Protective Effects of Selenium on Wheat Seedlings under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Growth Conditions
2.2. Experimental Treatments
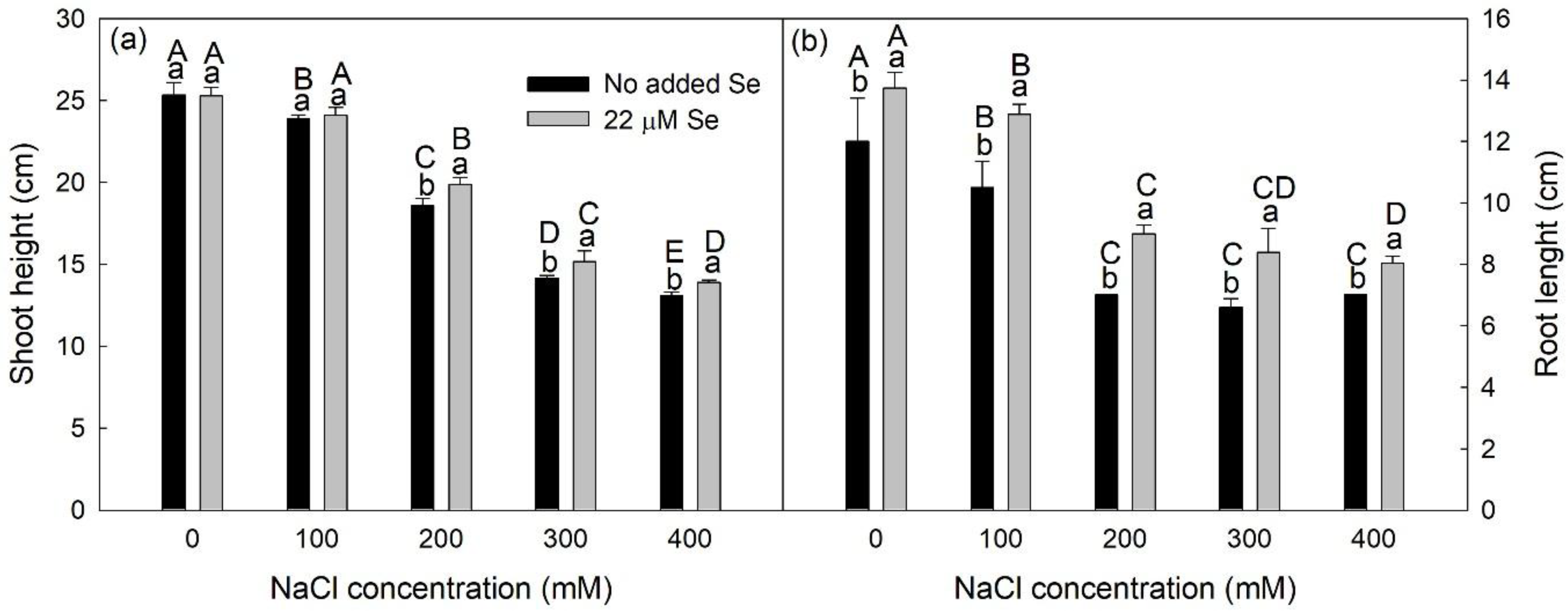
2.3. Growth Analysis
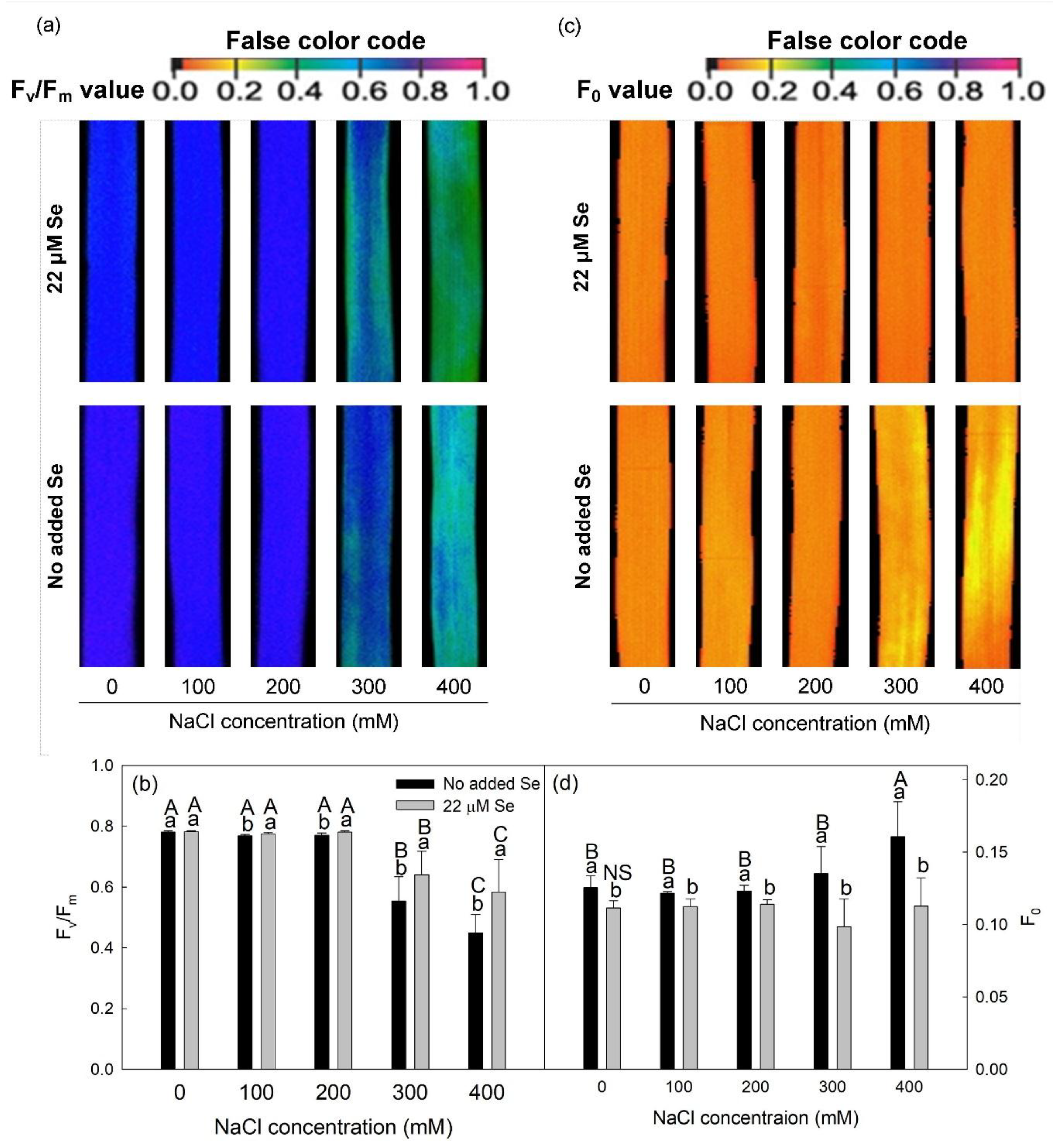
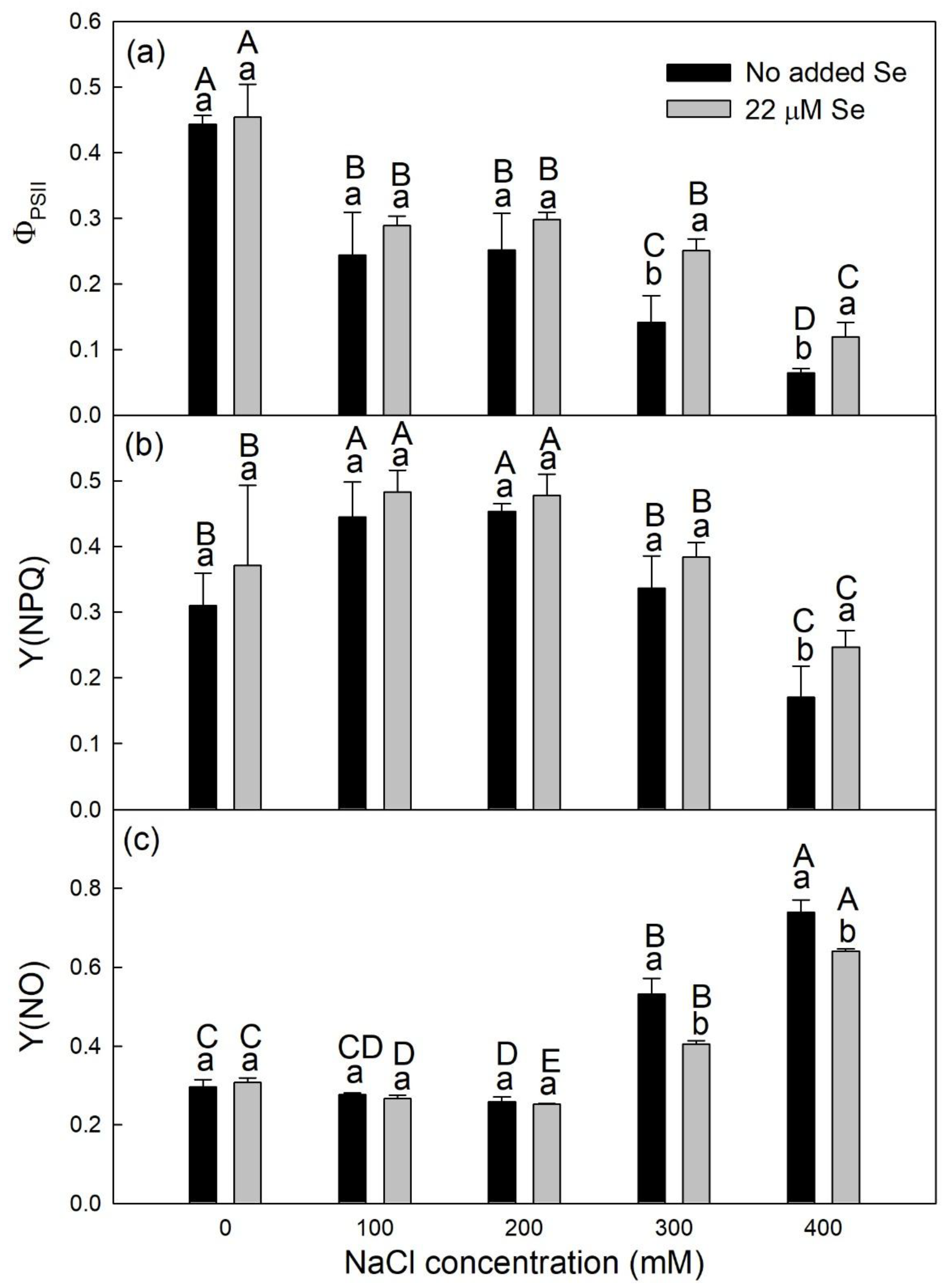
2.4. Measurements of ChlF
2.5. Measurement of Catalase (CAT) and Ascorbate Peroxidase (APX) Activities
2.6. Measurement of 1,1-Diphenyl-2-Picryl-Hydrazyl (DPPH)-Scavenging Capacity and the Reducing Power
2.7. Determination of Total Phenols, Total Flavonoids, and Anthocyanin Concentration
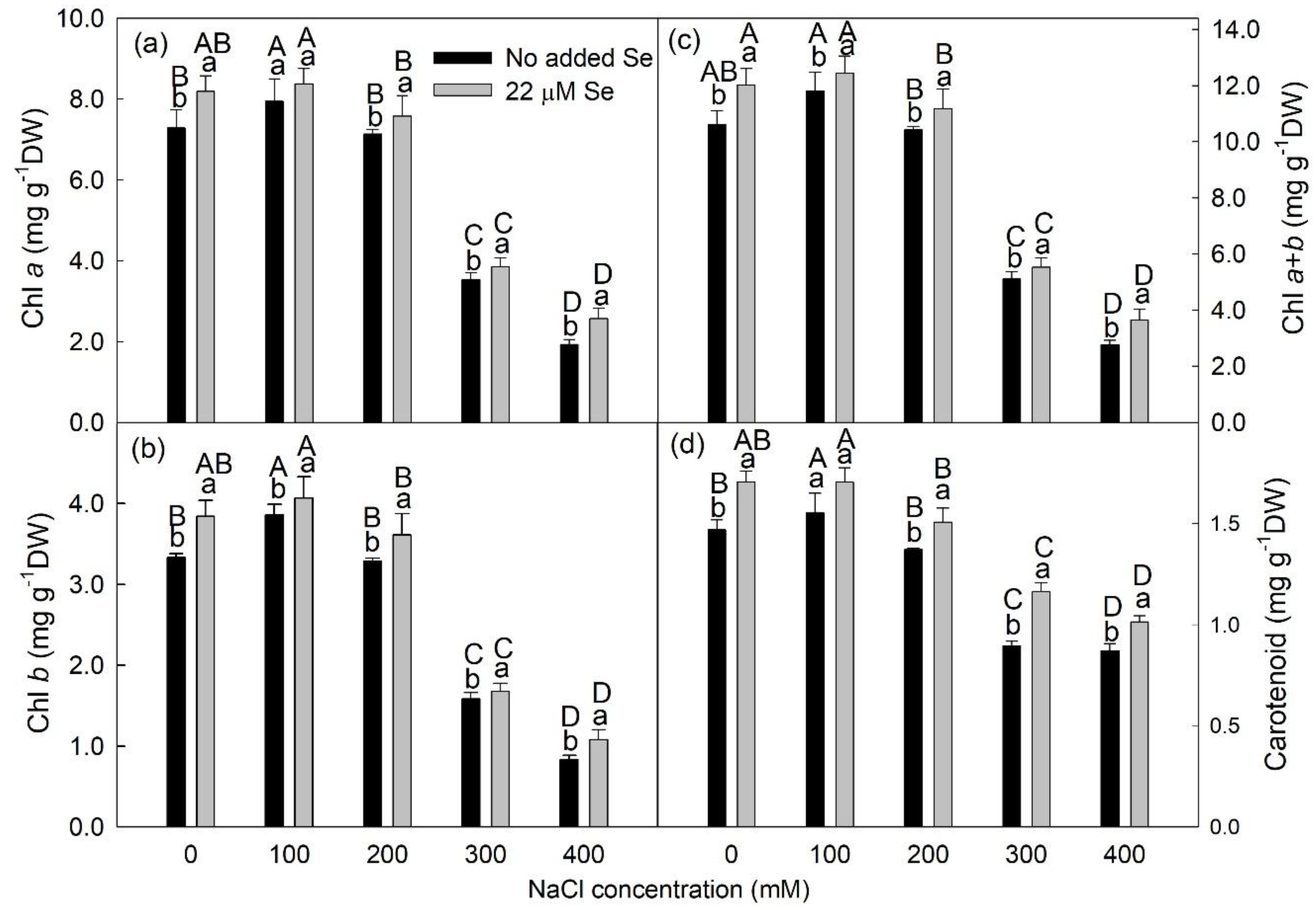
2.8. Determination of the Photosynthetic Pigment Concentrations
2.9. Statistical Analyses
3. Results
3.1. Growth Analysis
3.2. ChlF
3.3. Activities of CAT and APX, DPPH-Scavenging Capacity, and Reducing Power
3.4. Total Phenols, Total Flavonoids, and Anthocyanin Concentrations
3.5. Photosynthetic Pigments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Zhang, C.M.; Ma, J.; Zhang, Z.W.; Yuan, M.; Liu, W.J.; Zhang, H.Y.; Yuan, S. Comparison of phosphorylation and assembly of photosystem complexes and redox homeostasis in two wheat cultivars with different drought resistance. Sci. Rep. 2017, 7, 12718. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Selenium–An antioxidative protectant in soybean during senescence. Plant Soil 2005, 272, 77–86. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Hajiboland, R. Selenium supplementation stimulates vegetative and reproductive growth in canola (Brassica napus L.) plants. Acta Agric. Slov. 2012, 99, 13. [Google Scholar] [CrossRef]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A.U. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol Biochem 2015, 94, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.M.; Chen, S.F.; Shang, Z.G. Regulation of selenium on antioxidative enzymes activity in pepper leaves under high temperature stress. Acta Hort. Sinica 2005, 32, 35–38. [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef]
- Chu, J.; Yao, X.; Zhang, Z. Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol. Trace Elem. Res. 2010, 136, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mroczek-Zdyrska, M.; Wójcik, M. The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol. Trace Elem. Res. 2012, 147, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Breznik, B.; Germ, M.; Gaberscik, A.; Kreft, I. Combined effects of elevated UV-B radiation and the addition of selenium on common (Fagopyrum esculentum Moench) and tartary [Fagopyrum tataricum (L.) Gaertn.] buckwheat. Photosynthetica 2005, 43, 583–589. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; Ba, C. Antioxidant responses of wheat seedlings to exogenous selenium supply under enhanced ultraviolet-B. Biol. Trace Elem. Res. 2010, 136, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chu, J.; He, X.; Ba, C. Protective role of selenium in wheat seedlings subjected to enhanced UV-B radiation. Russ. J. Plant Physiol. 2011, 58, 283–289. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H. Association of antioxidative enzymes with the synergistic effect of selenium and UV irradiation in enhancing plant growth. Agric. Food Sci. Finl. 2000, 9, 177–186. [Google Scholar] [CrossRef]
- Xiaoqin, Y.; Jianzhou, C.; Guangyin, W. Effects of drought stress and selenium supply on growth and physiological characteristics of wheat seedlings. Acta. Physiol. Plant. 2009, 31, 1031–1036. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.; Waraich, E.; Khan, S. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Mona, I.N.; Gawish, S.M.; Taha, T.A.; Mubarak, M. Response of Wheat Plants to Application of Selenium and Humic acid under Salt Stress Conditions. Egypt. J. Soil Sci. 2017, 57, 175–187. [Google Scholar]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H.Y. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Kholodova, V.; Kuznetsov, V.V.; Yagodin, B. Selenium Regulates the Water Status of Plants Exposed to Drought. Dokl. Biol. Sci. 2003, 390, 266–268. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Beneficial Effects of Exogenous Selenium in Cucumber Seedlings Subjected to Salt Stress. Biol. Trace Elem. Res. 2009, 132, 259–269. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, L.; Wang, J.; You, Y. Influence of selenium on growth, lipid peroxidation and antioxidative enzyme activity in melon (Cucumis melo L.) seedlings under salt stress. Acta Soc. Bot. Pol. 2013, 82, 193–197. [Google Scholar]
- Sun, H.W.; Ha, J.; Liang, S.X.; Kang, W.J. Protective role of selenium on garlic growth under cadmium stress. Comm. Soil Sci. Plant Anal. 2010, 41, 1195–1204. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; Chapter 1; pp. 1–4. [Google Scholar]
- Yigit, E.; Akbulut, G.B.; Gok, Y.; Bayram, D. The effects of organic selenium on some physiological and biochemical parameters in Hordeum Vulgare L. and Triticum Aestivum L. exposed to salt stress. Fresen. Environ. Bull. 2012, 21, 743–747. [Google Scholar]
- Sattar, A.; Cheema, M.A.; Abbas, T.; Sher, A.; Ijaz, M.; Hussain, M. Separate and combined effects of silicon and selenium on salt tolerance of wheat plants. Russ. J. Plant Physiol. 2017, 64, 341–348. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res. 2004, 79, 209. [Google Scholar] [CrossRef]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chang, K.; Hsiung, T.; Lin, K.; Hunag, M.; Wu, C.; Yang, C. The antioxidant activity of methanol extract of black bean. Weed Sci. Bull. 2015, 36, 113–130. [Google Scholar]
- Mancinelli, A.; Yang, C.P.H.; Lindquist, P.; Anderson, O.; Rabino, I. Photocontrol of anthocyanin synthesis: III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Chang, K.W.; Yin, M.H.; Huang, H.M. Methods for the determination of the chlorophylls and their derivatives. Taiwania. 1998, 43, 116–122. [Google Scholar]
- Sun, Y.; Xu, W.; Fan, A. Effects of salicylic acid on chlorophyll fluorescence and xanthophyll cycle in cucumber leaves under high temperature and strong light. J. Appl. Ecol. 2006, 17, 399–402. [Google Scholar]
- Song, L.; Yue, L.; Zhao, H.; Hou, M. Protection effect of nitric oxide on photosynthesis in rice under heat stress. Acta physiol. Plant. 2013, 35, 3323–3333. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Jayanthi, P.; Lalitha, P. Reducing power of the solvent extracts of Eichhornia crassipes (Mart.) Solms. Int. J. Pharm. Pharm. Sci. 2011, 3, 126–128. [Google Scholar]
- Thiruvengadam, M.; Chung, I.M. Selenium, putrescine, and cadmium influence health-promoting phytochemicals and molecular-level effects on turnip (Brassica rapa ssp. rapa). Food Chem. 2015, 173, 185–193. [Google Scholar] [CrossRef]
- Sieprawska, A.; Kornaś, A.; Filek, M. Involvement of selenium in protective mechanisms of plants under environmental stress conditions–review. Acta Biol. Cracov. Bot. 2015, 57, 9–20. [Google Scholar] [CrossRef]
- Guerrero, B.; Llugany, M.; Palacios, O.; Valiente, M. Dual effects of different selenium species on wheat. Plant Physiol Biochem. 2014, 83, 300–307. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Sato, Y.; Murakami, T.; Funatsuki, H.; Matsuba, S.; Saruyama, H.; Tanida, M. Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J. Exp. Bot. 2001, 52, 145–151. [Google Scholar] [CrossRef]
- Gondim, F.A.; Gomes-Filho, E.; Costa, J.H.; Alencar, N.L.M.; Prisco, J.T. Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol. Biochem. 2012, 56, 62–71. [Google Scholar] [CrossRef]
- Ramos, S.J.; Yuan, Y.; Faquin, V.; Guilherme, L.R.G.; Li, L. Evaluation of genotypic variation of broccoli (Brassica oleracea var. italic) in response to selenium treatment. J. Agric. Food Chem. 2011, 59, 3657–3665. [Google Scholar] [CrossRef]
- Steyn, W.; Wand, S.; Holcroft, D.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Yuan, S.; Yuan, M.; Zhang, H.Y. Influence of stripe rust infection on the photosynthetic characteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Front. Plant Sci. 2015, 6, 779. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kong, L.; Wang, M.; Bi, D. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant. Growth Regul. 2005, 45, 155–163. [Google Scholar] [CrossRef]
| NaCl (mM) | Se treated | CAT Activity (μmol H2O2 min−1 mg−1 Protein) | APX Activity (μmol AsA min−1 mg−1 Protein) | DPPH Radical Scavenging Activity (%) | Reducing Power (BHT Equivalent g−1 DW) |
|---|---|---|---|---|---|
| 0 | No added | 1.53 ± 0.05 ns A | 0.22 ± 0.04 a A | 33.5 ± 1.1 b B | 19.2 ± 0.4 b A |
| 22 μM | 1.56 ± 0.12 ns A | 0.18 ± 0.01 b A | 43.2 ± 2.4 a A | 23.6 ± 0.4 a A | |
| 100 | No added | 1.39 ± 0.09 b A | 0.18 ± 0.02 ns AB | 36.0 ± 1.5 b A | 20.5 ± 0.9 b A |
| 22 μM | 1.79 ± 0.14 a A | 0.16 ± 0.03 ns A | 40.2 ± 0.2 a B | 23.1 ± 0.1 a A | |
| 200 | No added | 1.07 ± 0.19 b B | 0.17 ± 0.01 a AB | 28.8 ± 2.2 b C | 19.7 ± 0.5 b A |
| 22 μM | 1.62 ± 0.26 a A | 0.13 ± 0.01 b AB | 40.2 ± 1.0 a B | 21.7 ± 0.2 a B | |
| 300 | No added | 0.92 ± 0.00 ns BC | 0.13 ± 0.03 ns BC | 25.4 ± 0.2 b D | 19.2 ± 0.5 ns A |
| 22 μM | 0.98 ± 0.19 ns B | 0.10 ± 0.04 ns B | 31.9 ± 1.2 a C | 19.2 ± 0.7 ns C | |
| 400 | No added | 0.76 ± 0.02 ns C | 0.10 ± 0.02 ns C | 28.0 ± 0.2 ns CD | 15.2 ± 0.3 b B |
| 22 μM | 0.89 ± 0.14 ns B | 0.11 ± 0.04 ns B | 28.9 ± 1.7 ns D | 18.3 ± 0.4 a D |
| NaCl (mM) | Se Treated | Total Phenols Concentration (Gallic Acid Equivalent g−1 DW) | Total Flavonoids Concentration (A540 g−1 DW) | Anthocyanin Concentration (μmol g−1 DW) |
|---|---|---|---|---|
| 0 | No added | 60.9 ± 2.4 b A | 33.9 ± 3.0 b AB | 117 ± 2 b A |
| 22 μM | 65.1 ± 2.8 a A | 41.5 ± 1.0 a A | 126 ± 7 a A | |
| 100 | No added | 56.0 ± 0.9 b B | 36.0 ± 1.1 b A | 117 ± 5 b A |
| 22 μM | 63.4 ± 0.3 a AB | 42.9 ± 0.6 a A | 121 ± 1 a A | |
| 200 | No added | 56.5 ± 0.5 b B | 31.1 ± 2.4 b B | 99 ± 0 b B |
| 22 μM | 65.8 ± 2.5 a A | 37.9 ± 1.8 a B | 111 ± 2 a B | |
| 300 | No added | 58.1 ± 0.1 ns AB | 22.6 ± 1.3 b C | 76 ± 2 b C |
| 22 μM | 59.7 ± 1.7 ns AB | 27.1 ± 0.9 a C | 83 ± 0 a C | |
| 400 | No added | 51.9 ± 2.2 b C | 17.7 ± 0.7 b C | 58 ± 1 b D |
| 22 μM | 59.5 ± 3.2 a B | 21.8 ± 0.7 a D | 69 ± 5 a D |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, C.-Y.; Lin, K.-H.; Huang, W.-D.; Chen, C.-C. Protective Effects of Selenium on Wheat Seedlings under Salt Stress. Agronomy 2019, 9, 272. https://doi.org/10.3390/agronomy9060272
Lan C-Y, Lin K-H, Huang W-D, Chen C-C. Protective Effects of Selenium on Wheat Seedlings under Salt Stress. Agronomy. 2019; 9(6):272. https://doi.org/10.3390/agronomy9060272
Chicago/Turabian StyleLan, Chiu-Yueh, Kuan-Hung Lin, Wen-Dar Huang, and Chang-Chang Chen. 2019. "Protective Effects of Selenium on Wheat Seedlings under Salt Stress" Agronomy 9, no. 6: 272. https://doi.org/10.3390/agronomy9060272
APA StyleLan, C.-Y., Lin, K.-H., Huang, W.-D., & Chen, C.-C. (2019). Protective Effects of Selenium on Wheat Seedlings under Salt Stress. Agronomy, 9(6), 272. https://doi.org/10.3390/agronomy9060272








