Evaluation of Yield-Based Low Nitrogen Tolerance Indices for Screening Maize (Zea mays L.) Inbred Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Field Experiment
2.2. Phenotype Evaluation
2.3. Statistical Analysis
3. Results
3.1. Phenotypes for the Seven Screening Indices and Grain Yield under HN and LN Treatments of 31 Inbred Lines
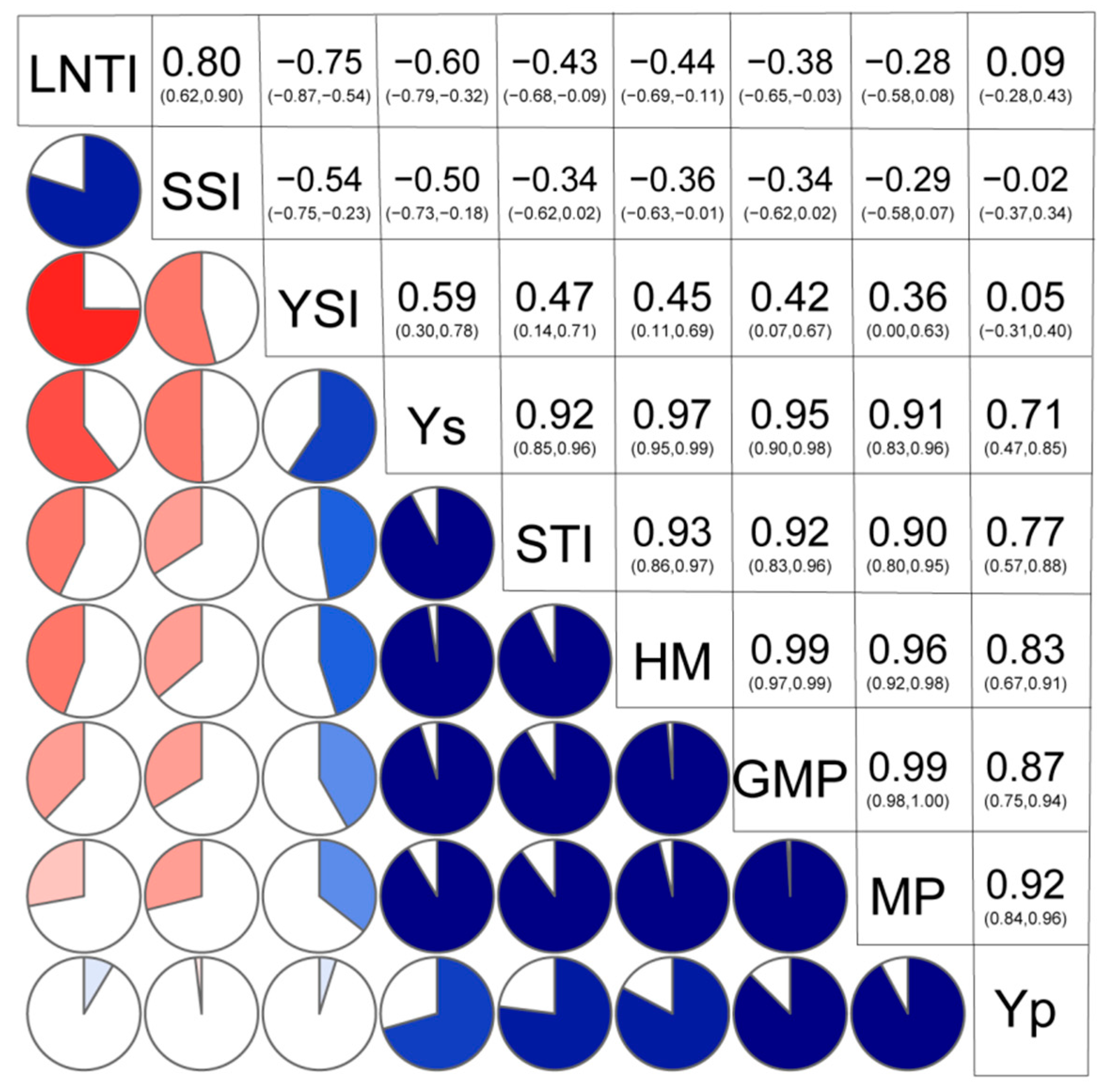
3.2. Correlation Analysis for the Seven Screening Indices and Grain Yield under HN and LN Treatments of 31 Inbred Lines
3.3. PCA for Seven Screening Indices and Grain Yield under HN and LN Treatments of 31 Inbred Lines
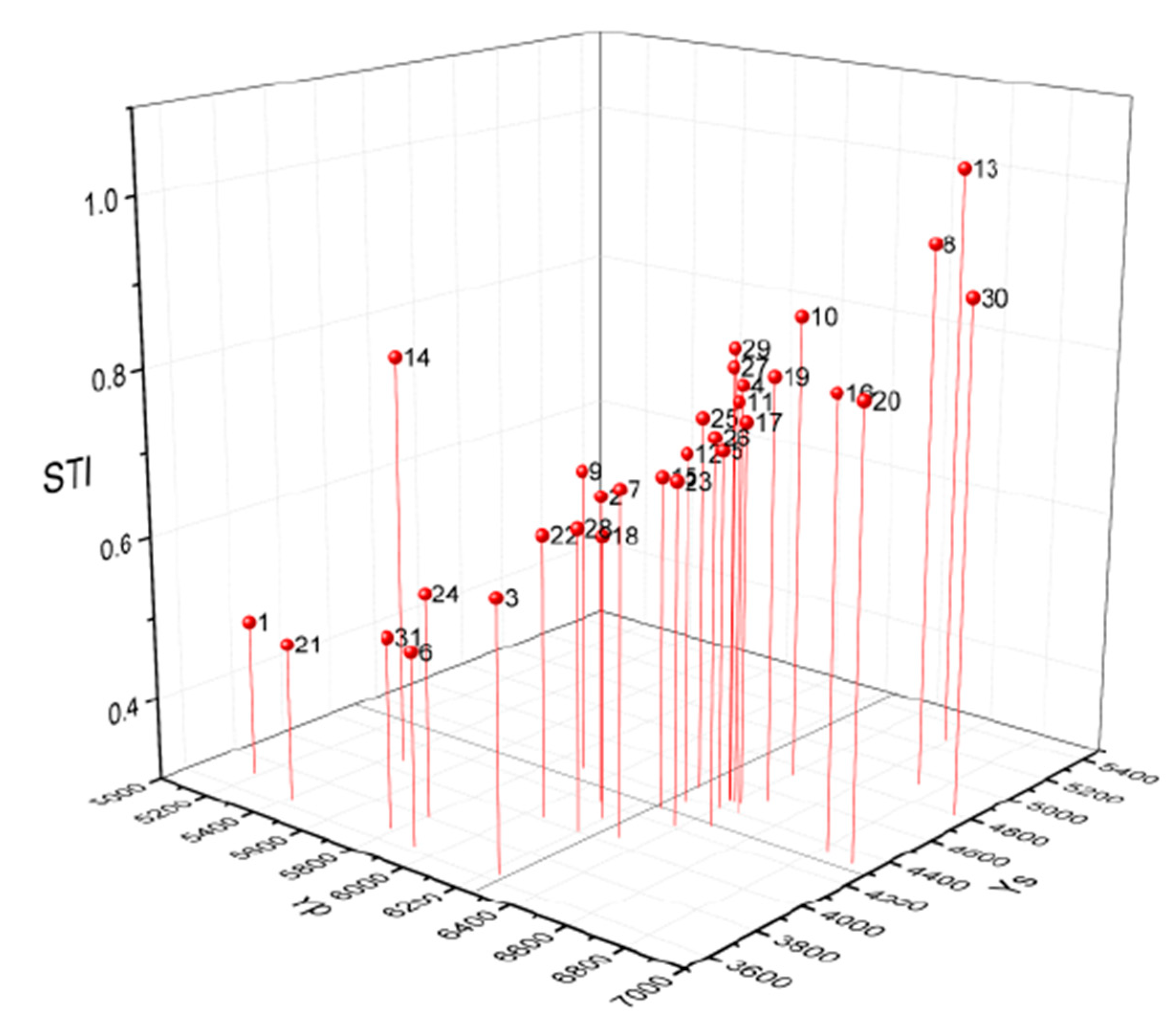
3.4. 3D Diagram for the STI Indices and Grain Yield under HN and LN Treatments of 31 Inbred Lines
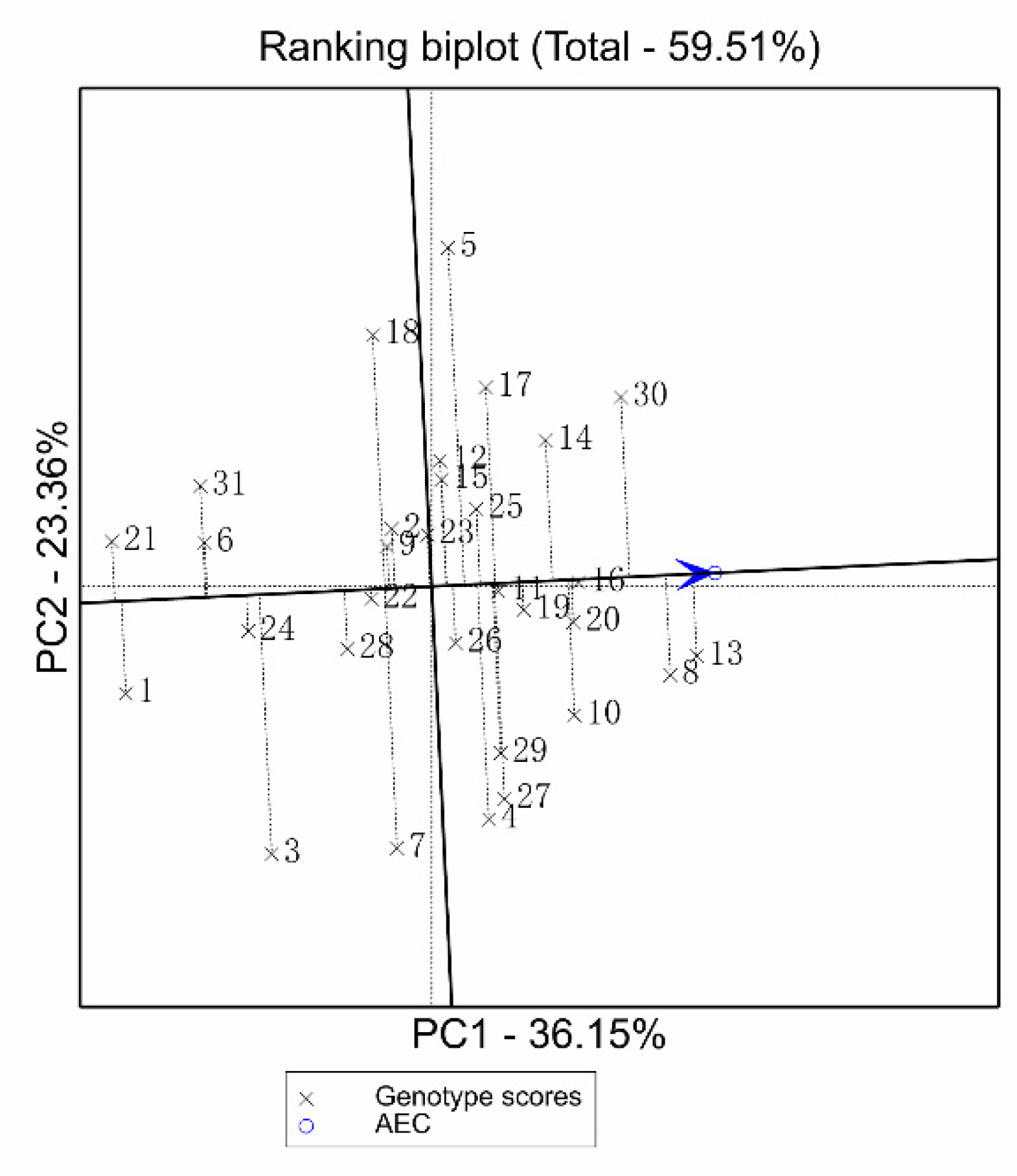
3.5. GGE Biplot for Seven Screening Indices and Grain Yield under HN and LN Treatments of 31 Inbred Lines
4. Discussion
4.1. Evaluation of Yield-Based Low Nitrogen Tolerance Indices
4.2. Analysis of the Low Nitrogen Tolerance Evaluation System for Maize
4.3. Selecting Low Nitrogen Tolerant Maize Accessions from Shaan A and Shaan B Groups
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trachsel, S.; Leyva, M.; Lopez, M.; Suarez, E.A.; Mendoza, A.; Montiel, N.G.; Macias, M.S.; Burgueno, J.; San, V.F. Identification of tropical maize germplasm with tolerance to drought, nitrogen deficiency, and combined heat and drought stresses. Crop Sci. 2016, 56, 3031–3045. [Google Scholar] [CrossRef]
- Hirel, B.; Le-Gouis, J.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Chu, Q.; Gu, R.; Yuan, L.; Liu, J.; Zhang, X.; Chen, F.; Mi, G.; Zhang, F. Identification of QTLs for plant height, ear height and grain yield in maize (Zea mays L.) in response to nitrogen and phosphorus supply. Plant Breeding 2012, 131, 502–510. [Google Scholar] [CrossRef]
- Giles, J. Nitrogen study fertilizes fears of pollution. Nature 2015, 433, 791. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.L.; Chen, D.L. Nitrogen fertilizer use in China-Contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 2002, 63, 117–127. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Ahmad, A.; Iqbal, M.; Gucel, S.; Ozturk, M. Nitrogen efficient rice cultivars can reduce nitrate pollution. Environ. Sci. Pollut. Res. 2011, 19, 607. [Google Scholar] [CrossRef]
- Liu, X.J.; Duan, L.; Mo, J.M.; Du, E.Z.; Shen, J.L.; Lu, X.K.; Zhang, Y.; Zhou, X.B.; He, C.E.; Zhang, F.S. Nitrogen depgosition and its ecological impacts in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Wen, X.Y.; Zhang, H.L.; Liu, X.H.; Chen, F. Net energy yield and carbon footprint of summer corn under different N fertilizer rates in the North China Plain. J. Integr. Agric. 2015, 14, 1534–1541. [Google Scholar] [CrossRef]
- Khan, F.U.; Mohammad, F. Application of stress selection indices for assessment of nitrogen tolerance in wheat (Triticum aestivum L.). J. Anim. Plant Sci. 2016, 26, 201–210. [Google Scholar]
- Cerón-Rojas, J.J.; Crossa, J.; Toledo, F.H.; Sahagún-Castellanos, J. A predetermined proportional gains eigen selection index method. Crop Sci. 2016, 56, 2436–2447. [Google Scholar] [CrossRef]
- Dekkers, J.C. Prediction of response to marker-assisted and genomic selection using selection index theory. J. Anim. Breed. Genet. 2015, 124, 331–341. [Google Scholar] [CrossRef]
- Mohammadi, R.; Armion, M.; Kahrizi, D.; Amri, A. Efficiency of screening techniques for evaluating durum wheat genotYpes under mild drought conditions. Int. J. Plant Prod. 2010, 4, 11–24. [Google Scholar]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campaline, R.G.; Ricciardi, G.L.; Borghi, B. Evaluation offield and laboratory predictors of drought and heat tolerance in winter cereals. Can. J. Plant Sci. 1997, 77, 523–553. [Google Scholar] [CrossRef]
- Farshadfar, E.; Sutka, J. Multivariate analysis of drought tolerance in wheat substitution lines. Cereal Res. Commun. 2003, 31, 33–40. [Google Scholar]
- Francisco, M.; Pangirayi, T.; John, D. S1 selection of localmaize landraces for low soil nitrogen tolerance in Zambia. Afr. J. Plant Sci. 2010, 4, 67–81. [Google Scholar]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Taiwan, 13–18 August 1992. [Google Scholar]
- Kristin, A.S.; Senra, R.R.; Perez, F.I.; Enriquez, B.C.; Gallegos, J.A.A.; Vallego, P.R.; Wassimi, N.; Kelley, J.D. Improving common bean performance under drought stress. Crop Sci. 1997, 37, 43–50. [Google Scholar]
- Jafari, A.; Paknejad, F.; Alahmadi, M.J. Evaluation of selection indices for drought tolerance of corn (Zea mays L.) hybrids. Int. J. Plant Prod. 2009, 3, 33–38. [Google Scholar]
- Homa, I.; Habibollah, S.; Babak, R.; Shahpoor, A. Ecaluation of salt tolerance in rice (Oryza Sativa L.) cultivars and line with emphasis on stress tolerance indices. Nature 2013, 441, 1153. [Google Scholar]
- Ganjeali, A.; Porsa, H.; Bagheri, A. Assessment of Iranian chickpea (Cicer arietinum L.) germplasms for drought tolerance. Agric. Water Manag. 2011, 98, 1477–1484. [Google Scholar] [CrossRef]
- Horn, L.; Shimelis, H.; Sarsu, F.; Mwadzingeni, L.; Laing, M.D. Genotype-by-environment interaction for grain yield among novel cowpea (Vigna unguiculata L.) selections derived by gamma irradiation. Crop J. 2017, 6, 306–313. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Oyekunle, M. Genetic analysis of grain yield and other traits of extra-early yellow maize inbreds and hybrid performance under contrasting environments. Field Crops Res. 2012, 129, 99–110. [Google Scholar] [CrossRef]
- Tonk, F.A.; Ilker, E.; Tosun, M. Evaluation of genotype × environment interactions in maize hybrids using GGE biplot analysis. Crop Breed. Appl. Biotechnol. 2011, 11, 01–09. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Yan, W.K.; Kang, M.S.; Ma, B.L.; Woods, S.; Cornelius, P.L. GGE Biplot vs. AMMI Analysis of GenotYpe-by-Environment Data. Crop Sci. 2007, 47, 641–653. [Google Scholar] [CrossRef]
- Bahrami, F.; Arzani, A.; Karimi, V. Evaluation of yield-based drought tolerance indices for screening safflower genotYpes. Agron. J. 2014, 106, 1219–1224. [Google Scholar] [CrossRef]
- Han, Y.P.; Zhao, X.; Teng, W.L.; Sun, M.M.; Zhang, H.J.; Li, W.B. Perform stability of fatty acids of soybean cultivar evaluated by GGE biplot. Soybean Sci. 2014, 33, 514–518. [Google Scholar]
- Dehghani, H.; Feyzian, E.; Mokhtar, J.; Abdolmajid, R.; Fenny, D. Use of GGE biplot methodology for genetic analysis of yield and related traits in melon (Cucumis melon L.). Can. J. Plant Sci. 2017, 92, 77–85. [Google Scholar] [CrossRef]
- Li, T.; Qu, J.Z.; Wang, Y.H.; Chang, L.G.; He, K.H.; Guo, D.W.; Zhang, X.H.; Xu, S.T.; Xue, J.Q. Genetic characterization of inbred lines from Shaan A and B groups for identifying loci associated with maize grain yield. BMC Genet. 2018, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Bouslama, M.; Schapaugh, W.T. Stress tolerance in soybean. Part 1: Evaluation of three screening techniques for heat and drought tolerance. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Kirkpatrick, L.A.; Feeney, B.C. A Simple Guide to IBM SPSS: For Version 22.0; Cengage Learning: Boston, MA, USA, 2014. [Google Scholar]
- Emebiri, L.C.; Matassa, V.; Moody, D.B. GENSTAT Programs for Performing Muir’s Alternative Partitioning of Genotype-by-Environment Interaction. J. Hered. 2005, 96, 78–79. [Google Scholar] [CrossRef]
- SAS, Institute, Inc. SAS/STAT User’s Guide, Version 6; SAS Institute Inc.: Cary, NC, USA, 2010. [Google Scholar]
- He, K.H.; Chang, L.G.; Dong, Y.; Cui, T.T.; Qu, J.Z.; Liu, X.Y.; Xu, S.T.; Xue, J.Q.; Liu, J.C. Identification of quantitative trait loci for agronomic and physiological traits in maize (Zea mays L.) under high-nitrogen and low-nitrogen conditions. Euphytica 2018, 214, 15. [Google Scholar] [CrossRef]
- Akçura, M.; Partigoç, F.; Kaya, Y. Evaluating of drought stress tolerance based on selections indices in Turkish bread wheat landraces. J. Anim. Plant Sci. 2011, 21, 700–709. [Google Scholar]
- Dencic, S.; Kastori, R.; Kobiljski, B.; Duggan, B. Evaluation of grain yield and its components in wheat cultivars and landraces under near optimal and drought conditions. Euphytica 2000, 113, 43–52. [Google Scholar] [CrossRef]
- Rehman, S.; Harris, P.J.C.; Ashraf, M. Stress environments and their impact on crop production. In Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches; Food Products Press: New York, NY, USA, 2005; pp. 3–18. [Google Scholar]
- Ceccarelli, S.; Grando, S.; Impiglia, A. Choice of selection strategy in breeding barley for stress environments. Euphytica 1998, 103, 307–318. [Google Scholar] [CrossRef]
- Shiranirad, A.H.; Abbasian, A. Evaluation of drought tolerance in rapeseed genotypes under non stress and drought stress conditions. Notulae Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 164–171. [Google Scholar] [CrossRef]
- Nouri, A.; Etminan, A.; Silva, J.A.T.; Mohammadi, R. Assessment of yield, yield-related traits and drought tolerance of durum wheat genotypes (Triticum turjidum var. durum Desf.). Aust. J. Crop Sci. 2011, 5, 8–16. [Google Scholar]
- Lyra, D.H.; de Freitas Mendonça, L.; Galli, G.; Alves, F.C.; Granato, Í.S.C.; Fritsche-Neto, R. Multi-trait genomic prediction for nitrogen response indices in tropical maize hybrids. Mol. Breed. 2017, 37, 80. [Google Scholar] [CrossRef]
- Fotyma, E.; Fotyma, M. The agronomical and physiological efficiency of nitrogen applied for arable crops in Poland. Fertil. Res. 1995, 43, 9–12. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef]
- Wu, Y.S.; Liu, W.G.; Li, X.H.; Li, M.S.; Zhang, D.G.; Hao, Z.F.; Weng, J.F.; Xu, Y.B.; Bai, L.; Zhang, S.H.; et al. Low-nitrogen stress tolerance and nitrogen agronomic efficiency among maize inbreds: Comparison of multiple indices and evaluation of genetic variation. Euphytica 2011, 180, 281–290. [Google Scholar] [CrossRef]
- Duan, W.; Wang, Q.; Zhang, H.; Xie, B.; Li, A.; Hou, F.; Dong, S.; Wang, B.; Qin, Z.; Zhang, L. Differences between nitrogen-tolerant and nitrogen-susceptible sweetpotato cultivars in photosynthate distribution and transport under different nitrogen conditions. PLoS ONE 2018, 13, e0194570. [Google Scholar] [CrossRef]
- Zhong, S.R.; Gong, S.Y.; Zhang, S.C.; Chen, R.X.; Liu, Q.Y.; Zhai, X.Q. Research progress on low nitrogen tolerance and nitrogen efficiency in crop plants. J. Nucl. Agric. Sci. 2018, 32, 1656–1663. [Google Scholar]
- Wang, B.X.; Wang, Y.H.; Chen, P.F.; Li, D.Y.; Feng, Z.Q.; Hao, Y.C.; Zhang, R.H.; Zhang, X.H.; Xue, J.Q. Combining ability of maize inbred lines from Shaan A Group and Shaan B group under different density conditions. Acta Agron. Sinica 2017, 43, 1328–1336. (In Chinese) [Google Scholar] [CrossRef]

| Code | Group1 | Genotype | Yp (kg ha−1) | Ys (kg ha−1) | GMP | HM | MP | STI | YSI | LNTI | SSI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shaan A | KA008 | 5184.35 | 3705.16 | 4392.91 | 4306.59 | 4471.99 | 0.49 | 0.71 | 0.24 | 0.95 |
| 2 | Shaan A | 2012KA-1 | 6107.00 | 4214.43 | 5091.76 | 4989.91 | 5203.15 | 0.67 | 0.68 | 0.28 | 0.93 |
| 3 | Shaan A | KA064 | 6235.58 | 3636.09 | 4653.10 | 4399.80 | 4969.88 | 0.62 | 0.62 | 0.42 | 1.19 |
| 4 | Shaan A | 2012KA-58 | 6466.16 | 4400.15 | 5318.04 | 5180.47 | 5385.22 | 0.81 | 0.67 | 0.27 | 0.97 |
| 5 | Shaan A | KA103 | 6401.76 | 4393.07 | 5315.92 | 5203.67 | 5444.96 | 0.73 | 0.71 | 0.24 | 0.80 |
| 6 | Shaan A | KA203 | 5920.33 | 3626.73 | 4589.25 | 4397.79 | 4805.05 | 0.53 | 0.64 | 0.36 | 1.09 |
| 7 | Shaan A | 2013KA-34 | 6317.18 | 4047.09 | 4987.97 | 4789.97 | 5223.45 | 0.71 | 0.65 | 0.33 | 0.85 |
| 8 | Shaan A | KA105 | 6734.33 | 4923.36 | 5780.16 | 5698.23 | 5887.49 | 0.95 | 0.70 | 0.22 | 0.67 |
| 9 | Shaan A | KA227 | 5899.12 | 4370.32 | 5089.30 | 5009.21 | 5178.11 | 0.67 | 0.71 | 0.21 | 0.61 |
| 10 | Shaan A | KA225 | 6410.79 | 4740.25 | 5530.16 | 5450.85 | 5628.76 | 0.86 | 0.69 | 0.25 | 0.72 |
| 11 | Shaan A | XCA-1 | 6399.93 | 4466.13 | 5372.68 | 5278.98 | 5481.75 | 0.78 | 0.70 | 0.26 | 0.90 |
| 12 | Shaan A | KA060 | 6291.86 | 4369.85 | 5267.45 | 5170.89 | 5377.20 | 0.72 | 0.69 | 0.27 | 1.00 |
| 13 | Shaan B | KB081 | 6591.79 | 5272.05 | 5918.44 | 5869.12 | 5995.13 | 1.01 | 0.98 | 0.15 | 0.55 |
| 14 | Shaan B | KB417 | 5450.83 | 4064.38 | 4727.30 | 4661.26 | 4792.66 | 0.80 | 0.76 | 0.19 | 0.87 |
| 15 | Shaan B | KB109 | 6274.73 | 4282.33 | 5179.44 | 5055.69 | 5323.33 | 0.70 | 0.69 | 0.30 | 1.09 |
| 16 | Shaan B | 91227 | 6824.74 | 4347.14 | 5441.65 | 5269.80 | 5630.59 | 0.83 | 0.65 | 0.36 | 1.07 |
| 17 | Shaan B | KB-7 | 6433.13 | 4461.62 | 5368.64 | 5258.73 | 5496.27 | 0.76 | 0.68 | 0.27 | 0.84 |
| 18 | Shaan B | KB020 | 6194.17 | 4118.80 | 5043.45 | 4902.05 | 5198.03 | 0.64 | 0.69 | 0.25 | 0.63 |
| 19 | Shaan B | 2013KB-37 | 6472.23 | 4532.60 | 5406.49 | 5284.90 | 5552.75 | 0.81 | 0.70 | 0.26 | 0.92 |
| 20 | Shaan B | 2013KB-47 | 6929.63 | 4325.99 | 5500.27 | 5344.82 | 5678.26 | 0.83 | 0.66 | 0.38 | 1.37 |
| 21 | Shaan B | KB043 | 5411.19 | 3643.14 | 4411.33 | 4276.45 | 4555.11 | 0.49 | 0.66 | 0.29 | 0.88 |
| 22 | Shaan B | Z140588 | 6061.74 | 4016.02 | 4922.82 | 4783.60 | 5077.77 | 0.64 | 0.68 | 0.32 | 1.07 |
| 23 | Shaan B | Z140580 | 6382.42 | 4212.83 | 5198.92 | 5070.63 | 5342.11 | 0.71 | 0.66 | 0.32 | 1.06 |
| 24 | Shaan B | 2013HXB-4 | 5807.66 | 3800.68 | 4710.44 | 4585.85 | 4837.65 | 0.57 | 0.66 | 0.34 | 1.12 |
| 25 | Shaan B | 2013ZZB-6 | 6256.82 | 4484.76 | 5322.61 | 5240.09 | 5418.73 | 0.75 | 0.71 | 0.27 | 1.17 |
| 26 | Shaan B | 2014KB-54 | 6465.35 | 4276.59 | 5259.68 | 5120.53 | 5417.12 | 0.76 | 0.66 | 0.32 | 1.06 |
| 27 | Shaan B | KB215 | 6382.60 | 4463.20 | 5309.47 | 5185.88 | 5471.44 | 0.82 | 0.69 | 0.27 | 0.89 |
| 28 | Checks | Zheng58 | 6198.42 | 4003.97 | 4972.65 | 4819.70 | 5140.92 | 0.66 | 0.65 | 0.33 | 0.94 |
| 29 | Checks | Chang7-2 | 6378.99 | 4471.96 | 5147.36 | 5344.59 | 5244.57 | 0.84 | 0.66 | 0.30 | 1.02 |
| 30 | Checks | PH6WC | 6950.85 | 4795.11 | 5789.19 | 5675.06 | 5931.18 | 0.91 | 0.69 | 0.25 | 0.77 |
| 31 | Checks | PH4CV | 5782.55 | 3674.20 | 4591.15 | 4436.34 | 4759.62 | 0.53 | 0.67 | 0.34 | 1.10 |
| Source of Variation | Df | F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yp | Ys | SSI | STI | MP | GMP | YSI | HM | LNTI | ||
| Genotype (G) | 30 | 12.053** | 7.192** | 3.037** | 20.545** | 11.36** | 13.301** | 2.334** | 10.524** | 2.616** |
| Year (Y) | 2 | 7.11** | 31.34** | 5.994** | 16.703** | 25.951** | 34.471** | 18.105** | 30.118** | 17.411** |
| Location (L) | 1 | 1012.76** | 4.196* | 0.764 | 247.582** | 304.328** | 219.199** | 261.056** | 97.121** | 256.633** |
| G × Y | 60 | 1.65** | 1.158 | 1.884** | 1.447* | 1.269 | 1.544* | 1.435* | 1.436* | 1.477* |
| G × L | 30 | 9.969** | 5.114** | 6.958** | 14.068** | 8.32** | 8.421** | 4.586** | 6.957** | 4.481** |
| G × L × Y | 60 | 2.742** | 1.726** | 2.532** | 2.303** | 2.683** | 2.843** | 1.307 | 2.366** | 1.31 |
| Contribution to Variation | Cumulative Percentage | Yp | Ys | GMP | MP | STI | SSI | YSI | HM | LNTI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ————%———— | ——kg ha−1—— | ||||||||||
| PC1 | 68.14 | 68.14 | 0.77 | 0.99 | 0.97 | 0.95 | 0.94 | −0.50 | 0.56 | 0.98 | −0.56 |
| PC2 | 22.89 | 91.03 | 0.61 | −0.06 | 0.21 | 0.29 | 0.12 | 0.70 | −0.64 | 0.15 | 0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; He, K.; Feng, Z.; Li, Y.; Chang, L.; Zhang, X.; Xu, S.; Liu, J.; Xue, J. Evaluation of Yield-Based Low Nitrogen Tolerance Indices for Screening Maize (Zea mays L.) Inbred Lines. Agronomy 2019, 9, 240. https://doi.org/10.3390/agronomy9050240
Zhao Z, He K, Feng Z, Li Y, Chang L, Zhang X, Xu S, Liu J, Xue J. Evaluation of Yield-Based Low Nitrogen Tolerance Indices for Screening Maize (Zea mays L.) Inbred Lines. Agronomy. 2019; 9(5):240. https://doi.org/10.3390/agronomy9050240
Chicago/Turabian StyleZhao, Zhixin, Kunhui He, Zhiqian Feng, Yanan Li, Liguo Chang, Xinghua Zhang, Shutu Xu, Jianchao Liu, and Jiquan Xue. 2019. "Evaluation of Yield-Based Low Nitrogen Tolerance Indices for Screening Maize (Zea mays L.) Inbred Lines" Agronomy 9, no. 5: 240. https://doi.org/10.3390/agronomy9050240
APA StyleZhao, Z., He, K., Feng, Z., Li, Y., Chang, L., Zhang, X., Xu, S., Liu, J., & Xue, J. (2019). Evaluation of Yield-Based Low Nitrogen Tolerance Indices for Screening Maize (Zea mays L.) Inbred Lines. Agronomy, 9(5), 240. https://doi.org/10.3390/agronomy9050240





