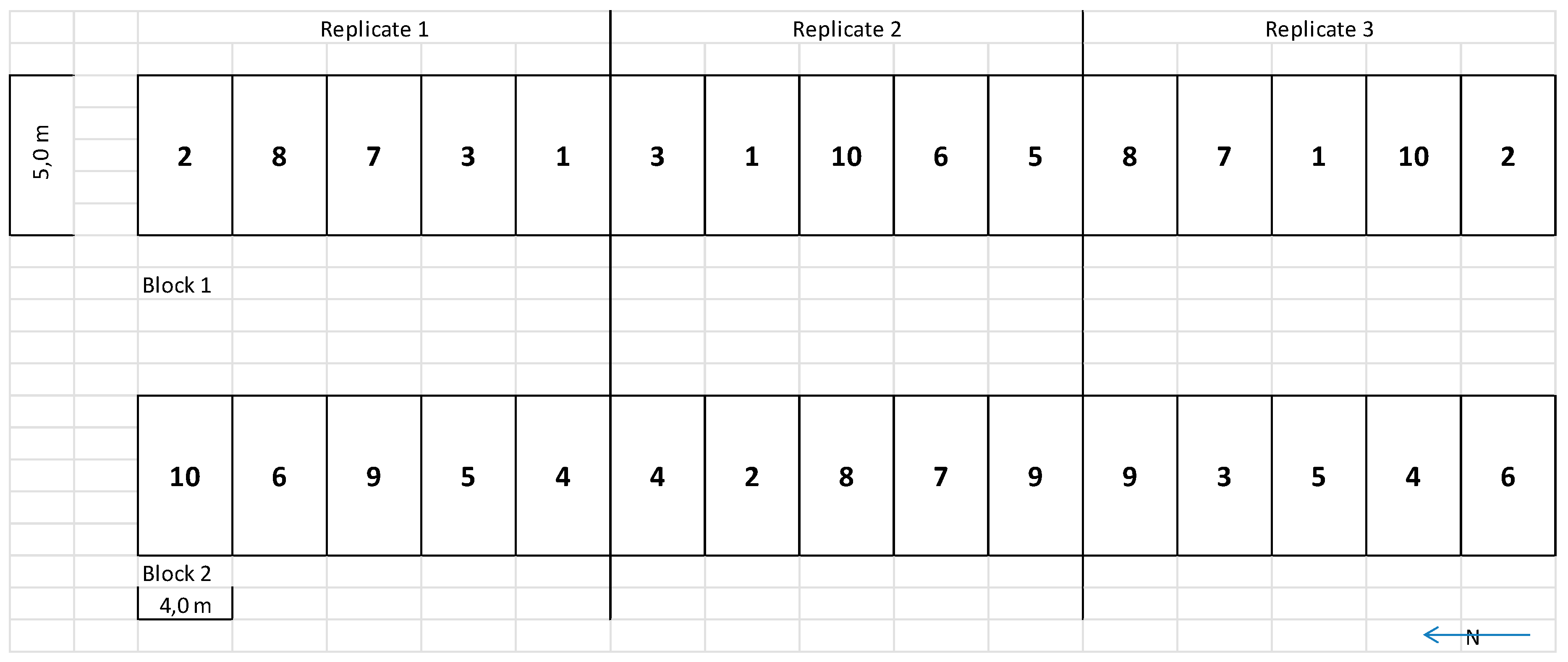
2.1. Field Site and Experimental Design
2.2. Treatments
2.3. Field Measurements and Sample Preparation
2.4. Statistical Analysis
3. Results
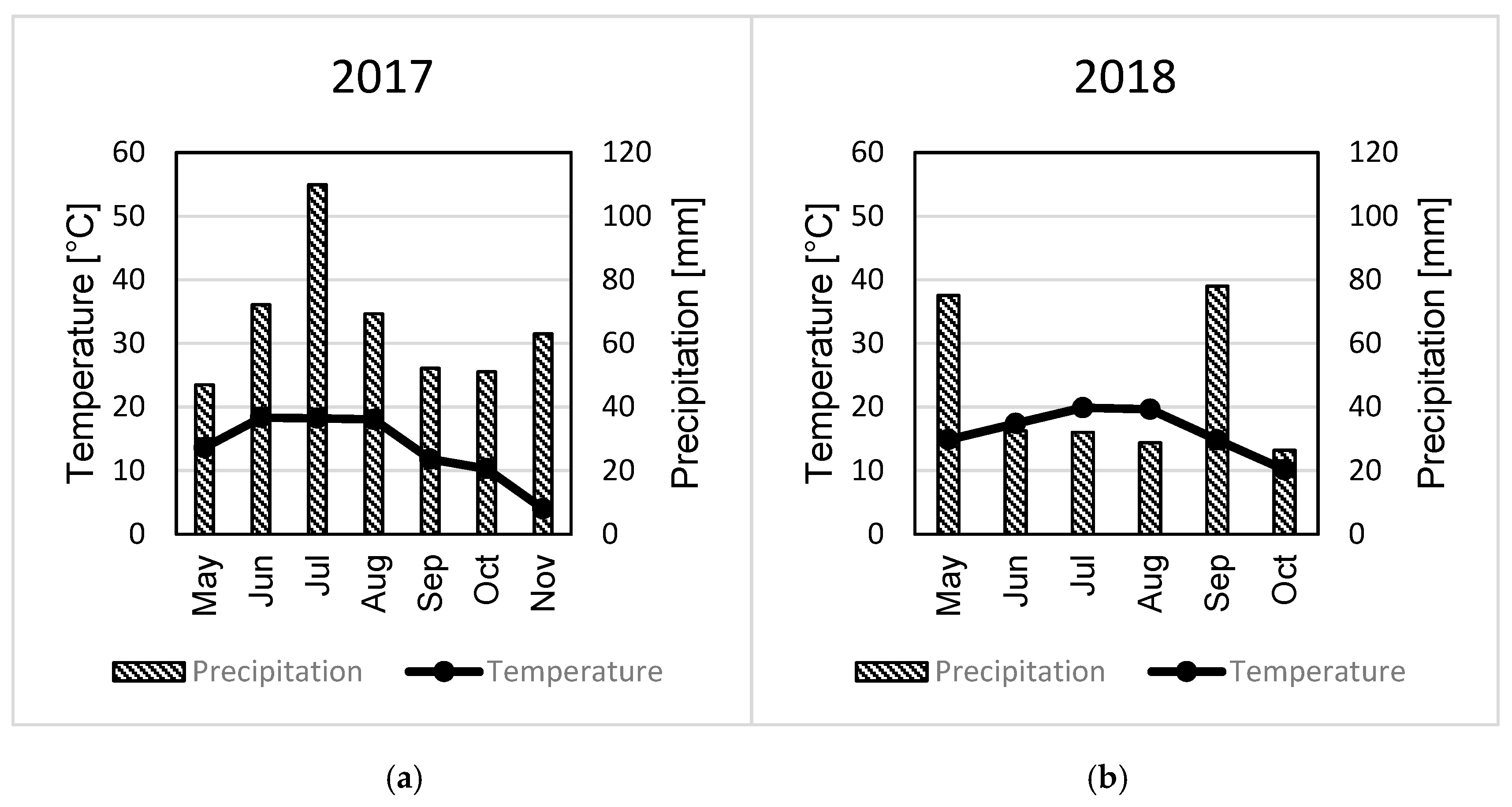
3.1. Tuber Yield Formation
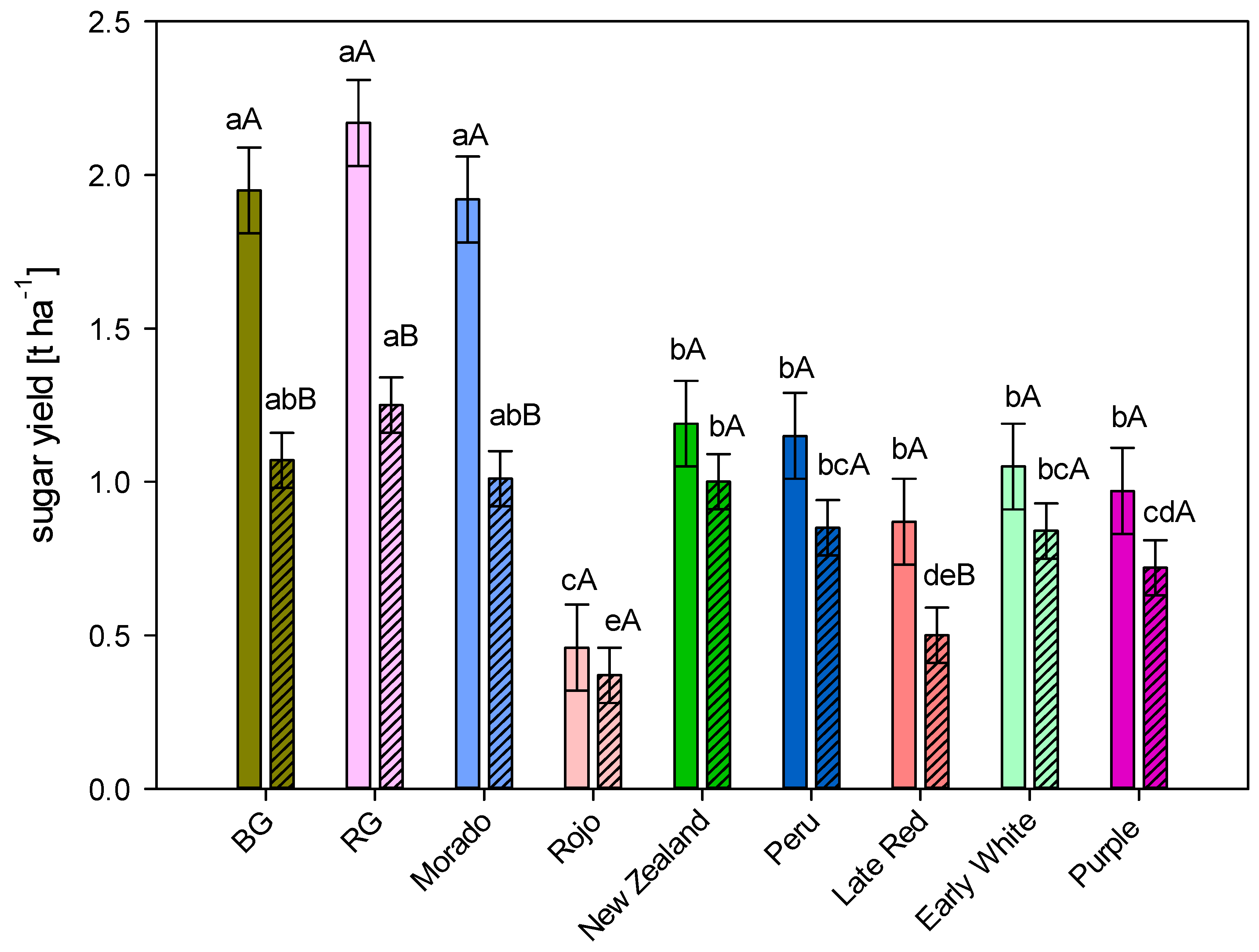
3.2. Sugar Yield
3.3. Sugar Composition
4. Discussion
4.1. Tuber Yields
4.2. Sugar Yield
4.3. Sugar Composition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Genotype | Single Tuber Weight (g) | Glucose (%) | Sucrose (%) | GF3 (%) | Total FOS (%) |
|---|---|---|---|---|---|
| ’BG’ | 267.4a | 3.29ab | 5.12a | 16.29ab | 40.72a |
| ‘RG‘ | 223.7ac | 1.71c | 3.24bc | 18.56a | 41.37a |
| ‘Morado’ | 205.9bc | 2.38bc | 2.98bc | 16.04ab | 37.04a |
| ‘Rojo’ | 172.1cd | 3.71a | 3.04bc | 6.55c | 17.83b |
| ‘New Zealand’ | 175.3cd | 2.19c | 2.58c | 13.30b | 30.61a |
| ‘Peru’ | 231.8ab | 4.56a | 5.44a | 15.16ab | 39.04a |
| ‘Late Red’ | 144.8d | 1.50c | 2.52c | 16.42ab | 35.60a |
| ‘Early White’ | 223.7ac | 3.67a | 4.10ab | 15.48ab | 34.63a |
| ‘Purple’ | 185.3bcd | 2.21bc | 3.22bc | 14.30ab | 31.05a |
| Factor | DF | p-Value for the F-test of the Corresponding Factor |
|---|---|---|
| Year × Rep | 4 | 0.1666 |
| G | 8 | <0.0001 |
| Year | 1 | 0.0002 |
| Year × G | 8 | 0.0001 |
References
- Hermann, M. Andean Roots and Tubers: Ahipa, Arracacha, Maca and Yacon; International Plant Genetic Resources Institute: Rome, Italy, 1997; Volume 21, pp. 191–243. [Google Scholar]
- Ohyama, T.; Ito, O.; Yasuyoshi, S.; Ikarashi, T.; Minamisawa, K.; Kubota, M.; Tsukihashi, T.; Asami, T. Composition of storage carbohydrate in tubers of yacon (Polymnia sonchifolia). Soil Sci. Plant Nutr. 1990, 36, 167–171. [Google Scholar] [CrossRef]
- Bredemann, G. Über Polymnia Sonchifolia Poepp. et Endl. (P. edulis Wedd.), die Yacon - Erdbirne; Botanische Oeconomie: Hamburg, Germany, 1948; pp. 65–85. [Google Scholar]
- Zardini, E. Ethnobotanical notes on “Yacon” Polymnia sonchifolia (Asteraceae). Econ. Bot. 1991, 40, 72–85. [Google Scholar] [CrossRef]
- Fernández, E.C.; Viehmannova, I.; Bechyne, M.; Lachman, J.; Milella, L. The cultivation and phenological growth stage of yacon [Smallanthus Sonchifolius (Poepp. et Endl.) H. Robinson]. Agric. Trop. Et. Subtrop. 2007, 40, 71–77. [Google Scholar]
- Delgado, G.T.C.; Tamashiro, W.M.d.S.C.; Marostica, M.R., Jr.; Pastore, G.M. Yacon (Smallanthus sonchifolius): A functional food. Plant Foods Hum. Nutr. 2013, 68, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.A.; Follett, J.M.; Douglas, M.H.; Deo, B.; Scheffer, J.J.C.; Littler, R.A.; Manley-Harris, M. Effect of environment and time of planting on the production and quality of yacon (Smallanthus sonchifolius) storage roots. N. Z. J. Crop. Hor. Sci. 2007, 35, 107–116. [Google Scholar] [CrossRef]
- Valentova, K.; Lebeda, A.; Dolezalova, I.; Jirovsky, D.; Simonovska, B.; Vovk, I.; Kosina, P.; Gasmanova, N.; Dziechciarkova, M.; Ulrichova, J. The biological and chemical variability of yacon. J. Agric. Food Chem. 2006, 54, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Manrique, I.; Parraga, A.; Hermann, M. Yacon Syrup: Principles and Processing. Available online: http://cipotato.org/wp-content/uploads/2014/07/1919-Yacon_Syrup.pdf (accessed on 19 July 2014).
- Graefe, S.; Hermann, M.; Manrique, I.; Golombek, S.; Buerkert, A. Effects of post-harvest treatments on the carbohydrate composition of yacon roots in the Peruvian Andes. Field Crop. Res. 2004, 86, 157–165. [Google Scholar] [CrossRef]
- Lachman, J.; Fernández, E.C.; Orsák, M. Yacon [Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson] chemical composition and use – a review. Plant Soil Environ. 2003, 49, 283–290. [Google Scholar] [CrossRef]
- Goto, K.; Fukai, K.; Hikida, J.; Nanjo, F.; Hara, Y. Isolation and Structural Analysis of Oligosaccharides from Yacon (Polymnia sonchifolia). Biosci. Biotechnol. Biochem. 2014, 59, 2346–2347. [Google Scholar] [CrossRef]
- de Almeida Paula, H.A.; Abranches, M.V.; de Luces Fortes Ferreira, C.L. Yacon (Smallanthus sonchifolius): A food with multiple functions. Crit. Rev. Food Sci. Nutr. 2015, 55, 32–40. [Google Scholar] [CrossRef]
- Campos, D.; Betalleluz-Pallardel, I.; Chirinos, R.; Aguilar-Galvez, A.; Noratto, G.; Pedreschi, R. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012, 135, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Fukai, K.; Miyazaki, S.; Nanjo, F.; Hara, Y. Distribution of carbohydrates and related enzyme activities in yacon (Polymnia Sonchifolia). Soil Sci. Plant Nutr. 1993, 39, 567–571. [Google Scholar] [CrossRef]
- Campbell, J.M.; Bauer, L.L.; Fahey, G.C.; Hogarth, A.J.C.L.; Wolf, B.W.; Hunter, D.E. Selected Fructooligosaccharide (1-Kestose, Nystose, and 1 F -β-Fructofuranosylnystose) Composition of Foods and Feeds. J. Agric. Food Chem. 1997, 45, 3076–3082. [Google Scholar] [CrossRef]
- Slimestad, R.; Seljaasen, R.; Meijer, K.; Skar, S.L. Norwegian-grown Jerusalem artichoke (Helianthus tuberosus L.): morphology and content of sugars and fructo-oligosaccharides in stems and tubers. J. Sci. Food Agric. 2010, 90, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.A. Maximising fructo-oligosacharide production in yacon. Agron. N. Z. 2002, 32, 49–55. [Google Scholar]
- de Andrade, E.F.; Leone, R.d.S.; Ellendersen, L.N.; Masson, M.L. Phenolic profile and antioxidant activity of extracts of leaves and flowers of yacon (Smallanthus sonchifolius). Ind. Crop. Prod. 2014, 62, 499–506. [Google Scholar] [CrossRef]
- Leidi, E.O.; Altamirano, A.M.; Mercado, G.; Rodriguez, J.P.; Ramos, A.; Alandia, G.; Sørensen, M.; Jacobsen, S.-E. Andean roots and tubers crops as sources of functional foods. J. Funct. Foods 2018, 51, 86–93. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006. Ein Rahmen für Internationale Klassifikation, Korrelation und Kommunikation. Erstes Update 2007. Deutsche Ausgabe; BGR: Hannover, Germany, 2008; ISBN 978-3-00-024824-5. [Google Scholar]
- Kamp, L.; Hartung, J.; Mast, B.; Graeff-Hönninger, S. Plant growth, tuber yield formation and costs of three different propagation methods of yacon (Smallanthus sonchifolius). Ind. Crop. Prod. 2019, 132, 1–11. [Google Scholar] [CrossRef]
- Kamp, L.; Hartung, J.; Mast, B.; Graeff-Hönninger, S. Impact of Nitrogen Fertilization on Tuber Yield, Sugar Composition and Nitrogen Uptake of Two Yacon (Smallanthus Sonchifolius Poepp. & Endl.) Genotypes. Agronomy 2019, 9, 151. [Google Scholar] [CrossRef]
- Fernández, E.C.; Viehmannová, I.; Bechyne, M.; Lachman, J.; Milella, L. Yacon [Smallanthus sonchifolius (Poeppig & Endlicher) H. Robinson]: A new crop in the Central Europe. Plant Soil Environ. 2006, 12, 564–570. [Google Scholar]
- Leemans, R.; Cramer, W. The IIASA Database for Mean Monthly Values of Temperature, Precipitation and Cloudiness on a Global Terrestrial Grid; Research Report; IIASA: Laxenburg, Austria, 1991; Volume 61, p. RR-91-018. [Google Scholar]
- Doo, H.S. Response of Different Seedlings to Growth and Yield in Yacon. Korean J. Crop Sci. 2002, 5, 356–360. [Google Scholar]
- Doo, H.S. Effect of Plant Density on Growth Response and Yield in Yacon. Korean J. Crop Sci. 2001, 10, 407–410. [Google Scholar]
- Kim, S.J. Domestic and Overseas Status and Future Trends of Yacon Production. Korean J. Int. Agric. 2012, 24, 417–424. [Google Scholar]
- Koike, A.; Murata, T.; Matsuda, Y.; Masuoka, C.; Okamoto, C.; Kabata, K. Cultivation and ensilage of yacon plants (Smallanthus sonchifolius [Poepp. & Endl.] H. Robinson) and the function of yacon silage. Grassl. Sci. 2009, 55, 6–10. [Google Scholar] [CrossRef]
- Cabrera, M.V. Manual del cultivo de yacon. Productores y mercados Del. Agro. De. La. Sierra. 2005. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=11&ved=2ahUKEwjIsLj8wN7iAhUF3aQKHceUBHgQFjAKegQIABAC&url=http%3A%2F%2Fwww.asocam.org%2Fsites%2Fdefault%2Ffiles%2Fpublicaciones%2Ffiles%2F74455093814a213d6976637f4f71ad5f.pdf&usg=AOvVaw3gvGyIMlYKr97e2OlelbDn (accessed on 10 June 2019).
- Losavio, N.; Lamascese, N.; Vonella, A.V. Water requirements and nitrogen fertilization in jerusalem artichoke (Helianthus tuberosus L.) grown under mediterranean conditions. Acta Hortic. 1997, 1, 205–209. [Google Scholar] [CrossRef]
- Müller, S.; Beer, K. The relationships between soil inorganic nitrogen levels and nitrogen fertilizer requirements. Agric. Ecosyst. Environ. 1986, 17, 199–211. [Google Scholar] [CrossRef]
- Hermann, M.; Freire, I.; Pazos, C. Compositional Diversity of the Yacon Storage Root. Impact Chang. World Program Rep. 1997, 98, 425–432. [Google Scholar]
- de Mastro, G.; Manolio, G.; Marzi, V. Jerusalem artichoke (Helianthus tuberosus L.) and chicory (Chicorium intybus L.): Potential crops for inulin production in the mediterranean area. Acta Hortic. 2004, 365–374. [Google Scholar] [CrossRef]
- FAO. Standard for Aubergines. 2018. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 10 June 2019).
- Brisco; Gracia. STANDARD FOR SWEET CASSAVA. CODEX STAN 238-2003. 2003. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 10 June 2019).
- FAO. Regional Standards for Yacon. 2017. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 10 June 2019).
- Onder, S.; Caliskan, M.E.; Onder, D.; Caliskan, S. Different irrigation methods and water stress effects on potato yield and yield components. Agric. Water Mgmt. 2005, 73, 73–86. [Google Scholar] [CrossRef]
- Bélanger, G.; Walsh, J.R.; Richards, J.E.; Milburn, P.H.; Ziadi, N. Nitrogen fertilization and irrigation affects tuber characteristics of two potato cultivars. Am. J. Potato Res. 2002, 79, 269–279. [Google Scholar] [CrossRef]
- Polreich, S. Establishment of a Classification Scheme to structure the Establishment of a Classification Scheme to structure the Post-Harvest Diversity of Yacon Storage Roots (Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson). Master’ Thesis, University of Kassel, Kassel, Germany, 2003. [Google Scholar]
- Douglas, J.A. Research and Development of Yacon Production in New Zealand. Acta Hortic. 2005, 670, 79–85. [Google Scholar] [CrossRef]
- Meijer, W.J.M.; Mathijssen, E.W.J.M.; Borm, G.E.L. Crop characteristics and inulin production of jerusalem artichoke and chicory. Stud. Plant Sci. 1993, 1991, 29–38. [Google Scholar]
- FAO. Ecuador Country Profile. Available online: http://www.fao.org/countryprofiles/index/en/?iso3 = ECU (accessed on 10 April 2019).
- FAO. Peru Country Profile. Available online: http://www.fao.org/countryprofiles/index/en/?iso3 = PER (accessed on 10 April 2019).
- Sprague, H.B.; Farris, N.F.; Colby, W.G. Effect of Soil Conditions and Treatment on Yields of Tubers and Sugar from the American Artichoke (Helianthus tuberosus). J. Am. Soc. Agrono. 1935, 392–399. [Google Scholar] [CrossRef]
- Delgado, G.T.C.; Thomé, R.; Gabriel, D.L.; Tamashiro, M.S.C.; Pastore, G.M. Yacon (Smallanthus sonchifolius)-derived fructooligosaccharides improves the immune parameters in the mouse. Nutr. Res. 2012, 32, 884–892. [Google Scholar] [CrossRef]
- Lebeda, A.; Dolezalova, I.; Valentova, K.; Gasmanova, N.; Dziechciarkova, M.; Ulrichova, J. Yacon (Smallanthus sonchifolius) - A traditional crop of the Andean Indians as a challenge for the future - The news about biological variation and chemical substances content. Acta Hortic. 2008, 127–136. [Google Scholar] [CrossRef]
- Khajehei, F.; Merkt, N.; Claupein, W.; Graeff-Hoenninger, S. Yacon (Smallanthus sonchifolius Poepp. & Endl.) as a Novel Source of Health Promoting Compounds: Antioxidant Activity, Phytochemicals and Sugar Content in Flesh, Peel, and Whole Tubers of Seven Cultivars. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Doo, H.S. Changes of chemical composition in tuberous root of yacon by different curing conditions. Korean J. Crop. Sci. 2000, 45, 79–82. [Google Scholar]
- Minamisawa, K. Fluctuations of oligofructan contents in tubers of yacon (Polymnia sonchifolia) during growth and storage. Jpn. Soc. Soil Sci. Plant Nutr. 1991, 43, 621–627. [Google Scholar]
| Genotype | Tuber Yield (t ha−1 FM) | DM (%) | Tuber Yield (t ha−1 DM) | Number of Tubers (per Plant) | Tuber Weight (g) | |
|---|---|---|---|---|---|---|
| 2017 | ||||||
| BG | 43.54aA ± 3.3 | 11.01efB ± 0.5 | 4.68bA ± 0.4 | 11.1bcA ± 1.0 | 314.00 ± 29.4 | |
| RG | 46.60aA ± 3.3 | 16.14aA ± 0.5 | 7.59aA ± 0.4 | 14.8aA ± 1.0 | 265.32 ± 29.4 | |
| Morado | 41.66aA ± 3.3 | 15.50abA ± 0.5 | 6.44aA ± 0.4 | 14.2aA ± 1.0 | 236.34 ± 29.3 | |
| Rojo | 22.94bcA ± 3.3 | 11.35eB ± 0.5 | 2.67dA ± 0.4 | 7.8dA ± 1.0 | 252.75 ± 29.4 | |
| New Zealand | 30.66bA ± 3.3 | 13.41cdB ± 0.5 | 4.08bcA ± 0.4 | 13.1abA ± 1.0 | 197.7 ± 29.4 | |
| Peru | 27.16bcA ± 3.3 | 9.40fB ± 0.5 | 2.58dA ± 0.4 | 8.5cdA ± 1.0 | 288.17 ± 29.7 | |
| Late Red | 19.21cA ± 3.3 | 14.23bcA ± 0.5 | 2.76dA ± 0.4 | 9.3cdA ± 1.0 | 174.9 ± 29.4 | |
| Early White | 23.71bcA ± 3.3 | 12.43deA ± 0.5 | 2.97cdA ± 0.4 | 8.3dA ± 1.0 | 231.9 ± 29.4 | |
| Purple | 21.91bcA ± 3.3 | 14.39bcB ± 0.5 | 3.10cdA ± 0.4 | 9.7cdA ± 1.0 | 202.99 ± 30.1 | |
| 2018 | ||||||
| BG | 12.75aB ± 1.0 | 14.03cA ± 0.6 | 1.76bcB ± 0.2 | 5.2cB ± 0.6 | 220.72 ± 26.6 | |
| RG | 11.75aB ± 1.0 | 16.03bA ± 0.6 | 1.89abB ± 0.2 | 5.6bcB ± 0.6 | 182.01 ± 26.0 | |
| Morado | 10.85aB ± 1.0 | 17.03abA ± 0.6 | 1.84abB ± 0.2 | 5.8bcB ± 0.6 | 175.48 ± 26.0 | |
| Rojo | 6.55bB ± 1.0 | 13.46cdA ± 0.6 | 0.88eB ± 0.2 | 6.8abA ± 0.6 | 91.43 ± 26.6 | |
| New Zealand | 12.36aB ± 1.0 | 17.71aA ± 0.6 | 2.22aB ± 0.2 | 7.9aB ± 0.6 | 152.8 ± 27.4 | |
| Peru | 11.36aB ± 1.0 | 12.02dA ± 0.6 | 1.35cdB ± 0.2 | 5.7bcB ± 0.6 | 175.38 ± 26.0 | |
| Late Red | 6.46bB ± 1.0 | 15.84bA ± 0.6 | 1.01deB ± 0.2 | 5.1cB ± 0.6 | 114.73 ± 26.0 | |
| Early White | 11.56aB ± 1.0 | 13.59cdA ± 0.6 | 1.57bcB ± 0.2 | 5.7bcB ± 0.6 | 215.45 ± 26.0 | |
| Purple | 10.06aB ± 1.0 | 16.49abA ± 0.6 | 1.66bcB ± 0.2 | 5.7bcB ± 0.6 | 167.6 ± 26.0 | |
| Results of statistical analysis | ||||||
| Factor | DF | p-value for the corresponding F test | ||||
| Year*Rep | 4 | 0.4384 | 0.0187 | 0.4352 | 0.9208 | 0.9241 |
| G | 1 | <0.0001 | 0.9366 | <0.0001 | <0.0001 | 0.0003 |
| year | 1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0032 |
| Year*G | 1 | <0.0001 | 0.0020 | <0.0001 | <0.0001 | 0.2237 |
| Genotype | Fructose | Glucose | Sucrose | GF2 | GF3 | GF4 | Total FOS | Total Sugars | |
|---|---|---|---|---|---|---|---|---|---|
| 2017 | |||||||||
| BG | 3.84bA ± 0.6 | 4.04 ± 0.6 | 4.00 ± 0.2 | 9.61 ± 0.9 | 13.21 ± 1.3 | 7.96acA ± 0.9 | 31.23 ± 2.9 | 41.43 ± 2.3 | |
| RG | 0.86cA ± 0.6 | 1.36 ± 0.6 | 1.97 ± 0.2 | 5.24 ± 0.9 | 11.21 ± 1.3 | 7.93acB ± 0.9 | 24.48 ± 3.0 | 28.67 ± 2.3 | |
| Morado | 1.13cA ± 0.6 | 2.58 ± 0.6 | 2.14 ± 0.2 | 5.10 ± 0.9 | 11.12 ± 1.3 | 8.98aB ± 0.9 | 25.58 ± 2.9 | 39.85 ± 2.3 | |
| Rojo | 5.61aA ± 0.6 | 3.97 ± 0.6 | 1.33 ± 0.2 | 1.30 ± 0.9 | 1.71 ± 1.3 | 2.47dB ± 0.9 | 5.05 ± 2.9 | 17.63 ± 2.3 | |
| New Zealand | 2.52bcA ± 0.6 | 3.08 ± 0.6 | 2.36 ± 0.2 | 5.19 ± 0.9 | 8.81 ± 1.3 | 6.78acB ± 0.9 | 20.68 ± 3.0 | 28.62 ± 2.3 | |
| Peru | 5.91aA ± 0.6 | 5.54 ± 0.6 | 4.34 ± 0.2 | 10.12 ± 0.9 | 12.23 ± 1.3 | 6.26cA ± 0.9 | 28.72 ± 3.0 | 44.52 ± 2.3 | |
| Late Red | 1.84cA ± 0.6 | 1.62 ± 0.6 | 1.98 ± 0.2 | 4.90 ± 0.9 | 12.61 ± 1.3 | 8.68abA ± 0.9 | 26.31 ± 3.0 | 31.76 ± 2.3 | |
| Early White | 2.39bcA ± 0.6 | 2.98 ± 0.6 | 2.96 ± 0.2 | 7.26 ± 0.9 | 12.57 ± 1.3 | 6.43bcA ± 0.9 | 25.81 ± 2.9 | 35.82 ± 2.3 | |
| Purple | 1.75cA ± 0.6 | 2.98 ± 0.6 | 2.97 ± 0.2 | 5.96 ± 0.9 | 11.25 ± 1.3 | 6.61bcB ± 0.9 | 23.77 ± 2.9 | 31.36 ± 2.3 | |
| 2018 | |||||||||
| BG | 2.44aB ± 0.3 | 2.55 ± 0.5 | 6.23 ± 0.8 | 21.56 ± 4.5 | 19.37 ± 3.0 | 9.74deA ± 0.8 | 50.22 ± 8.0 | 62.01 ± 8.2 | |
| RG | 1.08bA ± 0.3 | 2.07 ± 0.5 | 4.51 ± 0.8 | 16.99 ± 4.5 | 25.91 ± 3.0 | 15.34aA ± 0.8 | 58.26 ± 8.0 | 66.02 ± 8.2 | |
| Morado | 0.86bA ± 0.3 | 2.18 ± 0.5 | 3.83 ± 0.8 | 14.51 ± 4.5 | 20.95 ± 3.0 | 13.05bA ± 0.8 | 48.49 ± 8.0 | 55.27 ± 8.2 | |
| Rojo | 2.89aB ± 0.3 | 3.45 ± 0.5 | 4.75 ± 0.8 | 12.54 ± 4.5 | 11.39 ± 3.0 | 6.49fA ± 0.8 | 30.62 ± 8.0 | 41.12 ± 8.2 | |
| New Zealand | 0.67bB ± 0.3 | 1.32 ± 0.5 | 2.80 ± 0.8 | 10.39 ± 4.5 | 17.78 ± 3.0 | 12.35bcA ± 0.8 | 40.54 ± 8.0 | 45.41 ± 8.2 | |
| Peru | 1.80abB ± 0.3 | 3.57 ± 0.5 | 6.54 ± 0.8 | 24.00 ± 4.5 | 18.09 ± 3.0 | 7.80dfA ± 0.8 | 49.37 ± 14.3 | 62.39 ± 8.2 | |
| Late Red | 0.43bB ± 0.3 | 1.37 ± 0.5 | 3.06 ± 0.8 | 13.66 ± 4.5 | 20.23 ± 3.0 | 10.75cdA ± 0.8 | 44.86 ± 8.0 | 49.62 ± 8.2 | |
| Early White | 1.27bA ± 0.3 | 4.35 ± 0.5 | 5.24 ± 0.8 | 16.28 ± 4.5 | 18.39 ± 3.0 | 8.26efA ± 0.8 | 43.44 ± 10.0 | 53.80 ± 8.2 | |
| Purple | 0.72bA ± 0.3 | 1.44 ± 0.5 | 3.48 ± 0.8 | 11.08 ± 4.5 | 17.36 ± 3.0 | 9.68deA ± 0.8 | 38.32 ± 8.0 | 43.40 ± 8.2 | |
| Results of statistical analysis | |||||||||
| Factor | DF | p-Value for the F test of the corresponding factor | |||||||
| Year*Rep | 4 | 0.9828 | 0.9752 | 0.2029 | 0.4030 | 0.9209 | 0.9179 | 0.8986 | <0.0001 |
| G | 1 | <0.0001 | 0.0002 | 0.0009 | 0.1813 | 0.0025 | <0.0001 | 0.0236 | 0.3929 |
| year | 1 | 0.0006 | 0.0467 | 0.0014 | 0.0015 | 0.0009 | 0.0019 | 0.0011 | 0.0553 |
| Year*G | 1 | 0.0180 | 0.0784 | 0.1940 | 0.9366 | 0.6421 | 0.0095 | 0.8770 | 0.6907 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamp, L.; Hartung, J.; Mast, B.; Graeff-Hönninger, S. Tuber Yield Formation and Sugar Composition of Yacon Genotypes Grown in Central Europe. Agronomy 2019, 9, 301. https://doi.org/10.3390/agronomy9060301
Kamp L, Hartung J, Mast B, Graeff-Hönninger S. Tuber Yield Formation and Sugar Composition of Yacon Genotypes Grown in Central Europe. Agronomy. 2019; 9(6):301. https://doi.org/10.3390/agronomy9060301
Chicago/Turabian StyleKamp, Larissa, Jens Hartung, Benjamin Mast, and Simone Graeff-Hönninger. 2019. "Tuber Yield Formation and Sugar Composition of Yacon Genotypes Grown in Central Europe" Agronomy 9, no. 6: 301. https://doi.org/10.3390/agronomy9060301
APA StyleKamp, L., Hartung, J., Mast, B., & Graeff-Hönninger, S. (2019). Tuber Yield Formation and Sugar Composition of Yacon Genotypes Grown in Central Europe. Agronomy, 9(6), 301. https://doi.org/10.3390/agronomy9060301





